Back to Journals » Clinical Ophthalmology » Volume 11
Comparison of retina specialist preferences regarding spectral-domain and swept-source optical coherence tomography angiography
Authors Su GL, Baughman DM, Zhang Q, Rezaei K, Lee AY, Lee CS
Received 23 February 2017
Accepted for publication 13 April 2017
Published 15 May 2017 Volume 2017:11 Pages 889—895
DOI https://doi.org/10.2147/OPTH.S135479
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Grace L Su,1 Douglas M Baughman,2 Qinqin Zhang,3 Kasra Rezaei,2 Aaron Y Lee,2 Cecilia S Lee2
1Lewis Katz School of Medicine, Temple University, Philadelphia, PA, 2Department of Ophthalmology, 3Department of Bioengineering, University of Washington, Seattle, WA, USA
Purpose: The aim of this study was to compare physician preferences regarding the commercially available spectral-domain (SD) optical coherence tomography angiography (OCTA) and swept-source (SS) OCTA prototype device.
Design: Comparative analysis of diagnostic instruments was performed.
Patients and methods: Subjects at the University of Washington Eye Institute and Harborview Medical Center were prospectively recruited and imaged with the Zeiss SD OCTA (HD-5000, Angioplex) and Zeiss SS OCTA (Plex Elite, Everest) devices on the same day. The study included 10 eyes from 10 subjects diagnosed with a retinal/choroidal disease. Deidentified images were compiled into a survey and sent to retina specialists in various countries. The survey presented masked SD and SS images of each eye for each retinal sublayer side by side. Respondents were asked about their image preference and impact on clinical management. A priori and post hoc preferences for SD vs SS were collected.
Results: Fifty-four retina specialists responded to the survey. Median years in practice was 3.00 (interquartile range [IQR] 1.50–17.00). At baseline, 23 (48%) physicians owned an OCTA machine. The majority of physician responses showed a preference for the SS over SD OCTA, independent of the retinal pathology shown (n=454 overall responses, 74%). Nevertheless, the majority indicated that both SD and SS would be equally valuable in informing clinical decisions (n=374 overall responses, 61%).
Conclusion: These findings indicate that the majority of retina specialists surveyed prefer SS over SD OCTA based on image quality, regardless of the retinal pathology shown. Regarding the clinical utility of each modality, the majority of physicians perceive SD and SS as equally effective.
Keywords: swept-source optical coherence tomography angiography, spectral-domain optical coherence tomography angiography, physician preference
Background
Healthy retinal and choroidal vasculatures are essential for the normal functioning of the eye.1 Abnormal growth of blood vessels as seen in choroidal neovascularization (CNV) can result from a variety of ophthalmologic diseases, such as neovascular age-related macular degeneration (nAMD), high myopia, central serous chorioretinopathy (CSCR), and multifocal choroiditis.2 Quality retinal imaging techniques are critical in early detection and informing treatment decisions of these pathologies.
Optical coherence tomography angiography (OCTA) is a noninvasive imaging technique that utilizes the decorrelation motion contrast between sequential optical coherence tomography (OCT) B-scans to visualize retinal and choroidal blood flow at a fixed point without the usage of contrasting agent.3 OCTA provides both structural (OCT) and functional (angiography) information, showing exact delineation and size measurements of flow, which allows for both to be evaluated in tandem. This novel technology generates volumetric angiography images within seconds and has the potential for detecting abnormalities in blood flow, giving it utility in identifying diseases such as age-related macular degeneration (AMD) and diabetic retinopathy.2
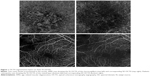
Currently available OCTA systems utilize the spectral-domain (SD) OCT software and operate at ~840 nm wavelength. However, visualization beneath the retinal pigment epithelium (RPE) is partially obscured due to the backscattering of light from the RPE–Bruch’s membrane complex.4,5 A newer OCTA prototype utilizes the faster swept-source (SS) OCT device that operates at a longer wavelength of ~1,050 nm, enabling enhanced light penetration into the deeper tissue. Other features of the SS OCT include a lower sensitivity roll-off, reduced fringe washout, and an ability to perform dual balanced detection,6 resulting in improved clarity and better visualization of the choroid on cross-sectional and en face imaging.7
With growing evidence of the involvement of the choroid in retinal pathogenesis and the rise of OCTA as an imaging modality, it is important to evaluate the clinical utility of the SS and SD OCTA technologies. The purpose of our study is to compare SD (Zeiss US commercial) and SS (Zeiss research prototype) OCTA with the goal of assessing retina physicians’ machine preferences and their effect on quality clinical management.
