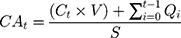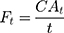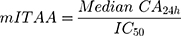Back to Journals » Journal of Pain Research » Volume 13
Comparison of Skin Permeation and Putative Anti-Inflammatory Activity of Commercially Available Topical Products Containing Ibuprofen and Diclofenac
Authors Pradal J
Received 18 May 2020
Accepted for publication 30 September 2020
Published 4 November 2020 Volume 2020:13 Pages 2805—2814
DOI https://doi.org/10.2147/JPR.S262390
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Dr Michael Schatman
Julie Pradal
GlaxoSmithKline Consumer Healthcare S.A, Nyon 1260, Switzerland
Correspondence: Julie Pradal
GSK Consumer Healthcare S.A., Route De l’Etraz 2, Nyon 1260, Switzerland
Tel +41 22 567 26 72
Email [email protected]
Purpose: The therapeutic effect of topical nonsteroidal anti-inflammatory drugs (NSAIDs) depends on the drug’s ability to penetrate and permeate the skin and subsequently inhibit cyclo-oxygenase (COX) isoforms responsible for pain and inflammation. Most commercially available topical NSAID formulations are clinically effective, but direct comparisons of anti-inflammatory activity including both skin absorption and inhibitory potency are lacking. This study examined the skin absorption of representative commercially available topical diclofenac- and ibuprofen-based formulations along with published potency values to determine formulations with superior anti-inflammatory activity.
Materials and Methods: Cumulative absorption and flux profiles of 12 commercially available topical NSAIDs (6 diclofenac-based and 6 ibuprofen-based) were evaluated in vitro using human skin in static Franz diffusion cells. Each formulation was applied as a single dose. In vitro permeation parameters and published COX-2 inhibition values were used to calculate a modified index of topical anti-inflammatory activity (mITAA).
Results: All diclofenac and ibuprofen formulations permeated human skin in vitro. The rate and degree of absorption differed between diclofenac and ibuprofen formulations and between formulations of the same drug. NSAID concentration within a product was not solely responsible for the permeation flux or degree of absorption. Ibuprofen formulations permeated the skin more rapidly and to a greater degree than diclofenac, but calculated mITAAs were higher for diclofenac.
Conclusion: Diclofenac exhibited superior anti-inflammatory activity as measured by the index. Differences beyond drug concentration, including excipients, drug salt form, and dosage form, contribute to differences in absorption and thus in anti-inflammatory activity. Both absorption and COX-2 inhibition potency are important for anti-inflammatory activity, but their priority depends upon the products being compared—with the same NSAID, absorption determines superiority; with different NSAIDs, superiority is determined by the balance between absorption and COX-2 potency. These findings should be considered when selecting a topical NSAID for treating patient pain and inflammation.
Keywords: cutaneous, NSAID, in vitro study, COX-2 inhibition, index of topical anti-inflammatory activity, ITAA
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) are often prescribed to manage acute and chronic pain in patients suffering from various musculoskeletal disorders, including osteoarthritis, rheumatoid arthritis, and trauma-related conditions such as sprains.1
Common NSAIDs include ibuprofen, diclofenac, and acetylsalicylic acid (aspirin). These drugs exert pain relief and reduce inflammation through inhibition of the cyclooxygenase (COX) isoforms COX-2, an inducible isoform of the enzyme that is typically upregulated in inflamed tissue, and COX-1, a constitutively expressed isoform that is generally more widely distributed.2 NSAIDs, such as ibuprofen and diclofenac, have different relative selectivity for COX-1 or COX-2, with diclofenac being a more selective inhibitor for COX-2 compared to COX-1, while ibuprofen inhibits both COX isoforms similarly.3 The clinical effectiveness of topical ibuprofen and diclofenac for the management of acute (eg, sprains/strains) and chronic pain has been previously demonstrated.4,5
Oral and intravenous NSAIDs are associated with risks for systemic adverse events including gastrointestinal, cardiovascular, and renal events;6–8 therefore, topical NSAIDs are often used as effective alternatives with a decreased risk of systemic adverse events.9–11 In addition to pharmacological potency against COX isoforms, drug absorption into the skin is a critical attribute for topical NSAIDs.12–15 These drugs must penetrate the stratum corneum and permeate through the epidermis and dermis to reach the site of inflammation and pain in sufficient amounts to exert a clinical effect. Penetration and permeation can be affected by numerous factors, including application site, formulation chemistry, and drug properties.14,16,17
While the clinical efficacy and in vitro skin permeability of certain topical NSAIDs have been evaluated previously, there have been no head-to-head comparisons of permeability and potency across multiple commercially available formulations using the same testing protocols. Because differences in efficacy (including intensity and durability of effect) and adverse reactions have been demonstrated with the use of different topically applied NSAIDs, it is important to understand the parameters and sources of potential differences when choosing an appropriate topical formulation for treating patient pain.
