Back to Journals » Neuropsychiatric Disease and Treatment » Volume 10
COMT Val158Met, but not BDNF Val66Met, is associated with white matter abnormalities of the temporal lobe in patients with first-episode, treatment-naïve major depressive disorder: a diffusion tensor imaging study
Authors Hayashi K, Yoshimura R , Kakeda S, Kishi T , Abe O, Umene-Nakano W, Katsuki A, Hori H, Ikenouchi-Sugita A , Watanabe K, Ide S, Ueda I , Moriya J, Iwata N , Korogi Y, Kubicki M, Nakamura J, Nishimura J, Goto N
Received 24 January 2014
Accepted for publication 27 February 2014
Published 25 June 2014 Volume 2014:10 Pages 1183—1190
DOI https://doi.org/10.2147/NDT.S61275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Kenji Hayashi,1 Reiji Yoshimura,1 Shingo Kakeda,2 Taro Kishi,3 Osamu Abe,4 Wakako Umene-Nakano,1 Asuka Katsuki,1 Hikaru Hori,1 Atsuko Ikenouchi-Sugita,1 Keita Watanabe,2 Satoru Ide,2 Issei Ueda,2 Junji Moriya,2 Nakao Iwata,3 Yukunori Korogi,2 Marek Kubicki,5 Jun Nakamura1
1Department of Psychiatry, 2Department of Radiology, University of Occupational and Environmental Health, Kitakyushu, Japan; 3Department of Psychiatry, Fujita Health University, Toyoake, Japan; 4Department of Radiology, Nihon University School of Medicine, Tokyo, Japan; 5Psychiatry Neuroimaging Laboratory, Brigham and Women's Hospital, Harvard Medical School, Boston, MA, USA
Abstract: We investigated the association between the Val158Met polymorphism of the catechol-O-methyltransferase (COMT) gene, the Val66Met polymorphism of the brain-derived neurotrophic factor (BDNF) gene, and white matter changes in patients with major depressive disorder (MDD) and healthy subjects using diffusion tensor imaging (DTI). We studied 30 patients with MDD (17 males and 13 females, with mean age ± standard deviation [SD] =44±12 years) and 30 sex- and age-matched healthy controls (17 males and 13 females, aged 44±13 years). Using DTI analysis with a tract-based spatial statistics (TBSS) approach, we investigated the differences in fractional anisotropy, radial diffusivity, and axial diffusivity distribution among the three groups (patients with the COMT gene Val158Met, those with the BDNF gene Val66Met, and the healthy subjects). In a voxel-wise-based group comparison, we found significant decreases in fractional anisotropy and axial diffusivity within the temporal lobe white matter in the Met-carriers with MDD compared with the controls (P<0.05). No correlations in fractional anisotropy, axial diffusivity, or radial diffusivity were observed between the MDD patients and the controls, either among those with the BDNF Val/Val genotype or among the BDNF Met-carriers. These results suggest an association between the COMT gene Val158Met and the white matter abnormalities found in the temporal lobe of patients with MDD.
