Back to Journals » Clinical Epidemiology » Volume 11
Completeness of RET testing in patients with medullary thyroid carcinoma in Denmark 1997–2013: a nationwide study
Authors Mathiesen JS , Kroustrup JP, Vestergaard P, Stochholm K, Poulsen PL, Rasmussen ÅK, Feldt-Rasmussen U, Schytte S, Londero SC, Pedersen HB, Hahn CH, Bentzen J, Möller S , Gaustadnes M, Rossing M, Nielsen FC, Brixen K, Frederiksen AL , Godballe C
Received 15 August 2018
Accepted for publication 7 November 2018
Published 10 January 2019 Volume 2019:11 Pages 93—99
DOI https://doi.org/10.2147/CLEP.S183268
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Jes Sloth Mathiesen,1,2 Jens Peter Kroustrup,3 Peter Vestergaard,3,4 Kirstine Stochholm,5,6 Per Løgstrup Poulsen,6 Åse Krogh Rasmussen,7 Ulla Feldt-Rasmussen,7 Sten Schytte,8 Stefano Christian Londero,8 Henrik Baymler Pedersen,9 Christoffer Holst Hahn,10 Jens Bentzen,11 Sören Möller,2,12 Mette Gaustadnes,13 Maria Rossing,14 Finn Cilius Nielsen,14 Kim Brixen,2 Anja Lisbeth Frederiksen,2,15 Christian Godballe1
On behalf of the Danish Thyroid Cancer Group (DATHYRCA)
1Department of ORL Head & Neck Surgery and Audiology, Odense University Hospital, Odense, Denmark; 2Department of Clinical Research, University of Southern Denmark, Odense, Denmark; 3Department of Clinical Medicine and Endocrinology, Aalborg University Hospital, Aalborg, Denmark; 4Steno Diabetes Center North Jutland, Aalborg, Denmark; 5Department of Internal Medicine and Endocrinology, Aarhus University Hospital, Aarhus, Denmark; 6Center for Rare Diseases, Aarhus University Hospital, Aarhus, Denmark; 7Department of Medical Endocrinology, Copenhagen University Hospital, Copenhagen, Denmark; 8Department of ORL Head and Neck Surgery, Aarhus University Hospital, Aarhus, Denmark; 9Department of ORL Head & Neck Surgery, Aalborg University Hospital, Aalborg, Denmark; 10Department of ORL Head & Neck Surgery, Copenhagen University Hospital, Copenhagen, Denmark; 11Department of Oncology, Herlev Hospital, Herlev, Denmark; 12Odense Patient data Explorative Network (OPEN), Odense University Hospital, Odense, Denmark; 13Department of Molecular Medicine, Aarhus University Hospital, Aarhus, Denmark; 14Center for Genomic Medicine, Copenhagen University Hospital, Copenhagen, Denmark; 15Department of Clinical Genetics, Odense University Hospital, Odense, Denmark
Background: The completeness of REarranged during Transfection (RET) testing in patients with medullary thyroid carcinoma (MTC) was recently reported as 60%. However, the completeness on a population level is unknown. Similarly, it is unknown if the first Danish guidelines from 2002, recommending RET testing in all MTC patients, improved completeness in Denmark. We conducted a nationwide retrospective cohort study aiming to evaluate the completeness of RET testing in the Danish MTC cohort. Additionally, we aimed to assess the completeness before and after publication of the first Danish guidelines and characterize MTC patients who had not been tested.
Methods: The study included 200 patients identified from the nationwide Danish MTC cohort 1997–2013. To identify RET tested MTC patients before December 31, 2014, the MTC cohort was cross-checked with the nationwide Danish RET cohort 1994–2014. To characterize MTC patients who had not been RET tested, we reviewed their medical records and compared them with MTC patients who had been tested.
