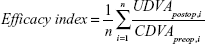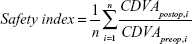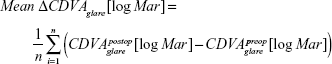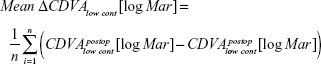Back to Journals » Clinical Ophthalmology » Volume 8
Comparison of clinical outcomes in PRK with a standard and aspherical optimized profile: a full case analysis of 100 eyes with 1-year follow-up
Authors Dausch D, Dausch B, Wottke M, van Langeweyde G
Received 22 April 2014
Accepted for publication 24 June 2014
Published 24 November 2014 Volume 2014:8 Pages 2251—2260
DOI https://doi.org/10.2147/OPTH.S66608
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Dieter Dausch,1,2 Burglinde Dausch,2 Matthias Wottke,3 Georg Sluyterman van Langeweyde3
1Chung-Ang University, Seoul, South Korea; 2Augen-Laser-Klinik Nürnberg, Nuremberg, Germany; 3Carl Zeiss Meditec AG, Jena, Germany
Purpose: One hundred eyes from 55 adult patients with myopia were retrospectively studied to determine the comparative safety, efficacy, and predictability of aberration smart ablation (ASA) and a new advanced ablation algorithm (Triple-A) using the MEL® 80 excimer laser.
Methods: Fifty myopic eyes with a manifest refraction spherical equivalent (MRSE) between -1.0 diopters (D) and -9.75 D were consecutively treated with photorefractive keratectomy ASA, and 50 myopic eyes with an MRSE between -1.38 D and -11.0 D with photorefractive keratectomy Triple-A. Uncorrected distance visual acuity, MRSE, the absolute value of the cylinder, corrected distance visual acuity, and postoperative complications at 1 month, 3 months, 6 months, and 12 months (1 year) were descriptively analyzed and compared at 1 year.
Results: After 12 months, the MRSE variance was statistically significantly better in patients triaged to receive Triple-A compared with patients receiving ASA (ASA, ±0.7 D; Triple-A, ±0.15 D; P<0.001). Furthermore, no patient in the Triple-A group had any cylinder postoperatively. Patients in the Triple-A treatment arm achieved a superior result. No statistically significant difference in the two treatment arms was noted for the analysis of the mean MRSE at 12 months (P=0.78).
Conclusion: Triple-A was more effective than standard aspherical surgical intervention in a number of treatment outcome parameters (eg, MRSE, astigmatism, efficacy index). The two surgical procedures were equivalent in terms of safety.
Keywords: aberration smart ablation (ASA), manifest refraction spherical equivalent, Triple-A advanced ablation algorithm, uncorrected distance visual acuity, corrected distance visual acuity, excimer laser, PRK, ablation profile
Introduction
In 1988, photorefractive keratectomy (PRK), a technique for laser refractive surgery, was introduced.1 The correction of myopic refractive errors with the MEL® 80 spot-scanning excimer laser (Carl Zeiss Meditec AG, Jena, Germany) with the aberration smart ablation (ASA) profile during PRK has been reported with good outcomes in terms of safety, efficacy, predictability, and the low incidence of vision-threatening complications.2–4 The ASA profile is an aspheric optimized ablation profile. It has an aspheric profile portion that is independent of the correction. In some cases, the ASA profile used still has distinct imperfections, such as an underablation on the peripheral cornea. The use of ASA in higher myopic corrections can induce a positive spherical aberration, (Z40 <0).5 Another detail of the ASA profile is that the use of this algorithm leads to a relatively higher ablation depth in low myopic corrections. This is because the asphericity has a constant amount over the total range of correction. In high myopic corrections, this amount is relatively low and leads to an induction of the higher order spherical aberration Z40.
To achieve a minimal ablation depth during the correction of low myopia, an improved ablation profile should have a minimal asphericity in low corrections that increases as the amount of the myopic correction increases. Moreover, the improved ablation profile should include a more powerful energy correction function that minimizes the induction of a spherical aberration generally. The Triple-A advanced ablation algorithm is an ideal ablation profile comprising these properties and was developed by Carl Zeiss Meditec AG. As a part of the corneal topography guided treatment modality it was initially introduced commercially on the CRS-Master (Carl Zeiss Meditec AG) at the American Academy of Ophthalmology meeting in Los Angeles, (CA, USA) in 2005. The CRS-Master is a planning station for customized vision correction. It is available in many countries worldwide; however, it is currently not available in the US and Japan.