Keywords: catechol-O-methyltransferase, brain-derived neurotrophic factor, 3-methoxy-4-hydroxyphenylglycol, homovanillic acid
Introduction
Catecholamines play an important role in the pathogenesis of major depressive disorder (MDD).1 Catechol-O-methyltransferase (COMT) is a methylation enzyme that plays a role in the degradation of noradrenaline and dopamine, by catalyzing the transfer of a methyl group from S-adenosylmethionine. Biochemical research has established that the enzyme activities in patients with MDD differ from those of nondepressed subjects.2 The COMT gene is located at 22q 11.21. In a multicenter European study, an association was found between the COMT gene Val158Met (G324A) functional polymorphism and MDD.3 The Val allele has been reported to result in three- to fourfold higher activity than the Met allele.4 One report suggests that there is an association between higher activity of the COMT gene Val158Val-type and a poor antidepressant treatment response.5 The Met-variants of COMT gene Val158Met were shown to be risk variants for depressed mood and low motivation in depressive Swedish men.6
Brain-derived neurotrophic factor (BDNF) is a molecular substrate of stress; data have demonstrated that BDNF expression is reduced by stress (an important risk factor for MDD and posttraumatic stress disorder)7 and correlates to hippocampus volume in patients.8 The levels of BDNF and its receptor, tropomyosin-related kinase B (TrkB) receptor, are decreased in regions of the hippocampus in postmortem tissue taken from suicide victims and patients with MDD, and in the serum of MDD patients.9–11 Researchers have investigated the BDNF gene for a single nucleotide polymorphism (SNP) that might be linked to MDD. The most common BDNF SNP in humans is at codon 66, resulting in the Val66Met protein variant, which prevents the activity-dependent release of BDNF.12 Men homozygous for the mutation might be at greater risk for MDD.13 It has been hypothesized that monoamine and BDNF are associated with the pathogenesis of MDD.14
The white matter (WM) abnormalities constitute one element of the pathogenesis of MDD.15–17 Various fiber tract alterations have been seen in MDD patients.18–22 Magnetic resonance imaging (MRI) is a noninvasive method used to examine WM abnormalities. Diffusion tensor imaging (DTI) is an MRI technique that can study the orientation and integrity of WM fiber tracts in vivo.23 DTI-based quantitative measures, such as fractional anisotropy (FA), represents intact myelin and axons, and has been shown to be a useful marker of the microstructural changes in WM.
Although several studies using a voxel-based DTI analysis demonstrated lower FA values in the frontal, temporal, and parietal lobes and the cerebellum of MDD patients,24–28 such an analysis is not a mainstream of statistical parametric mapping and is not officially supported. Therefore, there has not been a consensus about the optimal method to spatially normalize FA images and the size of the smoothing kernel. A voxel-wise approach of tract-based spatial statistics (TBSS) has been introduced. The TBSS method projects all subjects’ FA data onto an average FA tract skeleton before applying voxel-wise cross-subject statistics, and it minimizes the misalignment effects and is more robust and sensitive than voxel-based DTI analyses.18
The findings from individual reports of WM abnormalities in MDD patients indicate a widespread pattern of alterations, and the extent of WM abnormalities might be associated with clinical features. Indeed, the severity of illness and poorer treatment outcomes have been associated with increased WM pathology, indicating that patients with a greater illness burden are more likely to have microstructural damages.29 The most pronounced WM FA reductions have been observed in the main body and genu of the corpus callosum, consistent with some, but not all, DTI reports in MDD. FA values of the WM in the right frontal lobe, right fusiform gyrus, left frontal lobe, and right occipital lobe were also demonstrated to be reduced. Fiber tracking has shown that the main fascicles involved were the right inferior longitudinal fasciculus, right inferior fronto-occipital fasciculus, right posterior thalamic radiation, and interhemispheric fibers running through the genu and body of the corpus callosum.30
Carballedo et al31 recently reported that they observed a significant interaction, in the uncinated fasciculus, between a BDNF allele and diagnosis: patients carrying the BDNF Met allele had lower FA values in the uncinated fasciculus compared with healthy subjects carrying the Met allele. Kim et al32 reported an association between altered WM connectivity and COMT gene Val158Met polymorphism in panic disorder patients. We hypothesize that the COMT gene and the BDNF gene are associated with WM connectivity in MDD patients.
In the present study, therefore, we compared the status of polymorphism of the COMT gene or BDNF gene and DTI findings between drug-naïve MDD patients and age- and sex-matched healthy controls.
Subjects and methods
Subjects
Thirty first-episode, right-handed, treatment-naïve outpatients were recruited. Major depressive episodes were diagnosed using the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) according to the DSM-IV, text revision (TR) criteria. The severity of depression was evaluated using the 17-item Hamilton Rating Scale for Depression (HAMD17). Only those patients with a HAMD17 score ≥14 were eligible for the study. Exclusion criteria were: any history of neurological disease or other physical disease, and comorbidity with other mental disorders (no evidence of schizoaffective disorder, bipolar disorder, Axis II personality disorders, or mental retardation). In all, 17 subjects were male, and 13 were female. The age range was from 20 to 67 years, with a mean ± standard deviation (SD) age of 44±12 years. Similarly, 30 right-handed healthy subjects, 17 male and 13 female, with mean age 44±13 years were recruited from the community.