Results: Completeness of RET testing in the overall MTC cohort was 87% (95% CI: 0.81–0.91; 173/200). In the adjusted MTC cohort, after excluding patients diagnosed with hereditary MTC by screening, completeness was 83% (95% CI: 0.76–0.88; 131/158). Completeness was 88% (95% CI: 0.75–0.95; 42/48) and 81% (95% CI: 0.72–0.88) (89/110) before and after publication of the first Danish guidelines, respectively. Patients not RET tested had a higher median age at diagnosis compared to those RET tested. Median time to death was shorter in those not tested relative to those tested.
Conclusion: The completeness of RET testing in MTC patients in Denmark seems to be higher than reported in other cohorts. No improvement in completeness was detected after publication of the first Danish guidelines. In addition, data indicate that advanced age and low life expectancy at MTC diagnosis may serve as prognostic indicators to identify patients having a higher likelihood of missing the compulsory RET test.
Keywords: RET testing, medullary thyroid carcinoma, Denmark
Introduction
Medullary thyroid carcinoma (MTC) is a rare neuroendocrine tumor with an incidence of 0.19 per 100,000 per year and a prevalence of 3.8 per 100,000 inhabitants.1 MTC is divided into sporadic (75%) and hereditary (25%).1,2 The latter form occurs as part of multiple endocrine neoplasia (MEN)2, which is an autosomal-dominant inherited cancer syndrome, where virtually all patients develop MTC and variable proportions develop pheochromocytoma, primary hyperparathyroidism, cutaneous lichen amyloidosis, Hirschsprung’s disease, ganglioneuromatosis of the aerodigestive tract, and facial, ophthalmologic, and skeletal abnormalities.3 Conversely, the sporadic form is not known to have familial implications or predispositions to other endocrine neoplasia. It is thus important to distinguish hereditary MTC from sporadic MTC.
As germline mutations of the REarranged during Transfection (RET) proto-oncogene are present in practically all patients with hereditary MTC and absent in patients with sporadic MTC, distinction is best made by RET testing on peripheral blood.4 Presence of a RET germline mutation has serious implications for index cases and their families. Hence, index cases will need lifelong follow-up and screening for MEN2 manifestations other than MTC. Meanwhile, all first-degree relatives should be offered genetic counseling and genetic testing. If the relatives are positive of the family germline mutation, virtually all will require prophylactic thyroidectomy with the timing depending on the specific mutation. As for the index cases, relatives will also warrant lifelong follow-up for development of MEN2 manifestations. Thus, for several years, international guidelines have recommended RET testing of all patients with MTC.3,5,6
The first study aiming to determine the completeness of RET testing in MTC patients recently reported a completeness of 60% (86/142) in a single center.7 This completeness is remarkably discordant with both past and current recommendations.3,5,6 However, it is unknown if such low completeness is also the standard when examining a population-based cohort.
In Denmark, RET testing has been available since September 1994.8 Seven years later, in February 2002, the first Danish guidelines, recommending RET testing of all patients with MTC, were published.9 In the meantime, it is unknown if these guidelines have improved completeness of RET testing in MTC patients.
Consequently, we conducted the first population-based study aiming to evaluate the overall completeness of RET testing in a nationwide MTC cohort. Additionally, we aimed to assess the completeness before and after publication of the Danish guidelines and characterize MTC patients who had not been tested.
Methods
Design and setting
This investigation is a nationwide retrospective cohort study including all patients with newly diagnosed MTC in Denmark between January 1, 1997, and December 31, 2013.