Even though laser algorithms have been established to optimally treat all types of refractive errors, in some patients, the postoperative vision deteriorates under mesopic and lighting conditions.6,7 The aim of the current study was to compare PRK in myopic patients using the MEL® 80 excimer laser with the ASA or Triple-A ablation profile. Both ASA and Triple-A ablation profiles have been shown to be highly effective over a wide range of myopic corrections (−0.0 [diopters] D to −10.0 D).2–4,8 This retrospective study evaluated the safety, efficacy, and predictability of Triple-A (group B) and compared the same postoperative outcomes against patients triaged to receive standard ASA treatment (group A), both performed with the MEL® 80 excimer laser.
Materials and methods
Study design
The study was conducted at Augenpraxisklinik Amberg, Marienstrasse 3, Amberg, Germany. At 1 year, the safety index, efficacy index, the postoperative MRSE, and the cylinder of both groups were compared. Also, the best corrected distance visual acuity (CDVA) under glare and under low contrast of both groups were compared. The purpose of the study is to compare the separate inferential test of mean and variances of the parameters at 1 year. Only primary treatments were analyzed. The follow-up rate was 100%. Outcome measures were calculated according to the standardized graphs as originally defined by Waring.14 The logarithm of the minimum angle of resolution (logMAR) monocular UDVA, CDVA, and the manifest refraction were used to analyze the efficacy, safety, predictability, accuracy, stability, and the statistical calculation. Preoperative data, as well as 1-month, 3-months, 6-months, and 1-year postoperative data, were used for analysis (1 month, 3 months, 6 months, and 12 months for descriptive statistics and 12 months for comparison). Linear regression analysis was performed to analyze the predictability (manifest refraction spherical equivalent [MRSE] attempted versus MRSE achieved). Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) was used for data entry and analysis.
Inclusion criteria
Inclusion criteria included patients with age of 20 years or older with normal topography, a stable myopic spherical equivalent of –0.50 D or more, and a CDVA >20/32. Patients with a preoperative CDVA of 20/32 were included if at 1 year postoperation, the CDVA was 20/25 or better. The anatomical alteration of the cornea, lens, and macula of those eyes were not traceable. All treatments in the study are being targeted for plano. The ASA group patients were treated from June 2007–December 2009, while the Triple-A group patients were treated from January 2010–April 2011. Similar demographics resulted without selection.
Exclusion criteria
Patients with corneal scarring, autoimmune disorders, severe dry eyes, blepharitis, or lagophthalmos were excluded from the study.
Preoperative, postoperative measurements
Preoperative and postoperative measurements included uncorrected distance visual acuity (UDVA) and best CDVA at 1 month, 3 months, 6 months, and 1 year. Pachymetry and refraction with and without cycloplegia were performed in all patients before surgery. Keratometer readings, autorefraction, and visual acuity (VA) under glare and low-contrast were performed in all eyes, at each visit, using the Humphrey Automatic Refractor 515 (Carl Zeiss Meditec, Jena, Germany). Furthermore, slit lamp examination, and topographic measurements were performed in all eyes, at each visit. Intraocular pressure was measured by Goldmann applanation tonometry preoperatively and after each follow-up month. The severity of postoperative subepithelial haze was graded, according to a classification system devised by the authors.9,10
Surgical protocol
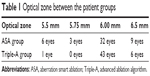
The optical zone varies between 5.5 mm and 6.5 mm, according to the scotopic pupil of the eyes (Table 1). The mean of the optical zone of the ASA group was 6.015±0.283 mm and 6.05±0.182 mm in the Triple-A group. Statistically, the mean of both groups turned out equal (P=0.34).
  | Table 1 Optical zone between the patient groups |
Because all of the measurements were performed at least under mesopic conditions and because the mean of the optical zones of both groups was equal, there was no consideration to do further analysis.
Before doing the surgical procedure, eyes were anesthetized with Novesine 0.4% eye drops (oxybuprocaine HCl 4 mg; OmniVision GmbH, Puchheim, Germany). Then, a lid-speculum was inserted. According to the desired treatment zone, the corneal surface was marked with an optical zone marker. Removal of the epithelium was done by using an Amoils brush (Innovative Excimer Solutions, Toronto, OT, Canada), according to the marked zone area. Debris was removed with an epithelium spatula of Weck-cell® (Beaver Visitec International, Waltham, MA, USA) sponge.