In this study, 12 commercially available topical NSAID products (6 ibuprofen-based and 6 diclofenac-based) were evaluated using in vitro human skin permeation assays. These assays were designed to determine the amount of drug reaching the first stratum of the dermis (400 μm dermatomed skin). The penetration of the drug, meaning the amount of drug released by the formulation going through the first layer of the skin (ie, the stratum corneum), has not been quantified.
Each formulation was applied at a dose intended to mimic recommended “in-use” conditions.18 These data were combined with previously published COX-2 inhibition potency values for diclofenac and ibuprofen to determine a modified index of topical anti-inflammatory activity (mITAA). The mITAA was based on the previously described ITAA,13 an index value that accounts for the biopharmaceutic and pharmacodynamic properties of a topical NSAID in order to estimate its intrinsic efficacy (ie, anti-inflammatory activity) and allow comparisons with other NSAIDs. To the author’s knowledge, this is the first direct comparison of diclofenac- and ibuprofen-containing commercially available topical formulations using this method.
Materials and Methods
Materials
Twelve commercially available topical NSAID products were used for this study (qualitative formulation compositions are summarized in Table 1). The 6 diclofenac-based products are hereafter termed Diclo-1 to Diclo-6, and the 6 ibuprofen-based products are termed Ibu-1 to Ibu-6.
 |
Table 1 Qualitative Composition of Diclofenac and Ibuprofen Products as Labelled on the Packaging |
In vitro Skin Permeation
Human Skin
Skin was obtained from the abdominal region of 6 patients during plastic surgery (patients provided informed written consent). Approval from 2 ethical committees was obtained (Lothian Research Ethics Committee, Edinburgh, UK (06/S1101/19), and West of Scotland Research Ethics Services, Glasgow, UK (08/S0704/30)). After collection, skin samples were frozen at −20°C until use. On the day of the experiment, skin preparations were thawed and dermatomed to ~400 µm thickness, starting from the stratum corneum. The barrier integrity of the skin samples was tested using an internal procedure. All samples that were used in this study exhibited an electrical resistance >10.9 kΩ and <35 kΩ according to the acceptance criteria for the procedure.
Sample Size
Split-thickness abdominal skin samples were provided by 6 human skin donors. Each formulation was applied to 2 replicate skin samples from each donor; therefore, 12 total skin samples were tested per formulation. The sample size was chosen to provide acceptable confidence in the estimation of the geometric mean ratios (ie, 95% confidence interval [CI] of −30%/+43% around the geometric mean ratio assuming a within-donor standard deviation of 0.40 on the log scale, based on previous internal data [data on file, not shown]).