Data sources
The Danish MTC cohort
This cohort formed the basis for the study. It initially comprised 476 patients diagnosed with histological (n=474) or cytological (n=2) MTC in Denmark between January 1960 and December 2014 and was constructed through three nationwide registries: the Danish Cancer Registry, the Danish Thyroid Cancer Database, and the Danish Pathology Register as described in detail elsewhere.1,10–13,36 The Danish Cancer Registry, the Danish Thyroid Cancer Database, and the Danish Pathology Register are considered to cover the whole country from 1987, 1996, and 1997, respectively. The MTC cohort consisted of an uncertain period from 1960 to 1996, where complete coverage could not be guaranteed, and a nationwide period from 1997 to 2014, where coverage of the entire country was considered complete. TNM staging in the MTC cohort was performed according to the seventh and eighth editions of the American Joint Committee on Cancer Staging Manual.14,15
The Danish RET cohort
This nationwide cohort was cross-checked with the Danish MTC cohort to determine the completeness of RET testing. The cohort initially contained all 1,583 patients RET tested in Denmark between September 1994 and December 2014. However, in the process of this study, we discovered three RET tested patients, whose test had initially been missed. Accordingly, the Danish RET cohort comprises 1,586 RET tested patients from 1994 to 2014. There was virtually no lag time between when a test was performed and when it was registered in the Danish RET cohort, as tests were registered instantly upon requisition. The cohort has been described and used on several occasions.8,16
Study participants
From the Danish MTC cohort, we extracted all 224 patients diagnosed in the nationwide period from January 1, 1997, to December 31, 2014. As RET testing post mortem is not considered a standard procedure in Denmark, we excluded all patients (n=4) diagnosed at autopsy.17 The original end date of the MTC cohort is similar to that of the RET cohort (December 31, 2014). Thus, to provide at least 1 year to detect RET testing in patients diagnosed during the last year (2013) of the MTC cohort, we excluded the patients (n=20) diagnosed from January 1, 2014, to December 31, 2014. This formed an overall MTC cohort comprising 200 patients, who were alive at the time of diagnosis in the study period from January 1, 1997, to December 31, 2013.
Virtually, all patients diagnosed with hereditary MTC by screening (asymptomatic) will have undergone predictive RET testing. Including such patients in the calculations may cause a false increase in completeness of RET tested MTC patients for the whole study period and for periods where large families with hereditary MTC were discovered. The latter could potentially bias a comparison of completeness between two periods. We therefore excluded patients diagnosed with hereditary MTC by screening (n=42). This yielded an adjusted MTC cohort of 158 patients diagnosed from January 1, 1997, to December 31, 2013.
Based on the publication date of the first Danish guidelines, February 7, 2002, we divided the adjusted MTC cohort into an early period comprising 48 patients diagnosed from 1, 1997, to February 6, 2002, and a late period comprising 113 patients diagnosed from February 7, 2002, to December 31, 2013.
For each patient, data were collected from the MTC and RET cohort on sex, date, and TNM stage at MTC diagnosis, RET test result and date of result, and survival time. The latter was calculated as the time from MTC diagnosis until death, emigration, or last follow-up (January 1, 2014), whichever came first. To explain why some patients had not been RET tested, we reviewed the medical records of these patients for any remarks of RET or genetic testing.
Statistical analyses
Completeness was calculated as the number of RET tested MTC patients divided by the total number of MTC patients and was given in percentage with 95% CI. Continuous data were presented as median and IQR. Categorical data were presented as absolute and relative frequencies. Continuous and categorical data were tested on univariate analysis with the Mann–Whitney–Wilcoxon-rank test and Fischer’s exact test as appropriate. In survival data, censoring was taken into account by the Kaplan–Meier method. All tests were two-sided, and P-values <0.05 were considered as significant. All analyses were done using Stata® 15.1 (StataCorp LP, College Station, TX, USA).
The investigation was approved by the Danish Health Authority (3-3013-395/3) and the Danish Data Protection Agency (18/17801).
Results
Overall completeness
In the overall MTC cohort, 87% (95% CI: 0.81–0.91; 173/200) of the patients diagnosed from January 1, 1997, to December 31, 2013, had been RET tested. Of the RET tested patients, 71% (122/173) had sporadic MTC and 29% (51/173) had hereditary MTC. In the latter patients, the following RET mutations were detected: C611W (n=3), C611Y (n=31), C618F (n=1), C618Y (n=3), C620R (n=4), C634Y+Y791F (n=1), L790F (n=1), A883F (n=1) and M918T (n=6). Several of these patients have been reported earlier.17–23
In the adjusted MTC cohort, in which patients diagnosed with hereditary MTC by screening had been excluded, the completeness of RET testing was 83% (95% CI: 0.76–0.88; 131/158).
