Back to Journals » Medical Devices: Evidence and Research » Volume 9
Computed tomography and magnetic resonance imaging contrast media injectors: technical feature review – what is really needed?
Authors Friebe M
Received 13 February 2016
Accepted for publication 15 March 2016
Published 15 July 2016 Volume 2016:9 Pages 231—239
DOI https://doi.org/10.2147/MDER.S106338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Michael Friebe
Institute of Medical Engineering, Otto-von-Guericke-University, Magdeburg, Germany
Abstract: There has been little technical innovation over the last few years for contrast media (CM) injectors that are used for diagnostic imaging (computed tomography [CT], magnetic resonance imaging [MRI], and hybrid imaging systems, such as positron emission tomography–CT or magnetic resonance–positron emission tomography) examinations. The medical need of CM for the enhancement of diagnostic images has been around for a long time, but the application of the CM into the blood stream comes with potential medical complications for the patient and requires a lot of operator experience and training. Most power injector systems that are currently used can do significantly more than what is typically required; this complexity however, adds error potential and cost. This paper focuses on the main features that CM injector systems should have and highlights the technical developments that are useful to have but which add complexity and cost, increase setup time, and require intensive training for safe use. CM injection protocols are very different between CT and MRI, with CT requiring many more variances, has a need for multiphase protocols, and requires a higher timing accuracy. A CM injector used in the MRI suite, on the other-hand, could only need a relatively time insensitive injection with a standard injection flow rate and a volume that is dependent on the patients’ weight. This would make easy and lightweight systems possible, which are able to safely and accurately perform the injection task, while allowing full MRI compatibility with relatively low cost investment and consumable costs.
Keywords: power injector, contrast media injection, injection protocols, MRI compatibility
Introduction
Today, contrast media (CM) injection is generally realized by using power injectors (mainly electromechanical or hydraulic piston or peristaltic roller drives) with two different injection volumes (in syringes, bags, or bottles).
The first volume (saline solution) has the task of opening the vein for the second volume to inject the CM, which is then followed again by a flush from the first volume thereby reducing the total CM consumption and increasing patient safety through reduced flow rates.
While this procedure can also be performed with a single injection volume of CM, fast imaging as in multislice detector computed tomography (CT) imaging or high-field magnetic resonance imaging (MRI) requires dual volume CM injector (CMI) systems.1
There are cost and handling issues with the consumables that are required for the injection (syringes, bags, bottle, tubing), problems with the actual injection pathway, and patient access (and the consumables for this).
And, the CMIs manufactured and sold today include features and components that increase operational complexity, such as the controlling computer system, hardware (eg, batteries, magnetic attraction when used in a MRI suite, servicing, electric safety), variables of the injection (amount of CM, timing, injection rate), patient safety, and the use of varying injection protocols.
CMI systems are relatively large and complex and need time and operator interaction for the individual patient setup – all for an apparently easy task of injecting a liquid into the patient’s bloodstream at the right point in time with respect to the imaging procedure.
Increased complexity means decreased patient safety and usually it also comes with increased investment and operational cost.
The innovations today very often add incremental improvements and usually do not consider completely new and possibly easier solutions that require more radical changes of the base system. The question should be on what is really needed to do the task safely and reliably, and with respect to concerns of an ever increasing cost for the health care delivery.
The focus of this paper is on electromechanical/hydraulic CT and particular MRI power injectors and intravenous injections, but a lot of the issues also apply for the injection problems and systems used for X-ray, hybrid imaging systems, and ultrasound applications.
A medium-sized hospital can easily own and operate ten or more CT/MRI CMIs with an average operational time before replacement of 7–8 years and an investment from US$10,000 (single syringe) to US$40,000 (multiphase, multicontrast media, etc).
In general, these systems are quite reliable, but require at least an annual maintenance and safety check and in case of high utilization a service every 6 months. The costs for these service and safety checks are typically in the order of US$1,000 per visit and another US$1,000 per year should be reserved for spare parts needed.
So, the total operational expenses for a typical hospital could be in the order of US$60,000 (US$40,000 for annual depreciation + US$20,000 for service and parts).
More of a cost problem are the required consumables that are needed for the different CMI systems for every patient examination (see Figure 1 for a typical set of consumables).