In vitro Skin Permeation Procedure
Static Franz diffusion cells (PermeGear, Hellertown, PA, USA) with an exposed skin area of 0.64 cm2 and a receptor chamber volume of ~5 mL were used to assess skin permeation in vitro. Phosphate buffered saline (PBS) containing bovine serum albumin (BSA) 5% (w/v) was used as the receptor fluid. These receptor fluid components were chosen to ensure drug solubility and avoid drug saturation in the receptor fluid. Based upon assessments during method validation, the solubility in receptor fluid was 5.15 mg/mL for diclofenac and 575 μg/mL for ibuprofen. The permeability study was performed under sink conditions for both drugs.
Skin samples were mounted between the donor and receptor chambers, the cells were positioned in a manifold heated to maintain a skin surface temperature of 32 ± 1°C, and receptor fluids were mixed using a magnetic stirrer flea. An electrical resistance barrier integrity assessment was performed, and skin samples not meeting acceptance criteria (electrical resistance >10.9 kΩ and <35 kΩ) were excluded.
To mimic the application of a single recommended dose (“in-use” conditions) and allow a fair comparison between products, the formulations were applied on the stratum corneum surface of excised human abdominal split-thickness skin at a single finite dose of 10 mg/cm2 for the tested products.19 Receptor fluid samples (300 µL) were collected at 0 (pre-dose), 2, 4, 8, 16, and 24h. The removed receptor fluid volume (300 µL) was replenished with fresh receptor fluid solution after each withdrawal.
All experimental studies were performed by Charles River Laboratories (Edinburgh, UK). Validated analytical methods were used to analyze receptor fluid samples by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Chromatographic separation for both drugs was done on a Poroshell EC-C18 column (50 mm × 2.1 mm, 2.7 μm spherical particles).
For diclofenac, the mobile phase consisted of a mixture of methanol/formic acid (100/0.5, v/v, A) and water/formic acid (100/0.5, v/v, B) using a gradient elution. The solvent flow rate was 500 µL/min. The determination of diclofenac in 5% BSA in PBS was validated over the range 1.00 to 10,000 ng/mL. The lower limit of quantification was 1 ng/mL. Assay accuracy and precision were ±20% and ≤20%, respectively.
For ibuprofen, the mobile phase consisted of a mixture of acetonitrile/ammonia (100/0.2, v/v, A) and water/ammonia (100/0.2, v/v, B) using a gradient elution. The solvent flow rate was 600 µL/min. The determination of ibuprofen in 5% BSA in PBS was validated over the range 5.00 to 5000 ng/mL. The lower limit of quantification was 5 ng/mL. Assay accuracy and precision were ±15% and ≤15%, respectively.
For both drugs, indomethacin (2.50 μg/mL) was used as an internal standard. The injection volume was 10 µL, and the column temperature was 60°C.
Data Analysis
Cumulative absorption (CA) of diclofenac and ibuprofen was determined at each time point (2, 4, 8, 16, and 24h) as follows:
where CAt = cumulative absorption at time t in ng/cm2, Ct = concentration of drug in the receptor fluid aliquot at time t, V = volume of the receptor chamber (~5 mL), Qi = amount of active pharmaceutical ingredient in the receptor fluid aliquot at time t, and S = surface area of the skin membrane (0.64 cm2).
Flux of diclofenac and ibuprofen for each formulation was determined at each time point (Ft) as follows:
Statistical Analysis
Human skin permeability values have previously been identified to have log-normal (or skewed non-normal) distribution,20 favoring the use of geometric means to compare the formulations. Log-transformed mean CA24h of each drug was compared between formulations using post hoc testing. Since both a fixed effect and a random effect were to be included in the model, a residual maxi-mum likelihood estimation-based mixed-effects model with formulation as a fixed effect and donor as a random effect was used. Because confidence interval (CI) for the geometric mean ratio is the recommended method for determining equivalence for skin permeation studies according to the EMA draft Guideline on Quality and Equivalence of Topical Products,21 CI was used instead of standard deviation for these analyses. 95% CIs for the geometric mean ratios were derived by back-transforming the CIs for the differences between formulations on the log-transformed scale obtained from the analysis. No adjustment for multiplicity was made and, for exploratory purposes, statistical significance was concluded if the 95% CI for the geometric mean ratio did not include 1.
