Back to Journals » OncoTargets and Therapy » Volume 9
Comparison of survival rates between 3D conformal radiotherapy and intensity-modulated radiotherapy in patients with stage Ⅲ non–small cell lung cancer
Received 10 October 2016
Accepted for publication 9 November 2016
Published 24 November 2016 Volume 2016:9 Pages 7227—7234
DOI https://doi.org/10.2147/OTT.S124311
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yao Dai
Moonkyoo Kong, Seong Eon Hong
Department of Radiation Oncology, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Republic of Korea
Purpose: Randomized trials showing a clear survival benefit of intensity-modulated radiotherapy (IMRT) over 3-dimensional conformal radiotherapy (3D-CRT) in the treatment of lung cancer are lacking. This study compared the survival rates of patients with stage III non-small cell lung cancer who were treated with either 3D-CRT or IMRT and analyzed the prognostic factors for survival.
Methods: From January 2008 to July 2015, 19 patients were treated with IMRT and 30 were treated with 3D-CRT in our institution. The choice between 3D-CRT and IMRT was determined by the physician based on tumor extent and general condition of the patients. The primary endpoint of this study was overall survival. The secondary endpoints were loco-regional recurrence-free survival, distant metastasis-free survival, and the incidence of radiation-induced lung and esophageal toxicities.
Results: The 1- and 2-year overall survival rates were 94.7% and 77.1% in the IMRT group and 76.7% and 52.5% in the 3D-CRT group, respectively. The overall survival rates of the IMRT group were higher than those of the 3D-CRT group; however, these differences were not statistically significant (P=0.072). Gross tumor volume was significantly associated with the overall survival rate. The 1- and 2-year loco-regional recurrence-free survival rates were 63.2% and 51% in the IMRT group and 67.5% and 48.1% in the 3D-CRT group (P=0.897), respectively. The 1- and 2-year distant metastasis-free survival rates were 78.9% and 68.4% in the IMRT group and 62.6% and 40.9% in the 3D-CRT group (P=0.120), respectively. Chemotherapy and treatment interruption were significantly associated with distant metastasis-free survival.
Conclusion: IMRT showed comparable or better overall survival compared with 3D-CRT in patients with stage III non-small cell lung cancer. To confirm the results of this study, further randomized prospective trials comparing IMRT with 3D-CRT are warranted.
Keywords: lung cancer, intensity-modulated radiotherapy, 3-dimensional conformal radiotherapy, survival rate, radiation toxicities, prognostic factor
Introduction
Radiotherapy (RT) is the standard treatment for patients with locally advanced non-small cell lung cancer. In recent years, intensity-modulated RT (IMRT) has become widely adopted for the treatment of lung cancer because of its ability to achieve a highly conformal dose distribution.1–4 Several planning studies have demonstrated theoretical dosimetric advantages of IMRT over 3-dimensional conformal RT (3D-CRT) in lung cancer.5–8 In addition, several studies have reported that the use of IMRT significantly reduces the rate of treatment toxicities in patients with lung cancer.9–11 However, data or randomized trials showing a clear survival benefit of IMRT over 3D-CRT in the treatment of lung cancer are lacking, and findings regarding survival remain generally inconclusive. This retrospective study of a single institution compared the survival rates of patients with stage III non-small cell lung cancer who were treated with either 3D-CRT or IMRT and analyzed the prognostic factors for survival.
Materials and methods
Inclusion criteria were histologically confirmed stage III non-small cell lung cancer, receipt of definitive RT with or without chemotherapy, Eastern Cooperative Oncology Group (ECOG) performance status ≤2, no previous history of thoracic RT, no distant metastasis, no previous or concurrent illness that would compromise completion of treatment, and available follow-up data. Patients who received postoperative or palliative RT were excluded. At this institution, IMRT for lung cancer was started in January 2008. From January 2008 to July 2015, 178 patients underwent RT for the treatment of non-small cell lung cancer. Of those patients, 49 met the inclusion criteria and were included in this study. The hospital records, laboratory results, and dose–volume histogram data extracted from computerized treatment planning records of all the study participants were retrospectively reviewed. The Institutional Review Board of Kyung Hee University Medical Center approved this study and waived the need for written informed consent. All research was carried out in compliance with the Helsinki Declaration.
