Back to Journals » Cancer Management and Research » Volume 9
Comparison of gefitinib as first- and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 or 19 del epidermal growth factor receptor mutation
Authors Patel N , Wu P, Zhang H
Received 2 April 2017
Accepted for publication 6 June 2017
Published 28 June 2017 Volume 2017:9 Pages 243—248
DOI https://doi.org/10.2147/CMAR.S138643
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Alexandra R. Fernandes
Nishant Patel,1 Pingping Wu,2 Haijun Zhang1
1Department of Oncology, Zhongda Hospital, Medical School, Southeast University, 2Department of Medical Oncology, Jiangsu Cancer Hospital, Nanjing, People’s Republic of China
Objectives: Gefitinib, a tyrosine kinase inhibitor (TKI) targeting epidermal growth factor receptor (EGFR), shows excellent clinical benefit in treating advanced non-small-cell lung cancer (NSCLC). The aim of this study was to compare the efficacy and toxicity of gefitinib as first-line therapy and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 (L858R) or exon 19 deletion of EGFR mutation.
Methods: We retrospectively analyzed the clinical data of 60 EGFR-mutated advanced lung adenocarcinoma patients from July 2011 to November 2015 who have received oral gefitinib 250 mg once daily. Gefitinib was taken until disease progression, intolerable toxicity or death.
Results: After a median follow-up of 792 days, one death had occurred. Among the 59 patients who survived, 17 patients progressed. Overall, the median progression-free survival (mPFS) was 10 months (95% confidence interval [CI]: 7.53–12.46 months, p<0.05). The response rate (RR) and disease control rate (DCR) were 33.33% and 71.66%, respectively. However, there was longer mPFS in the first line-therapy than that in the second-line therapy: in the first-line gefitinib therapy, mPFS was 12 months among 41 patients (95% CI: 9.58–14.41 months, p<0.05), and in the second-line therapy, mPFS was 7 months among 19 patients (95% CI: 1.31–12.68 months, p<0.05). Furthermore, in subgroup analyses examining different EGFR mutation types, we noted that mPFS was significantly longer for patients with exon 19 deletion than for those with positive exon 21in both the first-line therapy and second-line therapy.
Conclusion: Patients with advance lung adenocarcinoma who were selected by positive exon 21 or 19 deletion mutations had significantly longer mPFS in the first-line therapy than that in the second-line therapy when treated with gefitinib. EGFR mutation types may influence the response to gefitinib therapy.
Keywords: gefitinib, epidermal growth factor receptor, tyrosine kinase inhibitor, lung adenocarcinoma
Introduction
Lung cancer is the most common and fatal cancer worldwide. In the People’s Republic of China, there were 733,300 new cases of lung cancer as estimated in 2015, including 509,300 men and 224,000 women, which accounted for 17.09% of all new cancer cases.1 Lung cancer was also the leading cause of cancer death, contributing to 21.68% deaths among all cancers.2 The two main types of lung cancer are small-cell lung carcinoma (SCLC) and non-small-cell lung carcinoma (NSCLC). NSCLC is further divided into three subtypes: squamous-cell carcinoma, adenocarcinoma and large-cell carcinoma. Approximately 40% of lung cancers are adenocarcinoma, which is often diagnosed at an advanced stage with poor prognosis.3
Over the past years, enormous progress has been made in the treatment of lung cancer. In particular, the outgrowth of new chemotherapy agents, targeted therapeutics and immunotherapy drugs has remarkably improved the quality of life and prolonged the survival of patients with advanced NSCLC. In lung adenocarcinomas, the breakthrough of mutations in epidermal growth factor receptor (EGFR) was 15–20% in the generation of molecular and personalized therapeutics.4 The mutant EGFR (EGFRm+) in adenocarcinoma lung cancer is ~10% in the US and 30–50% in Asia.5 Instead of “one-size-fits-all” chemotherapy method, small-molecule EGFR tyrosine kinase inhibitors (TKIs) have been used as one personalized therapy, which is based on molecular characteristics of tumors. It is true that patients with EGFRm+ could benefit from the treatment with the first-generation EGFR TKIs of gefitinib and erlotinib and the second-generation EGFR TKIs of afatinib.6–9
However, it is arguable that the benefit from EGFR TKI is either equal to or inferior between the first-line therapy and second-line therapy and between positive exon 21 and 19 deletion mutations,10 and gefitinib is no exception. As far as gefitinib is considered, to date, there are no direct relative data. Thus, we chose related clinical cases to do this retrospective analysis to explore the potential differences of gefitinib as the first-line therapy and second-line therapy for advanced lung adenocarcinoma patients with positive exon 21 (L858R) or exon 19 deletion of EGFR mutation.