Estimation of mITAA
Previously, Cordero et al calculated ITAA using saturated solutions of NSAIDs, which provided a composite metric for anti-inflammatory activity that includes both a biopharmaceutic component (maximum flux) and a pharmacodynamic component (COX-2 inhibition potency).13 In the current study, maximum flux was not reached in all formulations within the 24h testing window due to the use of a single application of drug, which was intended to mimic “in-use” conditions. Therefore, the medians of cumulative absorptions observed at 24h (CA24h) were used to determine a modified ITAA (mITAA), as follows:
where IC50 values were taken from Esser et al22 and correspond to the drug concentration that induces 50% of the maximum effect. IC50 values reported by Esser et al were measured in human whole blood using production of prostaglandin E2 as a surrogate of COX-2 activity. The IC50 values for diclofenac and ibuprofen were 0.013 µM and 9 µM, respectively.
Results
Skin Permeation – Cumulative Absorption
Diclofenac and ibuprofen from all formulations permeated through human skin by 24h. In terms of absolute quantities, ibuprofen permeated in greater extent than diclofenac in all cases. Diclo-1 and Ibu-6 exhibited the highest CA24h values within their respective groups, with geometric mean CA24h values of 849 ng/cm2 and 25,282 ng/cm2, respectively (Figure 1).
 |
Figure 1 Cumulative absorption at 24h of topical diclofenac and ibuprofen products (geometric means and 95% confidence intervals). |
Skin permeation varied widely between formulations with the same drug and concentration. Geometric mean CA24h ranged from 119 to 747 ng/cm2 for products containing 1% diclofenac sodium or 1.16% diclofenac diethyl-amine (DEA) (Figure 1). The difference in geometric mean CA24h between diclofenac DEA (Diclo-5) and diclofenac sodium (Diclo-6) gel formulations was significant, with the sodium salt absorbing better (ratio of geometric mean CA24h: 0.16; 95% CI 0.10–0.26; Table 2). For the 1.16% diclofenac DEA formulations, while there was a significant difference between geometric mean CA24h for the gel formulation (Diclo-5) versus each of the emulsion formulations (Diclo-2, Diclo-3, and Diclo-4), with the emulsions absorbing better, the emulsions also contained propylene glycol which is a cosolvent with some permeation-enhancing properties.
 |
Table 2 Ratios (95% CIs) of Adjusted Geometric Means of Cumulative Absorption at 24h for Diclofenac and Ibuprofen Formulations |
Geometric mean CA24h ranged from 5944 to 9293 ng/cm2 for products containing 5% ibuprofen. The difference between geometric mean CA24h for 5% ibuprofen cream formulations (Ibu-1, Ibu-2) versus 5% ibuprofen gel formulations (Ibu-3, Ibu-4) was significant (Table 2), with greater absorption at 24h for the creams, although the creams also contained propylene glycol which has permeation-enhancing properties. There was also a significant difference between geometric mean CA24h for the 10% ibuprofen gel formulations (Ibu-5 and Ibu-6; ratio of geometric mean CA24h: 1.64; 95% CI 1.26 to 2.14), which could reflect the impact of formulation composition differences even when using the same dosage form.
Absorption was not proportional to drug concentration. Diclo-6 (1% diclofenac sodium) had a geometric mean CA24h of 747 ng/cm2, which was similar to Diclo-1 (2.32% diclofenac DEA) at 849 ng/cm2. This results in a geometric mean ratio of 0.88 (95% CI: 0.55 to 1.41; Table 2) despite a 2-fold difference in concentration. The ratio of geometric mean CA24h values for topical formulations containing 1.16% and 2.32% diclofenac and 5% and 10% ibuprofen (each 2-fold different) ranged from 2.5 to 7.1 and from 1.7 to 4.3, respectively (Table 2).