Completeness before and after guidelines
In the early period, 88% (95% CI: 0.75–0.95; 42/48) had been RET tested, while this was the case for 81% (95% CI: 0.72–0.88; 89/110) in the late period. Completeness of RET testing in the adjusted MTC cohort according to year of MTC diagnosis is depicted in Figure 1.
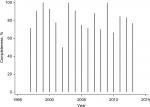  | Figure 1 Completeness of RET testing in patients with MTC in Denmark from 1997 to 2013. Abbreviations: RET, REarranged during Transfection; MTC, medullary thyroid carcinoma. |
Not RET tested
In the adjusted MTC cohort, 17% (27/158) of patients had not been RET tested (Table 1). For 20 patients, we found no reason for the lack of RET testing, as no medical record was available for two patients and no remark of testing was noted in the medical record of 18 patients. In three patients, testing was not performed due to advanced age. RET testing was planned in two patients but never performed for reasons unknown. Two patients had been tested for MEN1 instead of MEN2.
  | Table 1 Characteristics of 27 patients diagnosed with MTC in Denmark 1997–2013 but not RET tested before the end date of the Danish RET cohort, December 31, 2014 Notes: aStaging was based on the American Joint Committee on Cancer’s seventh and eighth editions.14,15 bSurvival was defined as the time from diagnosis of MTC until death, emigration, or last follow-up (December 31, 2014), whichever came first. Abbreviations: F, female; RET, REarranged during Transfection; M, male. |
Table 2 lists characteristics for those RET tested and those not RET tested. At the time of MTC diagnosis, those not RET tested had a higher median age and higher proportion of distant metastases compared to those RET tested. At the last follow-up, the median time to death was shorter in those not RET tested compared to those RET tested.
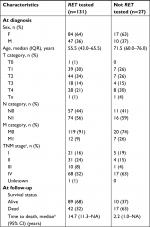  | Table 2 Characteristics of 158 patients evaluated for RET testing following diagnosis of MTC in Denmark from 1997 to 2013 Notes: Owing to rounding up, not all sums of percentages fit. aStaging was based on the American Joint Committee on Cancer’s seventh and eighth editions.14,15 bTaking censoring into account by the Kaplan–Meier method. No upper limit for the 95% CI due to too few events. Abbreviations: F, female; M, male; M, metastasis; MTC, medullary thyroid carcinoma; N, node; NA, not available; RET, REarranged during Transfection; T, tumor. |
Discussion
Although MEN2 is rare, recognition of the syndrome by RET testing is crucial, as early thyroidectomy before MTC has spread beyond the thyroid often confers the best chance for cure.13,24,37
In this nationwide study, we report a completeness of RET testing in >80% of patients diagnosed with MTC in Denmark from 1997 to 2013. We found no change in completeness when comparing completeness in periods before and after publication of the first Danish guidelines on the subject. Additionally, it seems that MTC patients with an advanced age and a short life expectancy have a higher likelihood of not being RET tested.
Limitations
The completeness estimate in this study is limited by the possibility that some patients from the MTC cohort (1997–2013) may have been tested after the end date of the RET cohort (1994–2014). This could result in a marginally higher completeness than reported. In our experience, however, most patients are tested within the first year of MTC diagnosis.
Our description of reasons for the lack of RET testing in MTC patients was limited by the retrospective collection of data from medical records. Medical records were available for 93% (25/27) of patients who had not been tested. However, 18 medical records contained no remark of RET testing, preventing us from drawing further conclusions in these patients. A reasonable thought, however, may be that RET testing in these cases was simply forgotten or not considered.