The DTI scans for all 60 subjects were performed on the day when each subject was enrolled. The 30 control subjects were interviewed by the same psychiatrists that interviewed the MDD patients, using the Structured Clinical Interview for DSM-IV, nonpatient edition. None of the control subjects had a history of serious medical or neuropsychiatric illness or a family history of major psychiatric or neurological illness in their first-degree relatives, and all were well matched with the patients in terms of age, sex, and years of education. All subjects were given complete information about the procedures. Written informed consent was obtained from all subjects via forms approved by the local Ethics Committee of the University of Occupational and Environmental Health, Kitakyushu, Japan.
Methods
Diffusion tensor images: MRI scanning protocol
All MRI examinations were performed using a 3T MRI system (Signa® EXCITE™ 3T; GE Healthcare, Little Chalfont, UK) with an eight-channel brain phased-array coil. DTIs were acquired by a single-shot, spin-echo planar sequence, with the following parameters: TR/TE =12,000/83.3 msec; 4 mm slice thickness; no gap; field of view =26 cm; number of excitations =1, spatial resolution =1.02×1.02×4 mm. Diffusion gradients (b-value of 1,000 sec/mm2) were always applied on two axes simultaneously around the 180-degree pulse. The diffusion properties were measured along 25 noncollinear directions. The spatial distortion of diffusion-weighted MRIs was corrected based on each T2-weighted echo-echo planar image (b=0 sec/mm2)33 using registration functional MRI of the brain (FMRIB) tools.
Image processing
Maps of FA were computed for all subjects from the DTIs, after eddy current correction and automatic brain extraction using the FMRIB Diffusion Toolbox, which is part of the FMRIB Software Library (The Oxford Centre for Functional MRI of the Brain, Oxford, UK).34 We performed a voxel-wise statistical analysis of the DTI data using TBSS35 (implemented in the FMRIB Software Library 4.1.6). The FA, radial diffusivity (RD), and axial diffusivity (AD) were created by fitting a tensor model to the raw diffusion data. Brain extraction was then performed using the Brain Extraction Tool 2.1.36
The FA data of all subjects were aligned into a common space by means of nonlinear registration.37 Next, a mean FA image was created and thinned to create a mean FA skeleton representing the centers of all tracts common to the group. This skeleton was thresholded at FA >0.2. Each subject’s aligned FA data were then projected onto this skeleton, and the resulting data were fed into a voxel-wise cross-subject statistical analysis. Subsequently, other relevant DTI output images (AD and RD) were projected onto the mean FA skeleton so that other diffusivity values could be compared between groups in the same spatial location.
We compared the DTI metrics between the MDD and control groups using a TBSS analysis.
Genotyping and serum catecholamine metabolites assay
Genomic DNA was extracted from peripheral leukocytes using a QIAamp® DNA Blood Kit (Qiagen, Venlo, the Netherlands) and was stored at −20°C until used for analysis. Genotyping for the presence of the BDNF Val66Met and COMT Val158Met polymorphisms was performed using direct sequencing in the region.
We analyzed the subjects’ plasma concentrations of homovanillic acid (HVA) and 3-methoxy-4-hydroxyphenylglycol (MHPG) by high-performance liquid chromatography with electrochemical detection (HPLC-ECD). The plasma HVA levels were analyzed by HPLC-ECD according to the method of Yeung et al,38 with slight modification. In brief, each cyano-bonded solid-phase extraction cartridge was preconditioned with methanol, followed by glass-distilled water. To each cartridge we added 0.3 mL of plasma sample or standard, and 0.1 mL of working internal standard solution (5 ng of 5-hydroxyindolecarboxylic acid in 0.01 M KH2PO4, pH 7.2). The samples were deproteinized with 1 mL of acetonitrile. After mixing by vortex and centrifugation (1,760× g, 4°C for 10 minutes), an aliquot (5 μL) of supernatant was allowed to pass through the cartridge slowly, under a mild vacuum (15 mmHg). The cartridge was washed with 0.2 mL of distilled water and extracted containing 1 mL of ethylacetate, and then an aliquot was evaporated to dryness under nitrogen gas. After dissolution in mobile phase (200 μL), a 10 μL portion of this solution was injected into the HPLC system. The detection limit was 0.5 ng/mL, and the calibration curve was linear up to 40 ng/mL. The intra- and interassay coefficients of variation were 6% and 8%, respectively. The recovery rate was more than 80%.