Methods
Patients
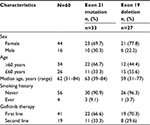
In the present retrospective analysis, we included 60 EGFRm+ advanced lung adenocarcinoma patients of Jiangsu Cancer Hospital, Nanjing, People’s Republic of China, from July 2011 to November 2015. Molecular pathological diagnosis confirmed that 60 patients had adenocarcinoma with positive EGFR mutation of exon 21 (n=33) or exon 19 deletion (n=27). The study was approved by the ethics committee of Jiangsu Cancer Hospital, and written informed consent was obtained from all the participants.
Treatment
Patients received oral gefitinib 250 mg once daily until disease progression, unacceptable toxicity or death. In the second-line therapy, gefitinib therapy was given to patients who had documented disease progression while receiving pemetrexed–platinum-based chemotherapy (four to six cycles). Further therapy after progression of the disease was at the physician’s discretion.
Assessments
Tumor response was assessed as complete response (CR), partial response (PR), stable disease (SD) and progressive disease (PD) based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.0 with computed tomography (CT) or magnetic resonance imaging (MRI). Progression-free survival (PFS) was defined as the time between first medication of gefitinib and first documented PD. Safety assessments were performed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.0.
Statistical analysis
Categorical variables were analyzed based on percentage, and continuous variables were expressed as median values. PFS was estimated using the Kaplan–Meier survival method and log-rank test for significance testing. The hazard ratio (HR) for risk factors was analyzed by univariate Cox regression analysis, and p<0.05 was considered to be statistically significant. All statistical analyses were performed by SPSS 21.0 version software (IBM Corporation, Armonk, NY, USA).
Results
Characteristics of patients
As shown in Table 1, there were 60 patients included in this study, 44 female patients and 16 male patients with a median age of 62 years (range, 31–84 years). Among them, 33 patients harbored positive exon 21 (L858R) mutation and 27 ones 19 deletion. All patients were treated with either the first-line gefitinib therapy (n=41) or second-line gefitinib therapy (n=19). Among all patients, a smoking history of never-smoker and ever-smoker was seen in 56 patients and four patients, respectively.
  | Table 1 Characteristics of patients |
Overall efficacy
After a median follow-up of 792 days (range, 406–1176 days), one death had occurred by the cutoff date (November 24, 2015), and 59 patients survived among 60 patients with advanced lung adenocarcinoma. Among them, 17 patients progressed, whereas 42 patients did not. Overall, the median progression-free survival (mPFS) was 10 months (95% confidence interval [CI]: 7.53–12.46 months, p=0.000004). The HR was 0.673 (95% CI: 0.514–0.883, p=0.004). The response rate (RR) and disease control rate (DCR) were 33.33% and 71.66%, respectively.
Comparison of gefitinib as the first-line therapy or second-line therapy
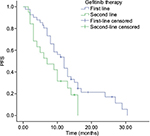
As shown in Figure 1, in the first-line gefitinib therapy, the mPFS was 12 months among 41 patients (95% CI: 9.58–14.41 months, p=0.002), whereas in the second-line gefitinib therapy, the mPFS was 7 months among 19 patients (95% CI: 1.31–12.68 months, p=0.002). The RR and DCR were 36.58%, 82.92% and 26.31%, 47.36% in the first-line therapy and second-line therapy, respectively. The HRs were 0.331 (95% CI: 0.157–0.698, p=0.004) and 0.134 (95% CI: 0.029–0.622, p=0.010) in the first-line therapy and second-line therapy, respectively. Obviously, there was a significantly longer mPFS in the first-line therapy than that in the second-line therapy of gefitinib for lung adenocarcinoma patients with EGFRm+ (p<0.05).
Comparison of gefitinib between positive exon 21 and 19 deletion mutation in the first-line therapy
As far as gefitinib is considered in the first-line gefitinib therapy, for positive exon 21, mPFS was 8 months among 22 patients (95% CI: 5.7–10.29 months) and for exon 19 deletion, mPFS was 16 months among 19 patients (95% CI: 13.32–18.67 months), which is shown in Figure 2. That is to say, lung adenocarcinoma patients with exon 19 deletion could achieve longer mPFS than patients with positive exon 21 in the first-line gefitinib therapy (p<0.05).