Some of the CMI systems use syringes with a recommended single use that are very often only replaced once a day (see Figure 1); however, despite the hygienic risk, others use bottles or bags that can contain larger amounts of CM and do not need to be replaced for every patient. Figure 2 shows the CMI system (CT Expres, Bracco Injeneering, Lausanne, Switzerland) using bottles instead of syringes.2–5
  | Figure 2 CT dual volume injector using bottles instead of syringes. Note: CT Expres, Bracco Injeneering, Lausanne, Switzerland. Abbreviation: CT, computed tomography. |
Tubing from the syringe/bag to the patient obviously needs to be replaced with every patient, and the total cost of injection consumables per patient can run from US$5 to US$25 plus the cost of the CM plus the running and depreciation cost of the CMI.
Using a total of 10,000 CT and MRI CM examinations per annum add up to additional consumable costs of between US$50,000 and US$250,000 per year plus the expenses for the CM.
So, investment as well as operational cost in combination with the required individual patient setup time is something that needs to be taken into consideration when selecting a CMI.6,7
Materials and methods
CT injection protocols
For CT injection protocols, timing is essential to achieve the desired contrast. A relatively standard volume of injection is between 75 and 150 mL (depending on patient weight) iodine-based CM using an injection rate of typically between 3 and 5 mL/s (using a 20-gauge flexible plastic cannula).8,9
The injection protocol also needs to be adapted to the type of CT scanner used (eg, number of slices) and is complimented with the injection of saline solution, which requires two working channels as a syringe or bag system for the CMI. As timing of the injection is critical with respect to the CT imaging, a computer control combined with a remote control is therefore essential.
Most commercially available CMI systems allow variation of the flow rate in steps of 0.1 mL/s from 1 mL/s all the way to 9.9 mL/s and allow the use of multiphase injection protocols.
CT injection protocols require the ability to adjust the timing and vary the flow rate, especially when advanced imaging is performed. The offered parameter adjustments are not needed however in most conventional examinations.6–9 With a minimum flow rate specification of 4 mL/s, a large majority of the normal CM imaging could be performed.
Even single syringe units for CM only are still sold for CT applications (Figure 3 shows an example system: APO 100, Apollo RT, Hong Kong, People’s Republic of China), and MRI injections are still commonly performed by handinjection mainly in private practice settings.
MRI injection protocols
A typical MRI CM injection (mainly gadolinium based that shortens the T1 relaxation time, Figure 4 shows an example of the effect of MRI contrast agent on the visualization of the pathology) is administered as a rapid intravenous bolus (depending on weight: ∼0.2 mL/kg) at a flow rate of 2 mL/s.10
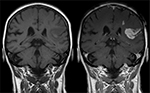  | Figure 4 Effect of MRI contrast agent. Note: T1-weighted images; left image without, right image with contrast medium administration show defect of the blood–brain barrier after stroke. Reproduced from wikimedia.org (http://creativecommons.org/licenses/by-sa/3.0/).10 Abbreviation: MRI, magnetic resonance imaging. |
So, for a 100 kg patient, ∼20 mL CM is used that is subsequently flushed with 20 mL saline solution at the same injection rate. Approximately 30 seconds after this, the MRI imaging protocols start.
The peak enhancement is ∼2 minutes after injection and holds on for several minutes.11
Table 1 summarizes the most commonly used gadolinium-based MRI CM and their recommended use according to the manufacturers’ recommendations.12–15 All manufacturers recommend an exclusive use of the CM with a flow rate of 1 or 2 mL/s and a volume that is dependent on the weight of the patient (0.2 mL/kg).
Since MRI CM application is not that time and flow rate sensitive, a relatively simple device with a dual volume injection drive with these two flow rates could be employed to achieve accurate and repeatable results, while greatly reducing the capital expenditure and operational costs.
In summary, for CMI, it is technically only required to have a driving unit that is capable of injecting a volume of ∼20 (MRI) or 150 mL (CT) CM with an injection of saline solution (50–150 mL) before and immediately following the CM injection at a typical flow rate of 2 (MRI) or 4 mL/s (CT).
Bags or bottles with higher volume, instead of syringes, could further ease operation and decrease setup time and cost of consumables.