Laser ablation in both groups (ASA group and Triple-A group) was conducted using a MEL® 80 excimer laser. The treated corneal surface was cooled with chilled balanced salt solution (eg, BSS®, Alcon, Fort Worth, TX, USA) (6°C). Then, a bandage lens (eg, Soft Lens; Bausch & Lomb Incorporated, Bridgewater, NJ, USA) was applied. For anti-inflammatory postoperative care (eg, Floxal® eye drops [Ofloxacin, 3mg]; Dr Gerhard Mann Chem-Pharm Fabrik GmbH, Berlin, Germany) were applied. Application of Floxal was conducted four times a day until complete reepithelialization had occurred. After lens removal, mild cortisone (ie, Efflumidex® Liquifilm eye drops [fluorometholone, 1 mg]; Allergan Inc., Irvine, CA, USA) was applied. The complete postoperative management is illustrated in Table 2. The duration of cortisone therapy depends on the preoperative refraction and the intensity of the haze.
  | Table 2 Postoperative management chart |
Based on our long clinical experience, after 3 months, about 75% of the treated eyes have no haze (grade 0), and about 25% of the treated eyes have only traces of minimal reticular or spotlike haze (grade 1). Therefore, we dispense with giving mitomycin C. For the classification of the postoperative haze, the established subjective method of Dausch et al was used.11
Statistical calculation
Microsoft Excel 2003 (Microsoft) and SAS Enterprise Guide 4.3 (SAS Institute Inc., Cary, NC, USA) were used for the statistical calculation.
The Kolmogorov–Smirnov test was used to check if both samples (ASA and Triple-A) were normally distributed. If both samples were normally distributed, the F-test and the two-sided t-test were used to analyze the variances and mean values.
If one of both samples was not normally distributed, then the two-sided Siegel–Tukey test, the two-sided Wilcoxon two-sample test, and the two-sided median test were used to analyze the variances and mean values.
Statistical significance was indicated by P<0.05.
To test the correlation between age and 1-year UDVA, the Spearman rank correlation was used. The Spearman rank correlation was also used to test the correlation between age and 1-year SEQ.
The efficacy index and the safety index were performed with the following formulas. According to these, the ratio between UDVA postoperatively and CDVA preoperatively was made per eye, and the average of the ratio was calculated:
| (1) |
| (2) |
Furthermore, the mean value of the differences between CDVA under glare and low contrast postoperatively and CDVA under glare and low contrast preoperatively were used for the analysis.
|
|
|
|
A Bonferroni adjustment was not applied for reasons of not increasing the type II error considerably and because of the controversy about its application.12,13
Results
The study population consisted of 55 patients, 29 in the ASA treatment arm (group A), and 26 in the Triple-A treatment arm (group B). There were 100 eyes total; 50 in each arm. All surgeries were performed by one surgeon (DD). No eye required or underwent any retreatment.
The preoperative demographics are shown in Table 3. The mean and the variance of the preoperative MRSE, sphere, and cylinder in ASA eyes were not significantly different from the mean and variance of the preoperative MRSE, sphere, and cylinder in Triple-A eyes (P=0.41, P=0.20, P=0.092 [mean]; P=0.52, P=0.28, P=0.997 [variance]). The mean age was significantly different (P=0.0024), but there was no correlation with respect to α=0.05 between age and 1-year UDVA or 1-year MRSE (P=0.32 for ASA and P=0.10 for Triple-A, or P=0.06 for ASA and P=0.53 for Triple-A).
The F-test and two-sided t-test were only used for the comparison of the sphere and MRSE preoperatively, because these variables turned out to be normally distributed for both groups (ASA and Triple-A).
All results of statistical calculation are listed in Table 9.
Efficacy
The average UDVA at 1 year postoperatively was better than the CDVA preoperatively (efficacy index =1.03±0.15) for the Triple-A group patients. In the ASA group, the efficacy index was 0.95±0.3. The variance and mean of both groups were statistically different (P=0.011 and P=0.015). At 1 year postoperatively, 86% of the ASA group patients had a UDVA of 20/32 or better. Preoperatively, the CDVA of 20/32 or better was found in 96% of patients. In the Triple-A group, all patients (100%) had at least a UDVA of 20/32. Preoperatively, the CDVA of 20/32 or better was 98%. Sixty-eight percent (68%) of patients in the ASA group had a UDVA of 20/20 or better; whereas, 80% of the Triple-A group patients had a UDVA of 20/20 or better. Preoperatively, 86% of the ASA group patients had a CDVA of 20/20 or better; whereas, 82% of the Triple-A group patients had a CDVA of 20/20 or better (Figure 1; Table 5).