Patients and methods
This study was conducted at the University of Washington with surveys sent to outside retina specialists. The research adhered to the tenets of the Declaration of Helsinki and the Health Insurance Portability and Accountability Act. This study was approved by the University of Washington Institutional Review Board, and written informed consent was obtained prior to OCTA imaging.
Subject selection
Subjects seen by the retina service between June and September 2016 were recruited. Subjects were eligible if they had been previously diagnosed with a retinal pathology by a retina specialist, which was confirmed via a comprehensive chart review. Those with non-vascular retinal diseases such as retinal tears and lattice degeneration were excluded. The retinal/choroidal pathologies were categorized a priori as 1) retinal disease, which included diabetic retinopathy and branch retinal vein occlusion (RVO), 2) nAMD, and 3) RPE disease, which included CSCR, punctate inner choroidopathy, and multifocal choroiditis.
Imaging acquisition
We used a commercialized Zeiss SD OCTA (HD-5000, Angioplex) that operates at a central wavelength of 840 nm and an A-line speed of 68 kHz. The bandwidth of the light source is 45 nm, contributing to an axial resolution of ~5 μm in the tissue and an estimated lateral resolution of ~15 μm at the retinal surface. We compared this with the Zeiss SS OCTA imaging system (Plex Elite, Everest), which utilizes a longer wavelength of 1,050 nm (1,000–1,100 nm full bandwidth) and operates at a faster speed of 100,000 A-lines per second. The axial and lateral resolutions are ~5 μm in the tissue and ~14 μm at the retinal surface.
All patients were imaged on the SD OCTA and SS OCTA within minutes of each other. All images were obtained as close to the fovea as possible. Since the 2 machines differ in the number of scan lines for a given retinal area, the SD and SS scans were taken over a 6×6 mm and 9×9 mm area, respectively, to assimilate the resolution in both machines. The subjects’ fixation was controlled using the internal fixation target.
Imaging analysis
Ten eyes with high-quality signal strength and a diverse variety of retinal pathologies were selected. The sublayers of the retina as segmented by the manufacturer’s default settings were shown, including the choriocapillaris, choroid, retina, superficial, deep, and avascular layers. The images were cropped and aligned to look visually equivalent but were otherwise unaltered. These scans were compiled into an online survey and sent to retina specialists in various countries, who were subsequently asked to send it to all of their colleagues. Surveys were initially sent to all retinal specialists for whom the authors could obtain an email address. Before completing the survey, retina specialists were informed via email of all pertinent information relative to study participation. Voluntary completion of the survey was considered consent to participate in all aspects of the study. The survey was designed as a web page opened within the users’ web browser and submitted online. The machine from which the image was generated was not revealed to the respondents throughout the study. Each eye was presented as a case preceded by a clinical vignette and shown side by side with its corresponding layer (Figure 1). Each sublayer could be viewed by scrolling or clicking “j” or “k” on the computer keyboard. The order of the SD vs SS images for each case and the laterality of the images were randomized. Only one survey result per IP address was included for the analysis to prevent repeated submissions.
At the beginning of the survey, we collected demographic data including physicians’ year of practice, type of practice, and practice location. We also asked about their OCTA purchase plan, OCTA preference, and opinions on clinical utility (Figure S1). Following each case, respondents were asked to select the image of higher quality in their assessment and which machine it came from. They were subsequently asked questions regarding clinical management preference (Figure S2).
Results
Subject demographics
Of the 15 image subjects selected, 13 completed the study, of which 10 subject eyes were selected. The remaining images were not used due to poorer imaging quality. One declined to participate due to language barriers, and another subject did not complete the study due to physical discomfort during imaging. The mean age of the study population was 59 (range =24–84) years, and 5 (50%) were male. Two subjects (20%) had retinal diseases, 4 subjects (40%) had AMD, and 4 subjects (40%) had RPE disease.
Survey responses
Surveys were emailed to retina specialists at multiple centers in various countries, including the USA, Europe, and Australia. Of the 54 retina specialists who filled out the survey, 32 (67%) practiced in academic medical centers, 16 (33%) in private practice, and 6 declined to answer. The physicians’ median years in practice was 3.00 (interquartile range [IQR] 1.50–17.00). In response to the OCTA purchase plan question, 23 (48%) already owned one, 8 (17%) planned on obtaining one within a year, and 17 (35%) were uncertain. In response to the question on the effect of OCTA on clinical management, the answers varied from definitely needing an OCTA (n=3, 6%), to sometimes (n=17, 35%), rarely (n=13, 27%), and maybe in the future (n=15, 31%). Prior to starting the survey, 23 (53%) retina specialists preferred an SS OCTA over an SD OCTA device if it were more affordable (Table 1).