Flux
All diclofenac formulations resulted in diclofenac penetration (through the stratum corneum) and permeation (through the remaining epidermis and first strata of dermis) through the skin by 8h, with Diclo-6 (containing 1% diclofenac sodium) delivering diclofenac earlier (at 4h) than the other formulations (Figure 2). There were two flux profile types: formulations exhibiting no flux plateau by 24h (Diclo-1, Diclo-2, Diclo-3, and Diclo-5) and formulations that plateaued around 16h (Diclo-4 and Diclo-6). Diclo-1 (containing 2.32% diclofenac DEA and 2 permeation enhancers) had the highest flux at 24h.
 |
Figure 2 Median fluxes of topical diclofenac (A) and ibuprofen (B) products. |
All ibuprofen formulations resulted in ibuprofen permeation flux through the skin beginning at 2h and reaching a plateau at 8h, except for Ibu-6, which continued to increase permeation flux through 24h (Figure 2). Formulations with higher ibuprofen concentrations (Ibu-5 and Ibu-6) had higher flux values than formulations with lower concentrations. There was little difference between the flux profiles of 5% ibuprofen formulations regardless of whether these were creams or gels.
mITAA
mITAA values ranged from 4 to 32 for diclofenac and from 0.37 to 2 for ibuprofen, with mITAA values for the diclofenac formulations being higher than those for the ibuprofen formulations in all cases (Figure 3).
 |
Figure 3 Modified index of anti-inflammatory activity for topical diclofenac and ibuprofen products. |
Among diclofenac DEA formulations, Diclo-1, with a higher drug concentration and 2 permeation enhancers, had a higher mITAA than formulations with lower drug concentrations and fewer permeation enhancers (Diclo-2, Diclo-3, Diclo-4, and Diclo-5). Diclofenac sodium (Diclo-6) had a higher mITAA than diclofenac DEA (Diclo-5) despite similar diclofenac concentrations and the same dosage form (gel).
Among ibuprofen gel formulations, higher drug concentration (10% ibuprofen; Ibu-5 and Ibu-6) was associated with higher mITAA values compared to gels with lower drug concentration (5% ibuprofen; Ibu-3 and Ibu-4). Five percent ibuprofen creams with permeation enhancers (Ibu-1 and Ibu-2) also had higher mITAA than ibuprofen gels (Ibu-3 and Ibu-4) of the same drug concentration.
Discussion
Following in vitro assessments that mimicked recommended application doses, there were broad differences in skin absorption, as measured by CA24h and flux, across formulations. This was true not only between the ibuprofen and diclofenac formulations but also within these groups.
It is known that if a drug presents a low IC50, the associated potency (related to mITAA) will be high. Moreover, ibuprofen permeated through human skin to a greater extent than diclofenac. These results are consistent with previous findings and are likely due, at least in part, to the lower molecular weight of ibuprofen (~206 g/mol) compared to diclofenac (~296 g/mol)23 and the pKa values of these drugs (3.9 for diclofenac vs 4.6 for ibuprofen).24–26 The degree of drug ionization influences the drug permeation rate. Indeed, the un-ionized species of a drug has a higher permeability coefficient than its respective ionized species. Hence, the pKa value of the drug, the pH of the formulation, and the physiological pH of the skin are essential parameters influencing drug permeation. In addition, higher concentrations of ibuprofen (5% and 10%) compared to diclofenac (1% and 2%) in these formulations must also contribute to the highest observed values for CA24h and flux.
Among formulations containing the same drug, there appear to be multiple factors contributing to differences in permeation that go beyond the concentration of drug in each formulation, which is supported by previous findings.27–30 These include the choice of excipients such as penetration enhancers, the drug salt that is used, and the dosage form.
For example, despite a 2-fold difference in diclofenac concentration, CA24h was 4 times greater for Diclo-1 (2.32% diclofenac DEA) than for Diclo-2 (1.16% diclofenac DEA). This difference may be explained by the presence of an additional permeation enhancer, oleyl alcohol,31 in Diclo-1.