Overall completeness
In the present study, we found a RET testing completeness of more than 80%. Recently, the first study aiming to determine the completeness of RET testing in MTC patients was published. The study was based on the Southern California Kaiser Permanente health care network and reported a completeness of 60% (86/142).7 Often such differences between Danish and US cohorts may be explained by a different access to health care systems. However, all patients from the Californian study obtained medical insurance from the Kaiser Permanente health care network and all patients in Denmark are provided with free public health care. Thus, there seems to be equal access to health care in the two study cohorts. Another potential explanation could be differences in the setup of RET testing in the two cohorts. After the diagnosis of MTC in Denmark, patients are offered RET testing at the surgical department or referred directly to another department that offers testing. The absent details of testing setup in the Kaiser Permanente study, however, prevent direct comparison. On the other hand, the Kaiser study described an outreach project, in which 32 MTC patients, who had not been RET tested, were scheduled for genetic counseling. However, only 25% (8/32) showed interest for their appointment and accepted RET testing. Although having no direct Danish comparison, one could speculate that the reluctance toward RET testing may have been higher in the Kaiser Permanente study cohort compared to the Danish cohort.
Recent institutional studies, with inclusion periods roughly similar to ours, have reported a RET testing completeness of 39%–66% in MTC patients undergoing thyroid surgery.25–27 In addition, in this context, the completeness in Denmark seems fairly high. However, relative to the recommendation of a completeness of 100%, the Danish completeness is not sufficient.
Completeness before and after guidelines
We saw no change in completeness of RET tested patients with MTC diagnosed before and after publication of the first Danish guidelines. Thus, judged solely from this, the Danish guidelines did not improve outcome, as is often a goal for clinical guidelines.28 On the other hand, as we also did not find a decrease in the completeness, the guidelines may have helped to maintain a roughly unchanged completeness in the late period compared to that in the early period. One may also argue that the completeness in the early period was unexpectedly high (88%), potentially due to a commendable awareness prompted by the discovery in 1993 and 1994 that mutations in the RET proto-oncogene cause hereditary MTC.29–33 To the best of our knowledge, no other study has investigated the temporal changes in RET testing completeness of MTC patients with respect to national guidelines. However, research groups from the US have conducted population-based studies exploring surgical practice in regard to MTC guidelines.34,35 Thus, a study of 2,033 MTC patients included from the Surveillance, Epidemiology, and End Results Program database 1973–2006 examined adherence to the 2009 American Thyroid Association guidelines and found that 41% of patients diagnosed with MTC from 1988 to 2006 received surgical treatment that was out of line with the guidelines.6,34 Additionally, a study of 609 MTC patients identified from the California Cancer Registry 1999–2012 reported that central neck dissection was underused despite recommendation of this procedure in both the 2009 and 2015 American Thyroid Association guidelines.35 Thus, there are indications that other parts of the clinical practice for MTC are also discordant with guidelines.
Not RET tested
To identify MTC patients having a higher likelihood of missing the recommended RET test, we performed a medical record review in patients who had not been RET tested. Although the circumstances of not RET testing were described in a limited number of medical records, there seems to be a fraction of patients (3/27) who were not RET tested due to advanced age. This is consistent with the higher age at diagnosis noted in those not tested relative to those tested. Although remaining speculative, this may also apply for some of the patients who were not tested and had a medical record with no remark of RET testing. Additionally, 39% (7/18) of patients with no remark of RET testing in the medical record lived less than 1 year from MTC diagnosis to death. Thus, some patients may simply not live long enough to undergo RET testing (as they often need to be referred to and convened by the RET testing department). This is supported by the lower median time from diagnosis to death found when comparing those not tested to those tested. In addition, we found a higher proportion of distant metastases among those not tested compared to those tested. The difference, however, disappeared after Bonferroni correction in a logistic regression model that also incorporated sex, age, T category, and N category as predictor variables. Altogether, this could indicate that a low life expectancy and an advanced age at MTC diagnosis may serve as prognostic indicators for identification of MTC patients having a higher likelihood of missing the compulsory RET test. The differences among the tested and untested patients with regard to median age at diagnosis and median time to death are substantial in a clinical context, where they may be used as markers to find patients needing special attention to secure that RET testing is performed. This may improve the completeness of RET testing. Another way to improve completeness could be to perform RET testing immediately when the patient attending the surgical outpatient clinic is informed about the diagnosis of MTC. Among the Danish thyroid cancer centers, this setup is used in one center, while the remaining three centers use a two-tier setup, in which patients after diagnosis are referred to another department, where testing is then performed.