Based on the 617 overall responses, retina specialists were significantly (n=454 overall responses, 74%) more likely to prefer SS OCTA over SD OCTA in terms of image quality (Table 2). However, this preference did not vary between retinal pathologies (retinal disease vs AMD vs RPE disease), and SS OCTA was predominantly preferred.
Regarding clinical management, the majority (n=374, 61%) reported that both SD and SS would be equally valuable in making clinical decisions. This was seen consistently across all pathologies. A greater number of responses (n=120, 20%) reported that only SS would be valuable, while a small minority (n=17, 3%) thought that only SD would be useful. A total of 104 (17%) overall responses reported that neither OCTAs would be useful in appropriate management (Table 2).
Prior to completing the survey, 12 of 43 (28%) respondents indicated that they preferred the SD OCTA, 8 (19%) preferred the SS, and 23 (53%) preferred the SS if it were more affordable as their choice of imaging (Table 3). At the conclusion of the survey, 11 of 45 (24%) respondents selected the SD OCTA, 13 (29%) chose the SS, and 21 (47%) chose the SS if it were more affordable. When analyzing changes in response upon termination of the survey, 5 of the 23 (23%) respondents who originally preferred “SS OCTA if it were more affordable” changed their response to solely “SS OCTA” without knowing the correct answers to the survey. Of the 12 respondents who originally chose “SD OCTA,” 8 (67%) remained the same, 1 (8%) changed their answer to “SS OCTA”, and 3 (25%) to “SS OCTA if were more affordable”. With regard to the accuracy of matching the image to the machine, 262 of 430 times (61%) the cases were correctly guessed.
Discussion
This study analyzed retina specialists’ OCTA preferences when comparing SS to SD OCTA technologies. We surveyed 54 retina specialists to determine what, if any, preferences were apparent based on the 10 clinical cases given. With regard to image quality, the majority of retina specialists selected the SS over the SD OCTA. This preference did not vary between retinal pathologies. While our results did not reveal a significant partiality in either machine in terms of its effect on anticipated clinical management, we demonstrated that most clinicians preferred the SS OCTA as an imaging device.
Our analysis did not reveal OCTA in having a significant role in clinical management outside the currently available technologies, possibly due to its novelty and cost.
However, current literature highlights the potential of OCTA in becoming an important imaging modality in evaluating common ophthalmologic diseases, including diabetic retinopathy,8 AMD,9,10 and RVO.11
Previous studies contrasting SS to SD prototypes have focused primarily on comparing physical parameters in specific diseases, such as measuring the choroidal thickness and detecting pathology.12 In a recent study, Novais et al12 compared the SS and SD OCTA technologies in their abilities to measure the total CNV area. They concluded that while both machines were able to identify feeder vessels, the SS OCTA yielded significantly larger CNV areas than did the SD OCTA in both type I and type II CNV, classified as when the neovascularization is below and above RPE, respectively. However, previous studies have not evaluated retina physicians’ current preference patterns in OCTA machines, the primary goal of our study.
This is the first study to our knowledge that makes a comparison of preference between the SS and SD OCTA technologies. The study was blinded by keeping the machine prototypes anonymous to minimize viewer bias. In addition, we imaged subjects with various pathologies and surveyed retina specialists from a variety of practices across multiple countries. Retina specialists’ preference of SS to SD OCTA did not vary across retinal or choroidal pathologies. This suggests that even the retinal diseases that had been previously imaged via SD, such as diabetic retinopathy8 and RVO,11 may show up more clearly via SS imaging. However, the analysis revealed that the majority found both machines to produce an adequate quality of imaging necessary to inform clinical management. We might have expected changes in management for the choroidal diseases, such as CNV secondary to AMD, as diseases beneath the RPE may be more easily visualized with SS imaging. Although there was no significant difference seen in anticipated clinical management, the improved delineation of the vasculature via the SS OCTA has the potential to advance our understanding of disease pathogenesis and consequently improve treatment.
Prior to starting the survey, about one-third of respondents indicated that they preferred the SD OCTA as an imaging technique. At the conclusion of the survey, there was a shift from the SD to solely the SS OCTA device irrespective of cost. This suggests an enhanced partiality for the SS images after repeated exposure, even with the randomization and anonymity of the machines. While the results indicated that neither machine had advantages over the other in terms its clinical utility, our analysis indicates a clear inclination for the SS OCTA device in terms of quality.