The subjects’ plasma MHPG levels were also analyzed by HPLC-ECD, according to the method of Minegishi and Ishizaki.39 In brief, the plasma was separated by centrifugation at 600× g at 4°C. Extraction was performed under a vacuum using Bond-Elut columns (Varian Medical Systems, Inc., Palo Alto, CA, USA) prepacked with 100 mg of C18-bonded silica (40 μm) in a 1 mL capacity disposable syringe. The columns, which were inserted into a vacuum chamber connected to an aspirator, were prepared by washing with 1 mL methanol followed by 1 mL of water. After the addition of 50 μL of a solution of vanillyl alcohol (internal standard equivalent to 5 ng/mL) to 1 mL of plasma, the samples were passed through the columns, followed by 0.75 mL of water to rinse off both residual samples and easily eluted hydrophilic compounds.
The adsorbed materials were eluted with 200 μL of methanol to a 0.1 M phosphate buffer (pH 4.8) mixture (40:60, v/v [volume/volume]). A 20 μL portion of this solution was injected into the HPLC system. The detection limit was 0.5 ng/mL, and the calibration curve was linear up to 40 ng/mL. The intra- and interassay coefficients of variation were 6% and 8%, respectively. The recovery rate was more than 80%.
Statistical analyses
The significance threshold for between-group differences was set at family-wise error (FWE)-corrected P<0.05; this was corrected for multiple comparisons across voxels by using the threshold-free cluster-enhancement option. The number of permutations was set to 20,000 in all voxel-wise analyses. The chi-square test was used to compare the number of patients with the COMT or BDNF genotype Val/Val, and the number of Met-carriers in both the MDD patient and the control groups. The unpaired t-test was used to compare the items of the HAMD17 scores, and the plasma levels of MHPG and HVA between the Val/Val group and Met-carriers in the MDD patient group. The unpaired t-test was also used to compare serum BDNF levels between the MDD patients and the healthy controls. A significance level of P<0.05 was used. Statistical procedures were performed using the Japanese version of SPSS, version 15 (SPSS Inc., Chicago, IL, USA).
Results
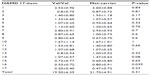
The genotype distributions of the COMT Val158Met polymorphism were determined in both the MDD patients and the control subjects, as shown in Table 1. The table provides the allele and genotype distributions as well as the chi-square and P-values of Hardy–Weinberg equilibria. As can be seen in Table 2, there were no significant differences among the MDD patients in each item of the HAMD17, with the exception that the responses to item 16 (weight loss) differed significantly between the COMT Val/Val group and the COMT Met-carriers.
  | Table 1 Gene distribution of COMT and BDNF in patients with MDD and healthy controls |
The analysis of the plasma levels of catecholamine metabolites (MHPG and HVA) revealed no significant differences between the MDD patients and the controls (MHPG was 5.3±1.0 ng/mL for MDD and was 5.4±1.2 ng/mL for controls [P=0.23]; HVA was 5.5±1.4 ng/mL for MDD and was 5.1±0.8 ng/mL for the controls [P=0.13]). In addition, no significant differences between the Val/Val group and the Met-carriers were found in plasma MHPG, a major metabolite of norepinephrine (5.0±1.4 ng/mL [Val/Val group]; 4.9±1.5 [Met-carriers] [P=0.85]) or in plasma HVA, a major metabolite of dopamine (8.5±2.7 ng/mL [Val/Val group]; 8.7±2.7 ng/mL [Met-carriers] [P=0.81]). The serum BDNF levels were significantly lower in the MDD patients (4.8±0.4 ng/mL) compared with the controls (5.6±0.5 ng/mL) (P=0.044).
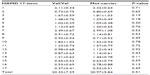
Regarding BDNF Val66Met, no significant differences between the Val/Val group and the Met-carriers were observed in plasma MHPG (5.0±1.4 ng/mL [Val/Val group]; 4.9±1.5 [Met-carriers] [P=0.85]), plasma HVA (5.3±1.1 ng/mL [Val/Val group]; 5.0±1.3 [Met-carriers] [P=0.54]), or serum BDNF (5.4±1.2 ng/mL [Val/Val group]; 4.8±1.6 [Met-carriers] [P=0.39]). No differences were observed in any items of the HAMD17 between the Val/Val genotype and the Met-carriers (Table 3).