In addition, there was a significant difference in CA24h between Diclo-5 and Diclo-6 (geometric mean ratio of Diclo-5: Diclo-6 of 0.16), despite similar drug concentrations (1.16% diclofenac DEA and 1.0% diclofenac sodium, respectively), qualitatively similar formulations, and the same dosage form (gel). These differences may be explained by the distinct salt forms of the drug in each formulation, as proposed by O’Connor et al,28 who observed that diclofenac sodium has a higher rate of membrane transport than diclofenac DEA owing to the higher saturation solubility of the sodium salt. In a prior publication27 from our group, the opposite outcome was observed for these diclofenac salt forms. Specifically, 7 times the amount of diclofenac DEA (1.16%) permeated human skin compared to diclofenac sodium (5%) despite the higher concentration of drug in the latter. Importantly, in that study, diclofenac DEA was contained in an emulsion formulation while diclofenac sodium was in a gel. Together, these results support the idea that composition, dosage form, salt form, and other factors can influence the permeation of diclofenac in different ways, with some enhancing it and others diminishing it.
The biggest contributor to increased absorption in the ibuprofen formulations was increased drug concentration. However, this does not explain the absorption and flux differences between the formulations with the highest concentration of ibuprofen, Ibu-5 and Ibu-6 (both 10% ibuprofen gels). Other formulation differences such as the gelling polymer (hydroxyethylcellulose versus carbomer) or another excipient may be responsible. Among 5% ibuprofen formulations, gel formulations (Ibu-3 and Ibu-4) had lower CA24h than creams (Ibu-1 and Ibu-2), in agreement with previous findings.29,30 This suggests that dosage form is an important variable for permeability in ibuprofen formulations.
The goal of this study was to determine superior NSAID topical formulations based upon mITAA, which provides an estimate of the intrinsic anti-inflammatory effectiveness of NSAIDs that includes both biopharmaceutic (permeability, as measured by CA24h) and pharmacodynamic (COX-2 inhibition) components. The mITAA allows for comparisons between different NSAIDs and between formulations of the same NSAID. When NSAIDs are different, mITAA depends upon both potency against COX-2 and absorption; therefore, the formulation with the best combination of these variables is deemed to have the greatest anti-inflammatory activity. In cases where IC50 values are widely different, as is true for diclofenac and ibuprofen, potency will be the primary driver of anti-inflammatory activity (unless the higher-potency drug absorbs very poorly or not at all and the lower-potency drug absorbs very well, making absorption critical). When IC50 values are similar between different NSAIDs, absorption will have a greater influence on anti-inflammatory activity. Similarly, when the NSAIDs are the same between formulations, meaning the potency will be the same, any difference in anti-inflammatory activity depends entirely on absorption; thus, the formulation with greater absorption will have greater anti-inflammatory activity, as reflected by a higher mITAA.
While higher CA24h corresponded with higher mITAA in formulations involving the same drug, this was not the case when comparing between drugs. All diclofenac formulations demonstrated greater mITAA than ibuprofen formulations despite lower absorption, which directly reflects diclofenac’s substantially greater potency as a COX-2 inhibitor (supported by multiple studies13,22,32). Because the IC50 of diclofenac is approximately 900 times lower than that of ibuprofen, cumulative absorption of ibuprofen-containing formulations would need to be 700 times greater than that of diclofenac products to compensate for this difference and achieve comparable anti-inflammatory activity. According to Fick’s law, for ibuprofen products containing 5% or 10% drug compared to 1% or 2% drug in diclofenac products, a 150- to 300-fold higher flux would be required to achieve the same anti-inflammatory activity as diclofenac products.