Table 2 shows some of the more commonly used MRI CMI systems and their main features.16–20
The drive mechanism of only one system (Figure 5, Empower MR, Bracco Injeneering) is hydraulic and really fully MRI compatible.21,22 All other units still do have some ferromagnetic components built in and while they state to be compatible to 3 T, translational attraction forces (mainly the power supply) require that these devices stay outside the 0.1 T (=1,000 Gauss) line.23
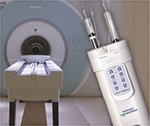  | Figure 5 Magnetic Resonance CMI operating at magnetic field strength of up to 9.4 T due to a hydraulic drive. Note: For other ultra-high field MRI issues see Scannexus.22 Abbreviation: CMI, contrast media injector. |
Moving toward the MRI magnet, this would generally be (with a new generation actively shielded 1.5 or 3 T) at ∼0.5–1 m from the bore opening of the magnet.
Results
What is required from a patient safety point of view, which after all is the most important issue?2,8,24,25
- Ease of use and low complexity is a key element of patient safety. If there are only few variables and few components, the user training is fast and the error margin low. An accidental flow rate of 8 versus 2 mL/s could for example cause venous rupture and with that severe problems for the patient.
- “Open” CM and saline volumes should not be used to prevent infections, sepsis, and contaminations.2,20,24,25
- Refilling bottle, bag, or syringe containers used in CMI systems should be avoided to eliminate the risk of contamination and infection.2,24
- Use of double volume injectors connected via a T-tube and an injection tube for the venous access to the patient that allows easy CM and saline filling should be the system of choice.24
- Standard (and easy) operating procedures for injection should be in place for every site taking local situations into consideration and regular training sessions should be conducted; this would, for example, include the use of gloves and hand disinfection prior to filling CM volume or saline volume and exchange of tubing sets.24–27
- Some CMI systems on the market offer optional extravasation protection (sensors that measure variances in skin surface or radio frequency identification device signals to warn if the injector needle is embedded in tissue instead of inside the vessel). While this is certainly advisable to have in CT due to the higher flow rates, it is not necessary for MRI CM injections. Extravasation happens only rarely, but can lead to severe swelling and pain.
- All systems should allow removing any air in the tubing by rotating the injector head and slowly pushing the liquid into the tubing with an optional air bubble detection sensor. Air in the blood stream could lead to severe respiratory problems and ultimately to death.26,27
- CMI systems should include sensors and safety measures to monitor overpressure.26,27
- An emergency-OFF button should be standard on the injector head and/or on the control panel.
- Optional sensors for contrast leaks could be helpful particularly since CM is very sticky.
- Some vendors now offer radio frequency identification device identifiers that prevent using single use syringes more than once. While it is recommended to not employ syringes/bottles/bags more than once, this can be part of a standard operating procedure.24 Clearly, the individual patient tubing has to be changed with every patient.
- Integrated should at least be the possibility to recall some of the usage data, such as amount of CM injected with what parameters at what time. This data is valuable for forensic reasons, but can also be used to calculate the total CM and consumables used.
Other not directly patient safety-related features that are optionally offered are Radiology Information Systems/Patient Archival and Communication System Data Management Systems connections and network integrations that allow to measure workflow efficiency and imaging optimization.28 This also allows management of CMI service issues and can track historical use of CM with respect to patients, diseases, and imaging protocols. This additional information is potentially helpful to manage a radiology operation, but not an essential feature.
The syringes and their cost – but also patient safety issues related to using these more than once – are definitely quite an important aspect. While multiuse saves setup time and reduces the cost per patient, it should be very carefully weighed with potential negative hygienic effects.
Especially in the US, prefilled syringes offered by the CM companies for selected CMI systems are getting more popular due to their fast setup and proven hygienic safety, while other CMI vendors are betting on larger volume bottles that reduce the preparation time and the cost of the consumables.2,7
Dual flow allows parallel injection of CM and saline solution and with that could help to provide new or enhanced contrast particularly for cardiac imaging. This is not needed for conventional examinations and if, then only for CT imaging.
CM viscosity is temperature-dependent; a warmer application temperature decreases the viscosity and flow resistance. Warming the CM before injection is therefore beneficial and some CMIs have such a feature built in.