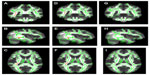
In the voxel-wise-based group comparison, no significant differences were observed regarding FA, AD, or RD, in all patients compared with the controls. We found a significant FA decrease (P<0.05) within the temporal lobe WM in the Met-carriers among the MDD patients compared with those of the healthy controls (Figure 1A–C), on the basis of the Johns Hopkins University (JHU) white-matter tractography atlas and the International Consortium for Brain Mapping DTI-81 WM labels (part of the FMRIB Software Library package). In the voxel-wise-based group comparison, there was no significant difference in FA, AD, or RD, between the MDD patients and the healthy controls.
After dividing the MDD patients into genotype subgroups, we found a significant FA decrease (P<0.05) in the temporal lobe among the MDD patients who were Met-carriers compared with the corresponding values among the healthy controls (Figure 1A–C). Significantly decreased AD in the temporal lobe (P<0.05) was also found in the Met-carrier MDD patients compared with the healthy controls (Figure 1D–F). Significantly decreased AD in the temporal lobe (P<0.05) was also found in the MDD patients compared with the controls (Figure 1D–F). Moreover, the genotype–diagnostic interaction effect on FA was seen in the same position (uncorrected P<0.05), although no voxels could survive after the correction for multiple comparisons (FWE <0.05). The results of the image analyses are shown in Table 4.
No significant differences were observed in FA or AD, at any brain regions, between the MDD patients with the COMT Val/Val genotype and the healthy controls with the COMT Val/Val genotype. No significant difference was observed in RD between the MDD patients and the healthy controls, both among the COMT Val/Val group and the COMT Met-carriers (Figure 1G–I). In addition, there were no significant differences regarding FA, AD, or RD between the Val/Val group and the Met-carriers among the MDD patients, and no correlations in FA, AD, or RD were observed, at any regions of the brain, between the MDD patients and the healthy controls, both those with the BDNF Val/Val genotype and the BDNF Met-carriers (Table 4).
Discussion
In the genotype comparison (significant genotype–diagnosis interactions), we found that the reduction of the FA values in the temporal lobe was significantly larger in the MDD patients compared with the healthy subjects. FA has been shown to have an increased sensitivity to WM damage, as its decrease has been reported in the normal-appearing WM of patients with MDD.24 The use of other DTI parameters, such as AD, which is related to axonal loss, and RD, which is associated with demyelination,37,40 may increase the specificity of DTI to particular microstructural abnormalities. The most noteworthy finding in the present study was that the FA and AD, but not the RD, in the temporal lobe in the Met-carriers with MDD were significantly decreased compared with those in the healthy controls. These results may indicate that neuronal degeneration (axonal loss) can occur in the temporal lobe of Met-carriers with MDD.
In contrast, Seok et al41 recently reported that FA reduction in the temporal lobe was significant only in the MDD patients with the Val/Val group of COMT Val158Met polymorphism. This finding indicates that MDD patients with a homozygote Val gene might have further abnormalities and brain pathological changes. Taken together, the above-described findings show that it is controversial whether the COMT Val158Met polymorphism is associated with structural changes of WM in the temporal lobe.
However, no significant differences were found in the plasma levels of MHPG and HVA between the present MDD patients and the control subjects. Depression is a heterogeneous condition characterized by multiple symptoms and subtypes. The different symptoms and subtypes are likely mediated by different neurocircuitry, and neurotransmitters such as noradrenaline, dopamine, and serotonin, and they might or might not be present in any particular individual with MDD. MDD might be characterized by an increase or a decrease in certain symptoms.
We reported that MDD patients with high plasma MHPG demonstrated severe anxiety and agitation, whereas those with low MHPG and/or HVA demonstrated severe psychomotor retardation.42,43 We suspect that this is one of the reasons that no significant between-group differences were found in the catecholamine metabolites, in the present study.