The initial diagnosis was pathologically confirmed in all the patients based on either bronchoscopic or percutaneous fine needle aspiration biopsy. Pretreatment evaluation consisted of complete history and physical examination, basic laboratory studies, liver function test, pulmonary function test, chest radiograph, chest computed tomography (CT), brain magnetic resonance imaging, and positron emission tomography (PET). The cancer stage was restaged for each patient according to the 7th edition of the American Joint Committee on Cancer staging system.
All the patients received CT-planned RT with either the 3D-CRT or IMRT technique. The choice between 3D-CRT and IMRT was determined by the physician based on tumor extent, pulmonary functional status, and general condition of the patient. Gross tumor volume (GTV) included gross extent of the primary tumor and grossly involved lymph nodes visualized on chest CT and PET. Elective nodal irradiation was not performed. Clinical target volume (CTV) included the GTV plus a 6–8 mm margin, and planning target volume (PTV) was generated by adding an additional 8–15 mm margin to the CTV to take into account target movement due to respiration. To reduce the movement of the target by respiration, all the patients were instructed to take shallow breaths. Prescription dose was determined by the physician based on PTV, the patient’s general condition, and probability of RT-induced toxicity. A daily dose of 1.8–2.5 Gy was delivered at five fractions per week, resulting in a total dose of 59.4–70.4 Gy. For standard comparison of different RT dose schedules, biologically equivalent doses were calculated using a linear quadratic model with α/β ratio of 10. A Clinac iX (Varian Medical System Inc., Palo Alto, CA, USA) was used for 3D-CRT and a TomoTherapy (Accuray Inc., Madison, WI, USA) was used for IMRT. Treatment plans were evaluated using dose–volume histograms and visual inspection of isodose curves. In general, if PTV was covered by 95% isodose curves, inhomogeneity for PTV ranged from 95% to 110%, and doses to critical normal organs were limited in their tolerances, then the plans were considered to be acceptable. The implementation and regimen of chemotherapy was individualized based on each patient’s performance status and compliance. With the exception of RT technique (3D-CRT vs IMRT), there were no major differences in treatment strategies among patients during the study period.
Patients were examined at least weekly during RT to monitor radiation-induced acute toxicity. Follow-up visits were scheduled 1 month after completion of RT and every 2–3 months thereafter. Visits were more frequent for those who experienced severe treatment-related complications or disease progression. At the time of follow-up visits, basic laboratory studies, chest radiograph, and chest CT scan were conducted. PET was also performed as needed. The primary endpoint of this study was overall survival. The secondary endpoints were loco-regional recurrence-free survival, distant metastasis-free survival, and incidence of radiation-induced lung and esophageal toxicities. Loco-regional recurrence was defined as an increase in the size of target lesions or the appearance of new lesions in the ipsilateral thorax, ipsilateral and/or contralateral hilum, mediastinum, and supraclavicular lymph node regions. Distant metastasis was defined as evidence of tumor in any other area. Radiation-induced lung and esophageal toxicities were graded using the Common Terminology Criteria for Adverse Events version 4.0.
Baseline characteristics between groups were compared using a chi-square test for discrete variables and an independent t-test for continuous variables. Actuarial survival rates were estimated using the Kaplan–Meier method, and comparisons between groups were performed using log-rank tests. Survival times were calculated from the date of lung cancer diagnosis to the date of event or final follow-up visit. Parameters with a P-value <0.50 in a univariate analysis were further assessed in a multivariate analysis using a Cox proportional regression hazard model. All tests were two-sided and P<0.05 was considered statistically significant. All analyses were performed using SPSS version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
During the study period, 19 patients were treated with IMRT and 30 were treated with 3D-CRT. Patient and tumor characteristics are summarized in Table 1. Eighteen patients received concurrent chemotherapy with a regimen of weekly paclitaxel plus carboplatin. Two of these patients received additional induction chemotherapy or consolidation chemotherapy, respectively. Seven patients underwent induction chemotherapy alone with a regimen of cisplatin plus etoposide. Three patients in the IMRT group experienced temporary RT interruption due to treatment-related toxicities, and the duration of interruption was 11, 5, and 4 days, respectively. Seven patients in the 3D-CRT group experienced temporary RT interruption, and the median duration of interruption was 7 days (range, 4–18 days). Total RT dose was higher and RT duration was shorter in the IMRT group than in the 3D-CRT group. However, there were no significant differences in other characteristics between the two groups. The median follow-up times of IMRT and 3D-CRT groups were 24.1 and 18.8 months, respectively.