Safety
One year following surgery, the safety index of the ASA group was 1.06±0.24 compared to 1.05±0.15 in the Triple-A group. Statistically, there was no difference (P=0.69). The CDVA remained unchanged in fewer patients in the ASA group compared to the Triple-A group (56% versus 70%) after 1 year. One line of CDVA was lost in 20% of ASA patients compared to 8% of the Triple-A patients. One line of CDVA was gained in 20% of ASA patients compared to 18% of the Triple-A patients. One patient in the ASA group gained two lines of CDVA compared to two patients in the Triple-A group. One month postoperatively, the safety index (1.04±0.17) of the Triple-A group was higher than the safety index (0.99±0.18) of the ASA-group (Figure 2; Table 6).
Predictability/accuracy
Results for the entire cohort of 50 eyes each are presented in Figure 3. The scatter plot shows the attempted versus achieved refraction at 1 year.
The MRSE postoperatively is significantly better for the Triple-A group at 1 year (P<0.0001). Also, 50% of the ASA and 86% of the Triple-A treated eyes had an MRSE between −0.13 D to +0.13 D after 1 year (Figure 4; Table 7).
  | Table 7 Accuracy MRSE distribution postoperatively |
Astigmatism
At 1 year postoperatively, 98% of the eyes treated with ASA had a normal cylinder of ≤0.5 D versus 100% in the Triple-A group. No patient had a normal cylinder >1.0 D at 1 year. The average cylinder in the ASA group was 0.06±0.18 D. In the Triple-A group, no patient had any cylinder at 1 year postoperatively (Figure 5; Table 8). The statistical analysis shows that the mean and variance were significantly different (P=0.0225; P=0.0232).
  | Table 8 Astigmatism absolute value distribution |
Stability
The stability of the MRSE is presented in Figure 6. The timeline depicts MRSE and one standard deviation represented by the error bars preoperatively, and at 1 month, 3 months, 6 months, and 1 year after surgery. In the ASA group, three eyes had a regression of the MRSE >0.5 D between 6 months and 1 year, postoperatively. In the Triple-A group, no eye had a regression >0.5 D. The average change in refraction between 1 month and 1 year was 0.29±0.66 D in the ASA group and 0.16±0.23 D in the Triple-A group. The statistical analysis showed a difference in terms of the variances (P<0.0001), and no difference in terms of the mean (P=0.232).
Visual acuity under glare
After 1 year, the mean value of the differences of the CDVA under glare for the ASA group was 0.08±0.21 logMAR and for the Triple-A group, 0.09±0.20 logMAR. Statistically, there was no difference between both groups with respect to mean and variance (P=0.41; P=0.96).
Visual acuity under low contrast
After 1 year, the mean value of the differences of the CDVA under low contrast for the ASA group was 0.03±0.20 logMAR and for the Triple-A group, 0.06±0.16 logMAR. Statistically, there was no difference between both groups with respect to mean and variance (P=0.89; P=0.77).
Discussion
Excimer lasers – such as the MEL® 80 – have revolutionized the field of laser eye surgery. They are used to reshape the cornea and correct refractive errors, such as myopia, hyperopia, and astigmatism. Ablation of the cornea with the MEL® 80 excimer laser to treat myopia has been found to be safe and effective, achieving LASIK outcomes that are better than the US Food and Drug Administration guidelines for refractive procedures.14,15
Surface ablation to treat myopia induces spherical aberrations.16–18 This is because the cornea is not completely spherical. Broad-beam ablation with lasers leads to an increase of spherical aberrations due to the asphericity of the cornea. Currently, the surgical focus is on treating myopic eyes with aspheric optimized ablation techniques.
Our study compared the outcome of ASA profile with the outcome of the Triple-A profile. Previous studies have shown that less spherical aberration is induced by modern ablation profiles than with conventional surface ablation techniques.8,19 Regarding refractive outcomes, the Triple-A group showed superiority. One year postoperatively, the mean MRSE (−0.02 D) was extraordinarily small, as well as the standard deviation (±0.15 D) of the same (Table 4). Recent studies also showed a mean MRSE of −0.12 D ±0.35 D,20 and −0.03 D ±0.15 D.21 Also, 1 year after the procedure, nearly all patients (96%) in the Triple-A group had an uncorrected visual acuity of 20/25 or better. This was not the case in the ASA group. Differences in VA under glare and under low contrast were not statistically significant. The cylinder correction for the Triple-A treated patients was much more successful than for the ASA-treated patients. No eye in the Triple-A group had a cylinder postoperatively.
Conclusion
The present study showed the Triple-A profile to be as good as a standard ablation algorithm as ASA in terms of safety, predictability (slope), stability, and VA under glare and low contrast. Compared with ASA, the Triple-A showed superiority with respect to cylinder corrections, efficacy and predictability (scatter), and accuracy; therefore, it led to high patient satisfaction.