In addition, each component of depressive symptoms might be related to the some brain regions and neurocircuits. Anhedonia, for example, has been found to be positively correlated with the ventromedial prefrontal cortex activity and negatively correlated with amygdala/ventral striatal activity in response to “happy” stimuli, using functional MRI (fMRI).44 Psychomotor symptoms have been associated with frontal and caudate abnormalities in depression.45 A recent fMRI study has shown that vulnerability to MDD is associated with temporofrontolimbic decoupling that is selective for self-blaming feelings.46
According to the meta-analysis of Liao et al30 using DTI, there are four consistent locations of decreased FA in patients with MDD: WM in the right frontal lobe, the right fusiform gyrus, the left frontal lobe, and the occipital lobe. Fiber tracking showed that the main fascicles involved were the right inferior fronto-occipital fasciculus, the right posterior thalamic radiation, and interhemispheric fibers running through the genu and the body of the corpus callosum.
Taken together, these results indicate that COMT gene Val158Met polymorphism did not reflect the plasma and cerebrospinal fluid levels of catecholamine metabolites. The weight loss item scores of the HAMD17 were significantly lower in the present COMT Val/Val group than in the Met-carriers. It might be possible that the higher activity of COMT leads to reduced physical activity by influencing the catecholaminergic pathways.
The specificity of WM hyperintensities to age-associated vascular depression47 reinforces the notion that MDD is a heterogeneous disorder. Although the data suggest that T2-weighted WM is related to late-onset MDD, findings suggestive of microstructural WM changes, as evinced by DTI, in young adults with MDD were reported by Li et al.48 The age-associated relationship between WM and MDD may preclude the use of this trait to identify young individuals at risk of developing MDD. Nevertheless, an understanding of the mechanisms by which microvascular lesions lead to depression may help elucidate important pathophysiological pathways and facilitate the development of new treatments. In the present study, however, no correlations were found with the BDNF Val/Met polymorphism in patients with MDD.
On the other hand, Carballedo et al31 reported that they observed a significant interaction between BDNF alleles in the uncinate fasciculus and diagnosis. In short, their patients with the BDNF Met allele had smaller FA in the uncinate fasciculus compared with the patients in the Val/Val group and compared with healthy controls with Met allele. In the present study, we did not examine the regions of the temporal lobe in detail. One of the reasons for the discrepancy between the results of Carballedo et al31 and those of the present study was that our MDD patients were at the early stage of depressive state and were drug-naïve.
Our finding that the serum BDNF levels in the MDD patients were lower than those in the healthy controls is in agreement with previous reports.11,49–51 We also found that the BDNF gene Val66Met polymorphism was not associated with serum BDNF levels in patients with MDD and that the BDNF Val66Met polymorphism is independent of the WM disturbance in MDD. Taken together, these results indicate that the BDNF gene Val66Met polymorphism is not critical for WM disturbances in patients with MDD.
The present study has several limitations. The sample size was too small to allow a second statistical analysis. The sample was heterogeneous, and the severity of illness was relatively moderate. A replication study that accounts for these limitations should be performed to confirm our preliminary results. Since the COMT gene Met-carriers showed more decreased body weight (Table 2), the possibility that the finding reflected the changed distribution in the brain could not be completely ruled out.
In conclusion, we observed an association between the COMT gene Val158Met polymorphism and the reduction of FA and AD, but not RD, in the temporal lobe of patients with MDD.
Acknowledgment
Professor Jun Nakamura was supported by a grant from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant number 21591494).
Disclosure
Professor Jun Nakamura has received grant support from Astellas Pharma, Tanabe-Mitsubishi Pharmaceutical Co Ltd, Otsuka Pharmaceutical Co Ltd, Eli Lilly and Company, Pfizer, Inc., and GlaxoSmithKline plc. The authors report no other conflicts of interest in this work.