Our study has several limitations. Our statistical power was diminished due to a small sample size. Furthermore, the patients represented only a subset of retinal pathologies. Future studies comparing SS OCTA with SD OCTA across additional diseases will better demonstrate the capabilities of the newer technology. This includes pathologies such as Vogt–Koyanagi–Harada13 and pathologic myopia,14 which increase and decrease the choroidal volume, respectively. Additionally, we selected images with the best signal strength; the remainder were not used in this study. With regard to the survey, response bias may have been present due to already having seen the patient’s diagnosis. Furthermore, those who owned an OCTA machine may have had an inherent preference compared to those who did not, which could have contributed additional bias. The median years in practice of survey respondents was also somewhat low, and our results may overrepresent the preferences of physicians in this demographic. Finally, we did not focus on other aspects of the imaging techniques, such as cost and machine handling, all of which influence a clinician’s preference in managing patients.
This study compares the clinical utility of SS OCTA to the SD OCTA system. Although the SS OCTA enables better visualization of the choroidoscleral interface, the majority of retina physicians did not feel that the SS OCTA offers additional advantages over SD OCTA in our study cases. However, SS OCTA was overall preferred over SD OCTA due to the higher quality of images. Therefore, SS OCTA may become useful for finding subclinical manifestations of various subretinal diseases in the future. As OCTA availability continues to expand, its clinical utility in detecting and visualizing a greater number of diseases will be better understood. Future studies that include a larger sample are warranted to further investigate the clinical applicability of the SD and SS OCTA technologies with the goal of improving disease management.
Acknowledgments
The authors would like to acknowledge Thellea Leveque MD MPH at the Department of Ophthalmology, University of Washington, for her contribution with OCT imaging of a patient in the study. The content of this manuscript has not been previously presented at a meeting. This work was supported by the National Eye Institute, Bethesda, MD [K23EY02492] (CSL), the Research to Prevent Blindness, Inc., New York, NY (CSL, AYL, KR), and Carl Zeiss Meditec, Inc. The funding source had no role in the design and conduct of the study’s collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author contributions
CSL had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: AYL, CSL. Acquisition, analysis, or interpretation of data: AYL, CSL, GLS, QZ, KR. Drafting of the manuscript: GLS. Critical revision of the manuscript for important intellectual content: AYL, CSL, KR, QZ, DMB. Statistical analysis: AYL, CSL, GSL. Obtaining funding: AYL, CSL, KR. Administrative, technical, or material support: AYL, KR, QZ. Study supervision: AYL, CSL. All authors contributed toward data analysis, drafting and critically revising the paper, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Huang Y, Zhang Q, Thorell MR, et al. Swept-source OCT angiography of the retinal vasculature using intensity differentiation-based optical microangiography algorithms. Ophthalmic Surg Lasers Imaging Retina. 2014;45(5):382–389. | ||
de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous. 2015;1(1):5. | ||
An L, Wang RK. In vivo volumetric imaging of vascular perfusion within human retina and choroids with optical micro-angiography. Opt Express. 2008;16(15):11438–11452. | ||
Saito M, Iida T, Nagayama D. Cross-sectional and en face optical coherence tomographic features of polypoidal choroidal vasculopathy. Retina. 2008;28(3):459–464. | ||
Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150(3):325–329. | ||
Drexler W, Liu M, Kumar A, Kamali T, Unterhuber A, Leitgeb RA. Optical coherence tomography today: speed, contrast, and multimodality. J Biomed Opt. 2014;19(7):071412. | ||
Adhi M, Liu JJ, Qavi AH, et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. Am J Ophthalmol. 2014;157(6):1272–1281. | ||
Ishibazawa A, Nagaoka T, Takahashi A, et al. Optical coherence tomography angiography in diabetic retinopathy: a prospective pilot study. Am J Ophthalmol. 2015;160(1):35–44. | ||
Palejwala NV, Jia Y, Gao SS, et al. Detection of nonexudative choroidal neovascularization in age-related macular degeneration with optical coherence tomography angiography. Retina. 2015;35(11):2204–2211. | ||
El Ameen A, Cohen SY, Semoun O, et al. Type 2 neovascularization secondary to age-related macular degeneration imaged by optical coherence tomography angiography. Retina. 2015;35(11):2212–2218. | ||
Kashani AH, Lee SY, Moshfeghi A, Durbin MK, Puliafito CA. Optical coherence tomography angiography of retinal venous occlusion. Retina. 2015;35(11):2323–2331. | ||
Novais EA, Adhi M, Moult EM, et al. Choroidal neovascularization analyzed on ultrahigh-speed swept-source optical coherence tomography angiography compared to spectral-domain optical coherence tomography angiography. Am J Ophthalmol. 2016;164:80–88. | ||
Rajendram R, Evans M, Rao NA. Vogt-Koyanagi-Harada disease. Int Ophthalmol Clin. 2005;45(2):115–134. | ||
Quaranta M, Arnold J, Coscas G, et al. Indocyanine green angiographic features of pathologic myopia. Am J Ophthalmol. 1996;122(5):663–671. |
Supplementary materials
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.