A more important hardware aspect is the power source and weight of the CMI systems. While the majority of the commercially available CT injectors are directly connected to a power outlet (weight between 5 and 25 kg), MRI injectors (weight 10–35 kg) usually have a battery integrated that has a tendency to be empty when you really need it. The injectors are not used for every patient, or are sometimes physically in the way when difficult patient examinations are performed (eg, interventions) and therefore need to be portable or moveable or mounted on a ceiling or wall. While mounting options are feasible for the CT injectors, it is almost impossible to do that in a radio frequency shielded MRI suite with no weight bearing walls and ceilings. The lighter a CMI is, the easier it can be moved and the lower the patient/operator hazards are.
The main reason for the heavy weight is because of the injector drive (electromechanical pump) and the power supply/battery unit. CT injectors are used in lead shielded environments that typically have several main power outlets. An operation without a battery as a power source is therefore possible, but an included battery provides higher movability and as there is no need for cables provides higher safety.
In an MRI room, there are typically no main power outlets and a battery powered injector drive is therefore the standard setup. Alternative solutions are hydraulic driven systems or could be new concepts of almost entirely mechanical driven systems (air, compressed air, spring loaded) with some electronic components that provide safety features and injection information.21,22
Especially for use in the MRI suite, electronic and metallic components are a potential safety and operational hazard and for ultimate patient safety, the device should be lightweight (<10 kg weight inside the MRI room) and entirely manufactured from nonferromagnetic materials with few to no electronic components to avoid accidents related to magnetic attraction forces (Figure 6) and to ensure that the MRI scanner operates properly.23,29
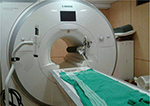  | Figure 6 3 T MRI systems – oxygen tank was pulled into the magnet. Notes: The MRI injector on the left was not a problem being located at ∼0.5 m from the magnet bore. For further details on MRI related accidents see MRI patient safety blog.29 Abbreviation: MRI, magnetic resonance imaging. |
Discussion
While dual volume CMI systems are technically not needed for most injection protocols (exceptions are very fast and cardiac CT imaging protocols), they are certainly standard and make CM injection easier and safer for the patient.2,24,26
Most of the CT and MRI injection protocols are standard with respect to flow rate, while the injection volume is weight-dependent (CT: 4 mL/s, 75–150 mL CM volume dependent on weight; MRI: 2 mL/s, 0.2 mL/kg patient weight). The starting point of the injection needs to be in line or in a certain timing relation with the start of CT or MRI imaging, and therefore, a remote control is advantageous.
CM injection in MRI is still often performed by hand, however, with a standard syringe through an intravenous access line. This is mainly because the injection protocol is not as time sensitive as for CT examinations. The image enhancement (mainly as an influence on the T1 timing) is present for several minutes. MRI power injection is much more stable and repeatable with respect to the flow rate and timing of injection, and therefore, the quality of imaging more predictable, which ultimately increases the procedure safety for the patient. Hand injections should therefore no longer be an alternative. Evaluation of the recommended injection protocols for gadolinium-based MRI CM (Table 1) shows that there is a standard injection volume based on the patient weight (0.2 mL/kg patient weight) and a standard injection flow rate (2 mL/s) with a recommended flush of saline solution after the CM injection with the same volume. Only for very selected procedures (eg, peripheral angiography), more complicated injection protocols are used. All commercially available MRI power injector systems (Table 2) consist of an electrically or hydraulically powered drive unit for the injection and a control computer, and in some cases, an additional battery stand or electronic box. These systems are able to inject in a very wide range of flow rates and can be programmed for virtually any protocol, but this is not needed and the complexity requires a significant training and application effort, and results in increased investment and operational cost.
Patient safety should be the key concern for the application of CM and feature reduction and limitation to standard protocols and ease of use could significantly decrease error potential, as well as investment and running cost. It should therefore be discussed whether MRI CM injections should be performed with a significantly simpler device.
CT CM injection is different in the respect that while there are typical protocols, there is no real standard as could be shown for MRI injection protocols. The timing of the CM is more critical and depends on the type of examination and CT scanner used. Faster CT scanners require faster injection protocols and more accurate timing. Also, more complicated injection protocols with multiphase injection of CM are used. This requires a computer controlled unit with a display and user-dependent injection protocol programming of flow rate, injection volumes, and injection sequences. Also, the flow rates are not as simple as for MRI and can vary between 1 and 7 mL/s, and in very selected cases even up to 9 mL/s.