References
Yoshimura R, Nakamura J, Shinkai K, Ueda N. Clinical response to antidepressant treatment and 3-methoxy-4-hydroxyphenylglycol levels: mini review. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28(4):611–616. | |
Karege F, Bovier P, Gaillard JM, Tissot R. The decrease of erythrocyte catechol-O-methyltransferase activity in depressed patients and its diagnostic significance. Acta Psychiatr Scand. 1987;76(3):303–308. | |
Massat I, Souery D, Del-Favero J, et al. Association between COMT (Val158Met) functional polymorphism and early onset in patients with major depressive disorder in a European multicenter genetic association study. Mol Psychiatry. 2005;10(6):598–605. | |
Lachman HM, Morrow B, Shprintzen R, et al. Association of codon 108/158 catechol-O-methyltransferase gene polymorphism with the psychiatric manifestations of velo-cardio-facial syndrome. Am J Med Genet. 1996;67(5):468–472. | |
Baune BT, Hohoff C, Berger K, et al. Association of the COMT val158met variant with antidepressant treatment response in major depression. Neuropsychopharmacology. 2008;33(4):924–932. | |
Åberg E, Fandiño-Losada A, Sjöholm LK, Forsell Y, Lavebratt C. The functional Val158Met polymorphism in catechol-O-methyltransferase (COMT) is associated with depression and motivation in men from a Swedish population-based study. J Affect Disord. 2011;129(1–3):158–166. | |
Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. | |
Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. Am J Psychiatry. 2000;157(1):115–118. | |
Castrén E, Rantamäki T. The role of BDNF and its receptors in depression and antidepressant drug action: Reactivation of developmental plasticity. Dev Neurobiol. 2010;70(5):289–297. | |
Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci. 2011;36(3):195–203. | |
Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry. Epub August 20, 2013. | |
Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. | |
Frielingsdorf H, Bath KG, Soliman F, Difede J, Casey BJ, Lee FS. Variant brain-derived neurotrophic factor Val66Met endophenotypes: implications for posttraumatic stress disorder. Ann N Y Acad Sci. 2010;1208:150–157. | |
aan het Rot M, Mathew SJ, Charney DS. Neurobiological mechanisms in major depressive disorder. CMAJ. 2009;180(3):305–313. | |
Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31(7):361–370. | |
Herrmann LL, Le Masurier M, Ebmeier KP. White matter hyperintensities in late life depression: a systematic review. J Neurol Neurosurg Psychiatry. 2008;79(6):619–624. | |
Kumar A, Cook IA. White matter injury, neural connectivity and the pathophysiology of psychiatric disorders. Dev Neurosci. 2002;24(4):255–261. | |
Kieseppä T, Eerola M, Mäntylä R, et al. Major depressive disorder and white matter abnormalities: a diffusion tensor imaging study with tract-based spatial statistics. J Affect Disord. 2010;120(1–3):240–244. | |
Korgaonkar MS, Grieve SM, Koslow SH, Gabrieli JD, Gordon E, Williams LM. Loss of white matter integrity in major depressive disorder: evidence using tract-based spatial statistical analysis of diffusion tensor imaging. Hum Brain Mapp. 2011;32(12):2161–2171. | |
Zhu X, Wang X, Xiao J, Zhong M, Liao J, Yao S. Altered white matter integrity in first-episode, treatment-naive young adults with major depressive disorder: a tract-based spatial statistics study. Brain Res. 2011;1369:223–229. | |
Cullen KR, Klimes-Dougan B, Mueller BA, et al. Altered white matter microstructure in adolescents with major depression: A preliminary study. J Am Acad Child Adolesc Psychiatry. 2010;49(2):173–183. | |
Zuo N, Fang J, Lv X, et al. White matter abnormalities in major depression: a tract-based spatial statistics and rumination study. PLoS One. 2012;7(5):e37561. | |
Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66(9):814–823. | |
Henderson CE, Higginson JS, Barrance PJ. Comparison of MRI-based estimates of articular cartilage contact area in the tibiofemoral joint. J Biomech Eng. 2011;133(1)014502. | |
Ma N, Li L, Shu N, et al. White matter abnormalities in first-episode, treatment-naive young adults with major depressive disorder. Am J Psychiatry. 2007;164(5):823–826. | |
Wu F, Tang Y, Xu K, et al. Whiter matter abnormalities in medication-naive subjects with a single short-duration episode of major depressive disorder. Psychiatry Res. 2011;191(1):80–83. | |