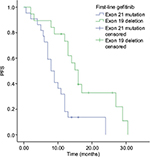  | Figure 2 Comparison of gefitinib between positive exon 21 and 19 deletion mutations in the first-line therapy. Abbreviation: PFS, progression-free survival. |
Comparison of gefitinib between positive exon 21 and 19 deletion mutation in the second-line therapy
When comparing the efficacy in the second-line therapy for patients with EGFRm+, as shown in Figure 3, for positive exon 21, mPFS was 4 months among 11 patients (95% CI: 2.05–5.94 months), while for exon 19 deletion mutation, mPFS was 14 months among eight patients (95% CI: 11.5–16.46 months, p<0.05).
  | Figure 3 Comparison of gefitinib between positive exon 21 and 19 deletion mutations in the second-line therapy. Abbreviation: PFS, progression-free survival. |
Safety
All patients were well tolerant to either the first-line gefitinib therapy or second-line gefitinib therapy between exon 21 and 19 deletion mutation with fewer Grade 3 or 4 adverse events, such as abnormal liver function (18.3%) and rash (3.33%). In the first-line gefitinib therapy, the most common Grade 1 or 2 adverse events in 21 mutation included rash (77.2%), abnormal liver function (61.3%), dry skin (54.5%), diarrhea (50%), fatigue (40.9%) and paronychia (27.2%) in non-hematological toxicity, whereas anemia (31.8%) and leukocytopenia (18.1%) were included in hematological toxicity as mentioned in Table 2. However, in 19 deletion, Grade 1 or 2 adverse events were observed to have similar effects with gradually less toxic levels than in exon 21 mutation (p<0.00048). However, in the second-line therapy, the most common Grade 1 or 2 adverse events in 21 mutation were more virulent compared to second-line 19 deletion (p<0.0024) or first-line 21 mutation (p<0.0015). Similarly, second-line 19 deletion adverse events were more harmful than the first-line 19 deletion adverse events because a higher percentage of adverse events occurred as per Table 2 (p<0.0044).
  | Table 2 Adverse events occurring in >10% of the gefitinib therapy Abbreviations: G1–2, grade 1–2; G≥3, grade ≥3; AST, aspartate aminotransferase; ALT, alanine aminotransferase. |
Risk factors
Whether sex, age, smoking history and EGFR mutation status were the risk factors for PFS were investigated using univariate Cox regression analysis. However, as shown in Table 3, consisting only of patients with positive exon 21 or exon 19 deletions of EGFR was the predictive factor for PFS of gefitinib (p<0.05)
Discussion
The Iressa Pan-Asia Study (IPASS) is the first study that demonstrated the benefit of first-line EGFR TKIs over platinum-based combination chemotherapy in patients with EGFR mutation.11 Five other Phase III studies (First-SIGNAL, NEJSG002, WJTOG 3405, OPTIMAL and EURTAC) comparing reversible EGFR TKIs with chemotherapy have also demonstrated the same findings.12–17 Some studies documented the effectiveness of EGFR TKIs in the second-line therapy too.18–20 Overall, in our study, the mPFS was significantly 10 months, which was consistent with the abovementioned previous studies. However, mPFS was not similar between the first-line gefitinib therapy and second-line therapy. There was longer mPFS in the first-line therapy (12 months) than that in the second-line therapy (7 months) of gefitinib for lung adenocarcinoma (p<0.05). Obviously, mPFS in the second-line gefitinib therapy was significantly inferior to that in the first-line therapy. That is to say, patients with EGFRm+ could achieve better benefit when treated with gefitinib sooner than later.
What is more, preclinical studies have shown that positive exon 21 or exon 19 deletion, the most sensitizing mutation types, has distinct biological properties that might affect response to different EGFR TKIs.21,22 EGFR exon 19 deletion lung adenocarcinoma might be distinct from positive exon 21 one, and these subgroups should be analyzed separately.23 In subgroup analyses examining different EGFR mutations of the present study, we noted that mPFS was significantly longer for patients with exon 19 deletion than that with positive exon 21 both in the first-line gefitinib therapy and in the second-line therapy. Consistent with the findings of afatinib by Yang et al,23 the subgroup analyses suggested that the mPFS benefit of gefitinib could be driven mainly by patients harboring exon 19 deletion. The cause of this difference in response to EGFR TKIs by EGFR mutation subtype is not known. In this context, patients with lung adenocarcinoma harboring positive exon 21 or exon 19 deletion should be stratified and analyzed separately in the future study.