Conclusion
Diclofenac demonstrates greater intrinsic anti-inflammatory activity than ibuprofen. Additionally, skin permeation can be impacted by formulation differences including the drug concentration and salt form, choice of excipients, and dosage form. Each of these factors should be considered when selecting a topical NSAID for treating patient pain and inflammation. When comparing formulations containing the same NSAID, the degree of absorption will dictate which formulation exhibits superior anti-inflammatory activity. When NSAIDs are different, this determination will depend upon the balance of absorption and COX-2 inhibition potency, with substantially greater absorption required to compensate when potencies are widely different, as is the case for diclofenac and ibuprofen. Thus, both absorption and COX-2 inhibition potency are important factors for anti-inflammatory activity for topically applied products, but the level of importance of each depends upon what products are being compared.
Acknowledgments
The author gratefully acknowledges Charles River Laboratories (Edinburgh, UK), which performed the in vitro study. The author wishes to acknowledge and thank Coralie Vallet, Maria-Stella Lombardi, Mako Araga and Guillaume Frappin for their contribution to this study. The author acknowledges Clotilde Cheignon for her review of the manuscript. Editorial assistance was provided by Nuventra, US, and funded by GlaxoSmithKline Consumer Healthcare, Nyon, Switzerland.
Disclosure
The author is an employee of GlaxoSmithKline Consumer Healthcare and reports no other conflicts of interest for this work.
References
1. Nair B, Taylor-Gjevre RA. Review of topical diclofenac use in musculoskeletal disease. Pharmaceuticals (Basel). 2010;3(6):1892–1908. doi:10.3390/ph3061892
2. Gunaydin C, Bilge SS. Effects of nonsteroidal anti-inflammatory drugs at the molecular level. Eurasian J Med. 2018;50(2):116–121. doi:10.5152/eurasianjmed.2018.0010
3. Van Hecken A, Schwartz JI, Depre M, et al. Comparative inhibitory activity of rofecoxib, meloxicam, diclofenac, ibuprofen, and naproxen on COX-2 versus COX-1 in healthy volunteers. J Clin Pharmacol. 2000;40(10):1109–1120.
4. Derry S, Conaghan P, Da Silva JA, Wiffen PJ, Moore RA. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2016;4:Cd007400.
5. Zacher J, Altman R, Bellamy N, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin. 2008;24(4):925–950. doi:10.1185/030079908X273066
6. McGettigan P, Henry D, Strom BL. Cardiovascular risk with non-steroidal anti-inflammatory drugs: systematic review of population-based controlled observational studies. PLoS Med. 2011;8(9):e1001098. doi:10.1371/journal.pmed.1001098
7. Salvo F, Fourrier-Reglat A, Bazin F, et al. Cardiovascular and gastrointestinal safety of NSAIDs: a systematic review of meta-analyses of randomized clinical trials. Clin Pharmacol Ther. 2011;89(6):855–866. doi:10.1038/clpt.2011.45
8. Brater DC. Anti-inflammatory agents and renal function. Semin Arthritis Rheum. 2002;32(3 Suppl 1):33–42. doi:10.1053/sarh.2002.37216
9. Altman R, Bosch B, Brune K, Patrignani P, Young C. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs. 2015;75(8):859–877.
10. Evans JM, McMahon AD, McGilchrist MM, et al. Topical non-steroidal anti-inflammatory drugs and admission to hospital for upper gastrointestinal bleeding and perforation: a record linkage case-control study. BMJ. 1995;311(6996):22–26. doi:10.1136/bmj.311.6996.22
11. Tiso RL, Tong-Ngork S, Fredlund KL. Oral versus topical Ibuprofen for chronic knee pain: a prospective randomized pilot study. Pain Physician. 2010;13(5):457–467.