The discussed technical features for both CT and MRI injectors are summarized in Table 3, highlighting the ones that a power injector “must have” (mainly patient safety-related and the ones needed for standard injection protocols) versus the ones that are optional. The optional ones are separated in “should have” and “nice to have” features, and there are also some listed that are not really needed, possibly used for differentiation purposes, that add little or no value to most users.
Health care expenditures and standardization are key concerns and goals going forward and making devices easier and simpler would be a way to possibly achieve this. Fewer buttons mean fewer user errors and fewer components result in reduced cost.
Conclusion
The CMI systems currently on the market are high-tech computer controlled devices with separate monitors (there is already not enough space for the existing monitors of the CT or MRI systems), that are capable of performing hundreds of different injection protocols. They are equipped with many optional features, that are not necessary for general imaging. Clinical research institutions have different needs and do require some of these offered capabilities for few selected examinations and for research purposes. The complexity provided comes with a high cost of investment and also comes with decreased patient safety.
Innovation in the segment of CM injection for CT and MRI systems in the last years added features to a basic concept that is in use for decades now. Some of the improvements clearly had a positive impact on the workflow and usability, particularly for the more challenging task of CT injections.
CMIs for use in the MRI suite were initially CT injectors adapted to the problems that come with working in a signal sensitive (shielded electronics needed) environment that does not tolerate ferromagnetic components. So, particularly for MRI injection, a complete redevelopment of a CMI system with a focus on ease of use and cost concerns could make sense.
Development activities should not only address new technology innovations with limited value but also try to simplify processes and procedures, especially when a human patient could benefit. With that a minimum viable MRI CMI system would consist of dual volume injection capabilities at one or two flow rates, simple timers to select the volume and time between the contrast media and saline injection. The ideal system should be lightweight and movable, easy to use and should avoid any user errors. It should be constructed of almost 100% non-ferrous material, if possible with an injection mechanism that does not require an electric charge. And finally it should allow connection to a local network or the possibility to store and retrieve forensic data. Such a system operated with bag or bottle CM/saline should be possible with a much reduced capital investment, lower operational cost, and increased patient safety. Such a system could be used as the standard system for CMI or be used in combination (for 90% of the more conventional procedures) with a more complex injector that is only used for advanced procedures.
That could also mean that combining high-end advanced systems that are movable with more standardized and feature-reduced systems in one imaging suite could potentially dramatically reduce the total operational cost of CM injection.
Acknowledgment
This research was partly funded by the German government grant (BMBF 03IPT7100X).
Disclosure
The author has codeveloped several CM injection systems that are commercially available and is the listed inventor of five patents in this field. The author reports no other conflicts of interest in this work.
References
Indrajit I, Sivasankar R, D’Souza J, et al. Pressure injectors for radiologists: a review and what is new. Indian J Radiol Imaging. 2015;25(1):2–10. | ||
Buerke B, Puesken M, Mellmann A, et al. Automatic MDCT injectors: hygiene and efficiency of disposable, prefilled, and multidosing roller pump systems in clinical routine. AJR Am J Roentgenol. 2011;197:W226–232. | ||
Medtron AG. MEDTRON CT consumable set. Available from: http://www.medtron.com/verbrauchsmaterial/sets.html. Accessed February 10, 2016. | ||
Bayer Vital GmbH. MEDRAD CT consumable set. Available from: https://www.radiologie.bayer.de/ct/verbrauchsmaterial-ct. Accessed February 10, 2016. | ||
Debiotech S.A. CT Expres. Available from: http://www.debiotech.com/newsite/page/index.php?page=product_01&id=1&id_prod=59#type=Brief. Accessed February 10, 2016. | ||