During the follow-up period, 14 patients (73.7%) in IMRT group and 16 patients (53.3%) in 3D-CRT group were still alive. The 1- and 2-year overall survival rates were 94.7% and 77.1% in IMRT group, and 76.7% and 52.5% in 3D-CRT group, respectively. The overall survival rates of IMRT group were higher than those of the 3D-CRT group, but these differences were not statistically significant (P=0.072) (Figure 1). Prognostic factors for overall survival were analyzed and are summarized in Table 2. In univariate analysis, age (P=0.035), ECOG performance status (P=0.044), and GTV (P=0.040) were significantly associated with the overall survival. In multivariate analysis, only GTV remained a significant prognostic factor for overall survival (hazard ratio, 4.985; 95% confidence interval, 1.569–15.839; P=0.017) (Figure 2). Smaller GTV was significantly associated with good overall survival. Non-current smoker (P=0.053), chemotherapy (P=0.051), and IMRT (P=0.063) were also associated with good overall survival, although these associations were not statistically significant.
During the follow-up period, 11 patients (57.8%) in IMRT group and 14 patients (46.7%) in 3D-CRT group experienced loco-regional recurrence. The 1- and 2-year loco-regional recurrence-free survival rates were 63.2% and 51% in IMRT group, and 67.5% and 48.1% in 3D-CRT group, respectively (P=0.897). Prognostic factors for loco-regional recurrence-free survival are summarized in Table 3. Both univariate and multivariate analyses did not identify any significant prognostic factor for loco-regional recurrence-free survival.
Seven patients (36.8%) in IMRT group and 14 patients (46.7%) in 3D-CRT group experienced distant metastases. The most common metastatic site was contralateral lung; among the 21 patients who experienced distant metastases, 15 developed distant metastases in contralateral lung. The 1- and 2-year distant metastasis-free survival rates were 78.9% and 68.4% in IMRT group and 62.6% and 40.9% in 3D-CRT group, respectively (P=0.120). Analysis of prognostic factors for distant metastasis-free survival is summarized in Table 4. In univariate analysis, there was no significant prognostic factor for distant metastasis-free survival. However, in multivariate analysis, chemotherapy (hazard ratio, 6.387; 95% confidence interval, 1.318–30.952; P=0.021) and RT interruption (hazard ratio, 0.244; 95% confidence interval, 0.060–0.995; P=0.049) were significantly associated with distant metastasis-free survival.
Radiation-related toxicities are summarized in Tables 5 and 6. Grade 4 toxicity was not observed, and no patient died from radiation-related toxicity. There were no differences in radiation-related lung toxicities between the IMRT and 3D-CRT groups. However, IMRT significantly decreased the incidence of esophagitis and esophageal stricture compared with 3D-CRT (P=0.042 for esophagitis and P=0.036 for esophageal stricture). All grade 3 esophageal toxicities were developed in patients with left-sided lung cancer.