The median age was 63 years for the patients included in this study, and never-smoker consisted of 93.3% of these patients. The major adverse events included rash, abnormal liver functions, dry skin, diarrhea, fatigue and anemia. Most of the adverse events were in the range of Grade 1 and 2. Therefore, the first-line gefitinib therapy in either 19 deletion or 21 mutation was more effective and tolerant for elderly patients compared to the second-line gefitinib therapy.
This finding suggests that for patients with advance lung adenocarcinoma who were selected by positive exon 21 or 19 deletion mutations, gefitinib could the preferred option, and EGFR mutation types may influence the response to gefitinib therapy.
Conclusion
Advance lung adenocarcinoma patients with EGFRm+ could have significantly longer mPFS when they were treated with gefitinib in the first-line therapy than that in the second-line therapy. EGFR mutation types may influence the response to gefitinib therapy, which was the predictive factor for PFS of gefitinib.
Acknowledgments
This paper was presented at the 2017 ASCO Annual Meeting held at Chicago, USA, on June 2–6, 2017, as a publication-only abstract with interim findings. The abstract was published in the Journal of Clinical Oncology in 2017 volume 35 supplement.
Disclosure
The authors report no conflicts of interest in this work.
References
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. | ||
Barlesi F, Gervais R, Lena H, et al. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: a multicenter phase II trial (GFPC 07-01). Ann Oncol. 2011;22(11):2466–2470. | ||
Siegelin D, Borczuk C. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94:129–137. | ||
Sequist LV, Bell DW, Lynch TJ, Haber DA. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J Clin Oncol. 2007;25(5):587–595. | ||
Zhang H. Three generations of epidermal growth factor receptor tyrosine kinase inhibitors developed to revolutionize the therapy of lung cancer. Drug Des Devel Ther. 2016;10:3867–3872. | ||
Zhang Q, Wang Z, Guo J, et al. Comparison of single-agent chemotherapy and targeted therapy to first-line treatment in patients aged 80 years and older with advanced non-small-cell lung cancer. Onco Targets Ther. 2015;8:893–898. | ||
Tiseo M, Bartolotti M, Gelsomino F, Bordi P. Emerging role of gefitinib in the treatment of non-small-cell lung cancer (NSCLC). Drug Des Devel Ther. 2010;4:81–98. | ||
Yang JC, Shih JY, Su WC, et al. Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol. 2012;13(5):539–548. | ||
Mok T, Yang JJ, Lam KC. Treating patients with EGFR-sensitizing mutations: first line or second line – is there a difference? J Clin Oncol. 2013;31(8):1081–1088. | ||
Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. | ||
Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. | ||
Maemondo M, Inoue A, Kobayashi K, et al; North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. | ||
Mitsudomi T, Morita S, Yatabe Y, et al; West Japan Oncology Group. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. | ||
Mitsudomi T, Morita S, Yatabe Y, et al. Updated overall survival results of WJTOG 3405, a randomized phase III trial comparing gefitinib (G) with cisplatin plus docetaxel (CD) as the first-line treatment for patients with non-small cell lung cancer harboring mutations of the epidermal growth factor receptor (EGFR). J Clin Oncol. 2012;30(15 suppl):7521. | ||
Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. | ||
Rosell R, Carcereny E, Gervais R, et al; Spanish Lung Cancer Group in collaboration with Groupe Français de Pneumo-Cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. | ||
Puijenbroek R, Bosquée L, Meert AP, et al. Gefitinib monotherapy in advanced nonsmall cell lung cancer: a large Western community implementation study. Eur Respir J. 2007;29(1):128–133. | ||
Popat S, Barbachano Y, Ashley S, Norton A, O’Brien M. Erlotinib, docetaxel, and gefitinib in sequential cohorts with relapsed non-small cell lung cancer. Lung Cancer. 2008;59(2):227–231. | ||
Ng R, Loreto M, Lee R, Leighl NB. Brief report: retrospective review of efficacy of erlotinib or gefitinib compared to docetaxel as subsequent line therapy in advanced non-small cell lung cancer (NSCLC) following failure of platinum-based chemotherapy. Lung Cancer. 2008;61(2):262–265. | ||
Okabe T, Okamoto I, Tamura K, et al. Differential constitutive activation of the epidermal growth factor receptor in non-small cell lung cancer cells bearing EGFR gene mutation and amplification. Cancer Res. 2007;67(5):2046–2053. | ||
Zhu JQ, Zhong WZ, Zhang GC, et al. Better survival with EGFR exon 19 than exon 21 mutations in gefitinib-treated non-small cell lung cancer patients is due to differential inhibition of downstream signals. Cancer Lett. 2008;265(2):307–317. | ||
Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16(2):141–151. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.