Alimenti A, Spohr L, Loubeyre P. Multidetector multiphase contrast-enhanced liver CT: prospective study comparing two contrast material injection rates on vascular and liver parenchyma enhancements in patients with varied cirrhotic status. J Comput Assist Tomogr. 2006;30;368–371. | ||
Xiaozhou M. Comparison of dual-syringe and syringeless power injectors in outpatient MDCT practice: impact on the operator’s performance, CT workflow, and operation cost. Annual Meeting Radiological Society of North America; 2009. Available from: http://dx.doi.org/10.1016/j.jacr.2012.04.007. | ||
American College of Radiology. ACR Manual on Contrast Media Version 10.1 (2015). Available from: http://www.acr.org/∼/media/37D84428BF1D4E1B9A3A2918DA9E27A3.pdf. Accessed February 1, 2016. | ||
Lell M, Jost G, Korporaal JG, et al. Optimizing contrast media injection protocols in state-of-the art computed tomographic angiography. Invest Radiol. 2015;50(3):161–167. | ||
wikimedia.org [webpage on the Internet]. Defect of the blood-brain barrier after stroke shown in MRI. Hellerhoff. 2010. Available from: https://commons.wikimedia.org/wiki/File:Bluthirnschranke_nach_Infarkt_nativ_und_KM.png. Accessed February 11, 2016. | ||
Gourtsoyiannis N, editor. Clinical MRI of the Abdomen. Heidelberg: Springer; 2011. | ||
emc. Bayer Magnevist. Available from: http://www.medicines.org.uk/EMC/medicine/1825/SPC/Magnevist/. Accessed February 2, 2016. | ||
Guerbet. Dotarem. Available from: http://www.guerbet-us.com/products/dotarem.html. Accessed February 2, 2016. | ||
Bracco Diagnostics. Multihance. Available from: http://braccoimaging.com/sites/braccoimaging.com/files/technica_sheet_pdf/MultiHance%20Prescribing%20Information.pdf. Accessed February 2, 2016. | ||
GE Healthcare. Omniscan. Available from: http://www3.gehealthcare.com/en/products/categories/contrast_media/omniscan#tabs/tab488621DABE7848C7837C18A88CFE9FFF. Accessed February 2, 2016. | ||
APOLLO RT. EPIC APO 100 CT Injector. Available from: http://www.apollo-rt.com/index.php/ct-injectors. Accessed March 10, 2016. | ||
Ulrich Medical. 3T tennessee. Available from: http://www.ulrichmedical.de/de/3t-tennessee.html. Accessed February 2, 2016. | ||
Medtron AG. Accutron MR3. Available from: http://www.medtron.de/en/magnetic-resonance-imaging/accutron-mr3.html. Accessed February 2, 2016. | ||
Nemoto. Sonic Shot 7. Available from: http://www.nemoto-do.co.jp/nemoto_en/pr_mr/moreinfo/ss_7.html. Accessed February 2, 2016. | ||
Bayer Vital GmbH. MEDRAD® Spectris Solaris™. Available from: http://www.radiologie.bayer.de/medizintechnik/mrt/medrad-solaris. Accessed February 2, 2016. | ||
Bracco Diagnostic Inc. EmpowerMR. Available from: http://braccoimaging.com/us-en/products-and-solutions/injectors/empowermr. Accessed February 2, 2016. | ||
Scannexus. Empower your MRI research, even at 9.4T. Maastricht, the Netherlands: scannexus; 2014 [May 12]. Available from: http://www.scannexus.nl/news/bericht-quot_empower_your_mri_research__even_at_9_4t_quot. Accessed February 3, 2016. | ||
Dula N, Shellock F. Evaluation of a power injection system in the 7-tesla MRI environment. Int J Imaging Syst Technol. 2015;25:50–55. | ||
Clark C. Safety and hygiene aspects of contrast media administration in Germany. hospital pharmacy europe. 2008. Available from: http://www.hospitalpharmacyeurope.com/featured-articles/safety-and-hygiene-aspectscontrast-media-administration-germany. Accessed February 1, 2016. | ||
Buerke B, Mellmann A, Kipp F, Heindel W, Wessling J. Hygienic aspects in radiology: what the radiologist should know? RöFo. 2012;184(12):1099–1109. | ||
Carlson JE, Hedlund LJ, Trenkner SW, Ritenour R, Halvorsen RA, Jr. Safety considerations in the power injection of contrast media via central venous catheters during computed tomographic examinations. Invest Radiol. 1992;27:337–340. | ||
Coyle D, Bloomgarden D, Beres R, Patel S, Sane S, Hurst E. Power injection of contrast media via peripherally inserted central catheters for CT. J Vasc Interv Radiol. 2004;15:809–814. | ||
Fornell D. Trends in contrast media injection systems. Imaging technology news. Available from: http://www.itnonline.com/article/trends-contrast-media-injection-systems. Accessed February 1, 2016. | ||
MRI Patient Safety Blog. Implementation > documentation in MRI safety. Available from: http://mripatientsafety.com/blog/?p=285. Accessed February 4, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.