Conclusion
The completeness of RET testing in MTC patients in Denmark seems to be higher than reported in other cohorts. No improvement in completeness was detected after publication of the first Danish guidelines recommending RET testing in all MTC patients. In addition, our data indicate that advanced age and low life expectancy at MTC diagnosis may serve as prognostic indicators to identify patients having a higher likelihood of missing the compulsory RET test.
Acknowledgments
This work was supported by the University of Southern Denmark, the Region of Southern Denmark, the Odense University Hospital, the Copenhagen University Hospital, the Danish Cancer Society, the Danish Cancer Research Foundation, and the A.P. Moller Foundation.
Disclosure
The research salary of Ulla Feldt-Rasmussen is sponsored by an unrestricted research grant from the Novo Nordic Foundation. The authors report no other conflicts of interest in this work.
References
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Incidence and prevalence of sporadic and hereditary MTC in Denmark 1960-2014: a nationwide study. Endocr Connect. 2018;7(6):829–839. | ||
Raue F, Kotzerke J, Reinwein D, et al. Prognostic factors in medullary thyroid carcinoma: evaluation of 741 patients from the German Medullary Thyroid Carcinoma Register. Clin Investig. 1993;71(1):7–12. | ||
Wells SA, Asa SL, Dralle H, et al. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25(6):567–610. | ||
Fink M, Weinhüsel A, Niederle B, Haas OA. Distinction between sporadic and hereditary medullary thyroid carcinoma (MTC) by mutation analysis of the RET proto-oncogene. “Study Group Multiple Endocrine Neoplasia Austria (SMENA)”. Int J Cancer. 1996;69(4):312–316. | ||
Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab. 2001;86(12):5658–5671. | ||
American Thyroid Association Guidelines Task Force, Kloos RT, Eng C, et al. Medullary thyroid cancer: management guidelines of the American Thyroid Association. Thyroid. 2009;19(6):565–612. | ||
Parkhurst E, Calonico E, Abboy S. Utilization of genetic testing for RET mutations in patients with medullary thyroid carcinoma: a single-center experience. J Genet Couns. 2018;27(6):1411–1416. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Distribution of RET mutations in multiple endocrine Neoplasia 2 in Denmark 1994-2014: a nationwide study. Thyroid. 2017;27(2):215–223. | ||
Andersen PH, Kroustrup JP, Feldt-Rasmussen UF. Multipel endokrin neoplasi: Screening, diagnostik, behandling og efterkontrol: Oversigt og vejledende retningslinjer udarbejdet af Dansk MEN-arbejdsgruppe under Dansk Endokrinologisk Selskab. Lægeforeningens forlag. 2002. | ||
Gjerstorff ML. The Danish Cancer Registry. Scand J Public Health. 2011;39(7 Suppl):42–45. | ||
Londero SC, Mathiesen JS, Krogdahl A, et al. Completeness and validity in a national clinical thyroid cancer database: DATHYRCA. Cancer Epidemiol. 2014;38(5):633–637. | ||
Erichsen R, Lash TL, Hamilton-Dutoit SJ, Bjerregaard B, Vyberg M, Pedersen L. Existing data sources for clinical epidemiology: the Danish National Pathology Registry and Data Bank. Clin Epidemiol. 2010;2:51–56. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Incidence and prevalence of multiple endocrine neoplasia 2B in Denmark: a nationwide study. Endocr Relat Cancer. 2017;24(7):L39–L42. | ||
Edge SB, Byrd DR, Compton CC. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010. | ||
Amin MB, Edge SB, Greene FL. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2017. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Founder Effect of the RETC611Y Mutation in Multiple Endocrine Neoplasia 2A in Denmark: a Nationwide Study. Thyroid. 2017;27(12):1505–1510. | ||