Disclosure
Matthias Wottke and Georg Sluyterman van Langeweyde, PhD, are employees of Carl Zeiss Meditec AG. The authors report no other conflicts of interest in this work.
References
Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. 1988;14(1):46–52. | ||
Frings A, Vidic B, El-Shabrawi Y, Ardjomand N. Laser-assisted subepithelial keratomileusis with mitomycin C for myopic astigmatism ≥2.00 diopters using a Zeiss MEL 80 Excimer. Int Ophthalmol. 2014;34(2):225–233. | ||
Mastropasqua L, Toto L, Zuppardi E, et al. Photorefractive keratectomy with aspheric profile of ablation versus conventional photorefractive keratectomy for myopia correction: six-month controlled clinical trial. J Cataract Refract Surg. 2006;32(1):109–116. | ||
Wygledowska-Promieńska D, Gierek-Ciaciura S, Mrukwa-Kominek E, Zawojska I, Rokita-Wala I. [Correction of myopia by PRK method with the use of MEL 80 excimer laser – initial report]. Klin Oczna. 2004;106(1–2):50–53. Polish. | ||
Malacara D. Optical Shop Testing. 2nd ed. NY: John H Wiley & Sons; 1978. | ||
Verdon W, Bullimore M, Maloney RK. Visual performance after photorefractive keratectomy. A prospective study. Arch Ophthalmol. 1996;114(12):1465–1472. | ||
O’Brart DP, Lohmann CP, Fitzke FW, et al. Disturbances in night vision after excimer laser photorefractive keratectomy. Eye (Lond). 1994; 8(Pt 1):46–51. | ||
Reinstein DZ, Archer TJ, Gobbe M. Combined corneal topography and corneal wavefront data in the treatment of corneal irregularity and refractive error in LASIK or PRK using the Carl Zeiss Meditec MEL 80 and CRS-Master. J Refract Surg. 2009;25(6):503–515. | ||
Dausch D, Klein R, Schröder E. [Photoablative, refractive keratectomy in treatment of myopia. A case study of 134 myopic eyes with 6-months follow-up]. Fortschr Ophthalmol. 1991;88(6):770–776. German. | ||
Dausch D, Dausch B, Klein R, Schröder E. Long-Term Results of Myopic Photorefractive Keratectomy with the Excimer Laser: Five to Six Year Follow-Up. Ophthalmic Practice. 1997;15(5). | ||
Dausch D, Klein R, Schröder E. The severity of haze classified in four grades. In: Ophthalmic Excimer Laser Surgery. Strasbourg: Editions Du Signe; 1991:101–102. | ||
Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316(7139):1236–1238. | ||
Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Behav Ecol. 2004;15(6):1044–1045. | ||
Waring GO 3rd. Standard graphs for reporting refractive surgery. J Refract Surg. 2000;16(4):459–466. | ||
Blum M, Kunert K, Gille A, Sekundo W. LASIK for myopia using the Zeiss VisuMax femtosecond laser and MEL 80 excimer laser. J Refract Surg. 2009;25(4):350–356. | ||
Hersh PS, Fry K, Blaker JW. Spherical aberration after laser in situ keratomileusis and photorefractive keratectomy. Clinical results and theoretical models of etiology. J Cataract Refract Surg. 2003;29(11): 2096–2104. | ||
Srivannaboon S, Reinstein DZ, Archer TJ, Chansue E. Spherical aberration from myopic excimer laser ablation for aspheric and non-aspheric profiles. Optom Vis Sci. 2012;89(8):1211–1218. | ||
AlMahmoud T, Munger R, Jackson WB. Advanced corneal surface ablation efficacy in myopia: changes in higher order aberrations. Can J Ophthalmol. 2011;46(2):175–181. | ||
Goes F. One year LASIK experience treating myopia with the Carl Zeiss MeditecMEL 80 LASER: Proceedings of the XXI Congress of the European Society of Cataract and Refractive Surgeons, Munich, Germany, 6–10 September 2003. Available from: www.pimos.net/fichiers/file/PDF/OneyearLASIKeyperience.pdf. Accessed February 5, 2014. | ||
Aslanides IM, Padroni S, Arba-Mosquera S. Aspheric photorefractive keratectomy for myopia and myopic astigmatism with the SCHWIND AMARIS laser: 2 years postoperative outcomes. J Optom. 2013;6(1):9–17. | ||
Gambato C, Catania AG, Vujosevic S, Midena E. Wavefront-optimized surface ablation with the Allegretto Wave Eye-Q excimer laser platform: 12-month visual and refractive results. J Refract Surg. 2011;27(11):792–795. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.