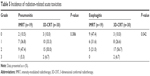  | Table 5 Incidence of radiation-related acute toxicities |
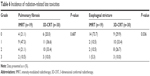  | Table 6 Incidence of radiation-related late toxicities |
Discussion
There have been no randomized prospective trials comparing the survival outcomes of IMRT and 3D-CRT in the treatment of non-small cell lung cancer. Previous studies using the US SEER-Medicare data did not find significant differences in survival outcomes between IMRT and 3D-CRT.12,13 A meta-analysis study also reported no significant survival differences.14 However, several single institution studies have reported that IMRT significantly improved the overall survival compared with 3D-CRT in the treatment of non-small cell lung cancer. Liao et al retrospectively analyzed the treatment outcomes of 496 patients with non-small cell lung cancer who were treated with either IMRT or 3D-CRT and reported that overall survival was significantly better in patients treated with IMRT.10 McCloskey et al also reported significantly better overall survival outcomes in patients treated with IMRT in their retrospective single institution study.15 In the present study, the 1- and 2-year overall survival rates were 94.7% and 77.1% in the IMRT group compared with 76.7% and 52.5% in the 3D-CRT group, respectively. Although the overall survival rates were higher in the IMRT group than in the 3D-CRT group, these differences were not statistically significant (P=0.072 in univariate analysis and P=0.063 in multivariate analysis). However, it is necessary to note the differences in patient characteristics between the two groups in this study. Patients in the IMRT group had a higher proportion of stage IIIB disease (52.6% vs 36.7%) and bigger GTV (median 112.5 cc vs 74.5 cc) than those in the 3D-CRT group. In the clinical field, patients whose tumors cannot be treated optimally with 3D-CRT are usually treated with IMRT. It is believed that these patient selection biases might be expected to militate against the IMRT group and strengthen the favorable survival outcomes of the IMRT group observed in this study. Patient selection biases have also been found in several previous studies.10,12,13,15,16 Therefore, comparison of survival outcomes between IMRT and 3D-CRT should be made with caution. In addition, meta-analysis studies and studies using US SEER-Medicare data enroll patients who might have inherently different characteristics from those in the general community. Because single institution studies enroll community-based populations, their results might offer valuable information regarding clinical outcomes in patients encountered in a community clinical setting. This study might offer additional information regarding survival outcomes in patients with stage III non-small cell lung cancer in the community clinical field. Further randomized prospective studies are warranted to confirm the results of this and previous single institution studies.
Because of the increased number of radiation beams, IMRT could expose a larger volume of lung tissue to low-dose radiation than 3D-CRT. Some studies reported that IMRT increases the amount of normal lung tissue exposed to a low dose of radiation and could potentially increase the risk of radiation pneumonitis.17,18 However, several studies have reported that IMRT did not increase the incidence of treatment toxicities in patients with lung cancer.9,10,16 This study conducted IMRT using helical tomotherapy. Because of helical radiation delivery method, low-dose radiation exposure of normal lung tissue is a greater concern in helical tomotherapy. However, this study showed no differences in radiation-related lung toxicities between IMRT and 3D-CRT groups. Moreover, despite the higher proportion of left lung cancer (52.6% vs 46.6%) and bigger GTV, IMRT significantly decreased the incidence of esophageal toxicity compared with 3D-CRT (Tables 5 and 6). Some previous studies also reported acceptable toxicities after IMRT (helical tomotherapy) in patients with lung cancer.19,20 It is believed that clinicians do not need be overly concerned about the likelihood of increased radiation toxicity after IMRT for lung cancer.
There were several limitations in this study. First, this study was retrospective and may therefore have inherent biases. For example, RT fractionation schedules were decided by the attending radiation oncologist rather than using a predetermined definite protocol, and the allocation of patients to either 3D-CRT or IMRT was not random. IMRT-treated patients usually received higher daily radiation dose, and consequentially, received RT for a shorter period. These biases may make it difficult to interpret the results obtained. In addition, because of incomplete patient medical records, some potential prognostic factors for overall survival such as changes in body weight could not be analyzed. Second, as the sample size was small, minor differences in the statistical analyses might not have been detected. Third, the patient characteristics were heterogeneous. And finally, the duration of the follow-up period was not long. Nonetheless, it is believed that this study provides valuable information regarding survival outcomes in patients with stage III lung cancer encountered in community clinical setting and contributes toward resolution of some inconclusive issues regarding the management of lung cancer.
Conclusion
In conclusion, IMRT showed comparable or better overall survival compared with 3D-CRT in patients with stage III non-small cell lung cancer. To confirm the results of this study, further randomized prospective trials comparing IMRT with 3D-CRT are warranted.
Acknowledgment
This research was supported by the Kyung Hee University Research Fund in 2016 (KHU-20161387).
Disclosure
The authors report no conflicts of interest in this work.