Godballe C, Jørgensen G, Gerdes AM, Krogdahl AS, Tybjaerg-Hansen A, Nielsen FC. Medullary thyroid cancer: RET testing of an archival material. Eur Arch Otorhinolaryngol. 2010;267(4):613–617. | ||
Mathiesen JS, Dossing H, Bender L. Medullary thyroid carcinoma in a 10-month-old child with multiple endocrine neoplasia 2B. Ugeskr Laeger. 2014;176(5a), pii. | ||
Emmertsen K. Screening for hereditary medullary cancer in Denmark. Henry Ford Hosp Med J. 1984;32(4):238–243. | ||
Mathiesen JS, Habra MA, Bassett JHD, et al. Risk profile of the RET A883F germline mutation: an international collaborative study. J Clin Endocrinol Metab. 2017;102(6):2069–2074. | ||
Mathiesen JS, Stochholm K, Poulsen PL, Vestergaard EM, Christiansen P, Vestergaard P. Aggressive medullary thyroid carcinoma in a ten-year-old patient with multiple endocrine neoplasia 2B due to the A883F mutation. Thyroid. 2015;25(1):139–140. | ||
Vestergaard P, Kroustrup JP, Rønne H, Eng C, Laurberg P. Neuromas in multiple endocrine neoplasia type 2A with a RET codon 611 mutation. J Endocr Genet. 1999;1(1):33–37. | ||
Hansen HS, Torring H, Godballe C, Jäger AC, Nielsen FC. Is thyroidectomy necessary in RET mutations carriers of the familial medullary thyroid carcinoma syndrome? Cancer. 2000;89(4):863–867. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Incidence and prevalence of multiple endocrine neoplasia 2A in Denmark 1901-2014: a nationwide study. Clin Epidemiol. 2018;10:1479–1487. | ||
Kwon H, Kim WG, Sung TY, et al. Changing trends in the clinicopathological features and clinical outcomes of medullary thyroid carcinoma. J Surg Oncol. 2016;113(2):152–158. | ||
Kwon H, Kim WG, Jeon MJ, et al. Dynamic risk stratification for medullary thyroid cancer according to the response to initial therapy. Endocrine. 2016;53(1):174–181. | ||
Cho YY, Jang HW, Jang JY, et al. Clinical outcomes of patients with hypercalcitoninemia after initial treatment for medullary thyroid cancer and postoperative serum calcitonin cutoffs for predicting structural recurrence. Head Neck. 2016;38(10):1501–1508. | ||
Weisz G, Cambrosio A, Keating P, Knaapen L, Schlich T, Tournay VJ. The emergence of clinical practice guidelines. Milbank Q. 2007;85(4):691–727. | ||
Donis-Keller H, Dou S, Chi D, et al. Mutations in the RET proto-oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2(7):851–856. | ||
Mulligan LM, Kwok JB, Healey CS, et al. Germ-line mutations of the RET proto-oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363(6428):458–460. | ||
Carlson KM, Dou S, Chi D, et al. Single missense mutation in the tyrosine kinase catalytic domain of the RET protooncogene is associated with multiple endocrine neoplasia type 2B. Proc Natl Acad Sci USA. 1994;91(4):1579–1583. | ||
Eng C, Smith DP, Mulligan LM, et al. Point mutation within the tyrosine kinase domain of the RET proto-oncogene in multiple endocrine neoplasia type 2B and related sporadic tumours. Hum Mol Genet. 1994;3(2):237–241. | ||
Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367(6461):375–376. | ||
Panigrahi B, Roman SA, Sosa JA. Medullary thyroid cancer: are practice patterns in the United States discordant from American Thyroid Association guidelines? Ann Surg Oncol. 2010;17(6):1490–1498. | ||
Kuo EJ, Sho S, Li N, Zanocco KA, Yeh MW, Livhits MJ. Risk factors associated with reoperation and disease-specific mortality in patients with medullary thyroid carcinoma. JAMA Surg. 2018;153(1):52–59. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Replication of newly proposed TNM staging system for medullary thyroid carcinoma: a nationwide study. Endocr Connect. Epub 2018 Dec 1. | ||
Mathiesen JS, Kroustrup JP, Vestergaard P, et al. Survival and long-term biochemical cure in medullary thyroid carcinoma in Denmark 1997–2014: A nationwide study. Thyroid. In press 2019. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