12. Cordero JA, Alarcon L, Escribano E, Obach R, Domenech J. A comparative study of the transdermal penetration of a series of nonsteroidal antiinflammatory drugs. J Pharm Sci. 1997;86(4):503–508. doi:10.1021/js950346l
13. Cordero JA, Camacho M, Obach R, Domenech J, Vila L. In vitro based index of topical anti-inflammatory activity to compare a series of NSAIDs. Eur J Pharm Biopharm. 2001;51(2):135–142. doi:10.1016/S0939-6411(00)00149-1
14. Goh CF, Lane ME. Formulation of diclofenac for dermal delivery. Int J Pharm. 2014;473(1–2):607–616. doi:10.1016/j.ijpharm.2014.07.052
15. Heyneman CA, Lawless-Liday C, Wall GC. Oral versus topical NSAIDs in rheumatic diseases: a comparison. Drugs. 2000;60(3):555–574. doi:10.2165/00003495-200060030-00004
16. Hadgraft J, Whitefield M, Rosher PH. Skin penetration of topical formulations of ibuprofen 5%: an in vitro comparative study. Skin Pharmacol Appl Skin Physiol. 2003;16(3):137–142. doi:10.1159/000069759
17. Hagen M, Baker M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr Med Res Opin. 2017;33(9):1623–1634. doi:10.1080/03007995.2017.1352497
18. OECD. Guidance Notes on Dermal Absorption. 2019.
19. OECD. OECD Guidelines for the Testing of Chemicals: Skin Absorption: In vitro Method. 2004.
20. Williams AC, Cornwell PA, Barry BW. On the non-gaussian distribution of human skin permeabilities. Int J Pharm. 1992;86(1):69–77. doi:10.1016/0378-5173(92)90032-W
21. Ema CHMP. Draft Guideline on Quality and Equivalence of Topical Products. 2018.
22. Esser R, Berry C, Du Z, et al. Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol. 2005;144(4):538–550. doi:10.1038/sj.bjp.0706078
23. Fini A, Laus M, Orienti I, Zecchi V. Dissolution and partition thermodynamic functions of some nonsteroidal anti-inflammatory drugs. J Pharm Sci. 1986;75(1):23–25. doi:10.1002/jps.2600750106
24. Brown MB, Martin GP, Jones SA, Akomeah FK. Dermal and transdermal drug delivery systems: current and future prospects. Drug Deliv. 2006;13(3):175–187. doi:10.1080/10717540500455975
25. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359–370. doi:10.1111/j.1467-2494.2006.00344.x
26. Sengupta C. Diclofenac sodium. In: Anti-Inflammatory and Anti-Rheumatic Drugs, Volume II: Newer Anti-Inflammatory Drugs. Boca Raton, FL: CRC Press; 1985:49–63.
27. Pradal J, Vallet CM, Frappin G, Bariguian F, Lombardi MS. Importance of the formulation in the skin delivery of topical diclofenac: not all topical diclofenac formulations are the same. J Pain Res. 2019;12:1149–1154. doi:10.2147/JPR.S191300
28. O’Connor KM, Corrigan OI. Comparison of the physicochemical properties of the N-(2-hydroxyethyl) pyrrolidine, diethylamine and sodium salt forms of diclofenac. Int J Pharm. 2001;222(2):281–293. doi:10.1016/S0378-5173(01)00717-7
29. Khullar R, Kumar D, Seth N, Saini S. Formulation and evaluation of mefenamic acid emulgel for topical delivery. Saudi Pharm J. 2012;20(1):63–67. doi:10.1016/j.jsps.2011.08.001
30. Stahl J, Wohlert M, Kietzmann M. The effect of formulation vehicles on the in vitro percutaneous permeation of ibuprofen. BMC Pharmacol. 2011;11(1):12. doi:10.1186/1471-2210-11-12
31. Kim MJ, Doh HJ, Choi MK, et al. Skin permeation enhancement of diclofenac by fatty acids. Drug Deliv. 2008;15(6):373–379. doi:10.1080/10717540802006898
32. Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. Curr Med Res Opin. 2010;26(7):1715–1731. doi:10.1185/03007995.2010.486301
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.