References
Mell LK, Mehrotra AK, Mundt AJ. Intensity-modulated radiation therapy use in the U.S., 2004. Cancer. 2005;104(6):1296–1303. | ||
Shirvani SM, Jiang J, Gomez DR, Chang JY, Buchholz TA, Smith BD. Intensity modulated radiotherapy for stage III non-small cell lung cancer in the United States: predictors of use and association with toxicities. Lung Cancer. 2013;82(2):252–259. | ||
Kim YJ, Song SY, Jeong SY, et al. Definitive radiotherapy with or without chemotherapy for clinical stage T4N0-1 non-small cell lung cancer. Radiat Oncol J. 2015;33(4):284–293. | ||
Ricardi U, Badellino S, Filippi AR. Stereotactic radiotherapy for early stage non-small cell lung cancer. Radiat Oncol J. 2015;33(2):57–65. | ||
Grills IS, Yan D, Martinez AA, Vicini FA, Wong JW, Kestin LL. Potential for reduced toxicity and dose escalation in the treatment of inoperable non-small-cell lung cancer: a comparison of intensity-modulated radiation therapy (IMRT), 3D conformal radiation, and elective nodal irradiation. Int J Radiat Oncol Biol Phys. 2003;57(3):875–890. | ||
Liu HH, Wang X, Dong L, et al. Feasibility of sparing lung and other thoracic structures with intensity-modulated radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1268–1279. | ||
Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58(4):1258–1267. | ||
Wu VW, Kwong DL, Sham JS. Target dose conformity in 3-dimensional conformal radiotherapy and intensity modulated radiotherapy. Radiother Oncol. 2004;71(2):201–206. | ||
Yom SS, Liao Z, Liu HH, et al. Initial evaluation of treatment-related pneumonitis in advanced-stage non-small-cell lung cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2007;68(1):94–102. | ||
Liao ZX, Komaki RR, Thames HD Jr, et al. Influence of technologic advances on outcomes in patients with unresectable, locally advanced non-small-cell lung cancer receiving concomitant chemoradiotherapy. Int J Radiat Oncol Biol Phys. 2010;76(3):775–781. | ||
Lopez Guerra JL, Gomez DR, Zhuang Y, et al. Changes in pulmonary function after three-dimensional conformal radiotherapy, intensity-modulated radiotherapy, or proton beam therapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83(4):e537–e543. | ||
Harris JP, Murphy JD, Hanlon AL, Le QT, Loo BW Jr, Diehn M. A population-based comparative effectiveness study of radiation therapy techniques in stage III non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;88(4):872–884. | ||
Chen AB, Li L, Cronin A, Schrag D. Comparative effectiveness of intensity-modulated versus 3D conformal radiation therapy among medicare patients with stage III lung cancer. J Thorac Oncol. 2014;9(12):1788–1795. | ||
Hu X, He W, Wen S, et al. Is IMRT superior or inferior to 3DCRT in radiotherapy for NSCLC? A meta-analysis. PLoS One. 2016;11(4):e0151988. | ||
McCloskey PM, Atallah S, Coate L, et al. Comparison of 3D conformal radiation therapy (3DCRT) and intensity modulated radiation therapy (IMRT) in stage III non-small cell lung cancer (NSCLC). Int J Radiat Oncol Biol Phys. 2012;84(3 Suppl):S597–S598. | ||
Noh JM, Kim JM, Ahn YC, et al. Effect of radiation therapy techniques on outcome in N3-positive IIIB non-small cell lung cancer treated with concurrent chemoradiotherapy. Cancer Res Treat. 2016;48(1):106–114. | ||
Gopal R, Tucker SL, Komaki R, et al. The relationship between local dose and loss of function for irradiated lung. Int J Radiat Oncol Biol Phys. 2003;56(1):106–113. | ||
Yorke ED, Jackson A, Rosenzweig KE, et al. Dose-volume factors contributing to the incidence of radiation pneumonitis in non-small-cell lung cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys. 2002;54(2):329–339. | ||
Kong M, Hong SE. Clinical outcome of helical tomotherapy for inoperable non-small cell lung cancer: the Kyung Hee University Medical Center experience. Asian Pac J Cancer Prev. 2014;15(4):1545–1549. | ||
Kim Y, Hong SE, Kong M, Choi J. Predictive factors for radiation pneumonitis in lung cancer treated with helical tomotherapy. Cancer Res Treat. 2013;45(4):295–302. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