Ouyang X, Tao HJ, Liu HH, et al. White matter integrity deficit in treatment-naïve adult patients with major depressive disorder. East Asian Arch Psychiatry. 2011;21(1):5–9. | |
Jia Z, Huang X, Wu Q, et al. High-field magnetic resonance imaging of suicidality in patients with major depressive disorder. Am J Psychiatry. 2010;167(11):1381–1390. | |
de Diego-Adeliño J, Pires P, Gómez-Ansón B, et al. Microstructural white-matter abnormalities associated with treatment resistance, severity and duration of illness in major depression. Psychol Med. Epub August 21, 2013. | |
Liao Y, Huang X, Wu Q, et al. Is depression a disconnection syndrome? Meta-analysis of diffusion tensor imaging studies in patients with MDD. J Psychiatry Neurosci. 2012;38(1):49–56. | |
Carballedo A, Amico F, Ugwu I, et al. Reduced fractional anisotropy in the uncinate fasciculus in patients with major depression carrying the met-allele of the Val66Met brain-derived neurotrophic factor genotype. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):537–548. | |
Kim B, Yoo E, Lee JY, et al. The effects of the catechol-O-methyltransferase val158met polymorphism on white matter connectivity in patients with panic disorder. J Affect Disord. 2013;147(1–3):64–71. | |
Haselgrove JC, Moore JR. Correction for distortion of echo-planar images used to calculate the apparent diffusion coefficient. Magn Reson Med. 1996;36(6):960–964. | |
Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. | |
Smith SM, Jenkinson M, Johansen-Berg H, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. | |
Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. | |
Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: application to breast MR images. IEEE Trans Med Imaging. 1999;18(8):712–721. | |
Yeung PK, Buckley SJ, Pedder SC, Dingemanse J. Determination of 3,4-dihydroxyphenylacetic acid and 5-hydroxyindoleacetic acid in human plasma by a simple and rapid high-performance liquid chromatography assay. J Pharm Sci. 1996;85(4):451–453. | |
Minegishi A, Ishizaki T. Determination of free 3-methoxy-4-hydroxyphenylglycol with several other monoamine metabolites in plasma by high-performance liquid chromatography with amperometric detection. J Chromatogr. 1984;311(1):51–57. | |
Brett M, Leff AP, Rorden C, Ashburner J. Spatial normalization of brain images with focal lesions using cost function masking. Neuroimage. 2001;14(2):486–500. | |
Seok JH, Choi S, Lim HK, Lee SH, Kim I, Ham BJ. Effect of the COMT val158met polymorphism on white matter connectivity in patients with major depressive disorder. Neurosci Lett. 2013;545:35–39. | |
Ueda N, Yoshimura R, Shinkai K, Nakamura J. Plasma levels of catecholamine metabolites predict the response to sulpiride or fluvoxamine in major depression. Pharmacopsychiatry. 2002;35(5):175–181. | |
Shinkai K, Yoshimura R, Ueda N, Okamoto K, Nakamura J. Associations between baseline plasma MHPG (3-methoxy-4-hydroxyphenylglycol) levels and clinical responses with respect to milnacipran versus paroxetine treatment. J Clin Psychopharmacol. 2004;24(1):11–17. | |
Stuhrmann A, Dohm K, Kugel H, et al. Mood-congruent amygdala responses to subliminally presented facial expressions in major depression: associations with anhedonia. J Psychiatry Neurosci. 2013;38(4):249–258. | |
Der-Avakian A, Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35(1):68–77. | |
Green S, Lambon Ralph MA, Moll J, Deakin JF, Zahn R. Guilt-selective functional disconnection of anterior temporal and subgenual cortices in major depressive disorder. Arch Gen Psychiatry. 2012;69(10):1014–1021. | |
Sneed JR, Rindskopf D, Steffens DC, Krishnan KR, Roose SP. The vascular depression subtype: evidence of internal validity. Biol Psychiatry. 2008;64(6):491–497. | |
Li L, Ma N, Li Z, et al. Prefrontal white matter abnormalities in young adult with major depressive disorder: a diffusion tensor imaging study. Brain Res. 2007;1168:124–128. | |
Bocchio-Chiavetto L, Bagnardi V, Zanardini R, et al. Serum and plasma BDNF levels in major depression: a replication study and meta-analyses. World J Biol Psychiatry. 2010;11(6):763–773. | |
Fernandes BS, Gama CS, Ceresér KM, et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res. 2011;45(8):995–1004. | |
Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry. 2008;64(6):527–532. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.