Back to Journals » Clinical Ophthalmology » Volume 10
Comorbidity and health care visit burden in working-age commercially insured patients with diabetic macular edema
Authors Kiss S, Chandwani HS, Cole AL, Patel VD, Lunacsek OE , Dugel PU
Received 1 June 2016
Accepted for publication 15 September 2016
Published 7 December 2016 Volume 2016:10 Pages 2443—2453
DOI https://doi.org/10.2147/OPTH.S114006
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Szilárd Kiss,1 Hitesh S Chandwani,2 Ashley L Cole,2 Vaishali D Patel,2 Orsolya E Lunacsek,3 Pravin U Dugel4
1Department of Ophthalmology, Weill Cornell Medical College, New York, NY, 2Global Health Economics and Outcomes Research, Allergan, Inc., Irvine, CA, 3Global Health Economics and Outcomes Research, Xcenda, LLC, Palm Harbor, FL, 4Retinal Consultants of Arizona and USC Eye Institute, Phoenix, AZ, USA
Purpose: To examine the comorbidity profile and update estimates of health care resource utilization for commercially insured, working-age adults with diabetic macular edema (DME) relative to a matched comparison group of diabetic adults without DME. Additional comparisons were made in the subgroup of pseudophakic patients.
Patients and methods: A retrospective matched-cohort study of commercially insured diabetic adults aged 18–63 years was conducted using medical and outpatient pharmacy claims (July 1, 2008–June 30, 2013). Outcomes included diabetes-related and ocular comorbidities and health care resource utilization (any health care visit days, outpatient visit days, inpatient visit days, emergency room visits, eye care-related visit days, unique medications) in the 12-month post-index period.
Results: All diabetes-related and ocular comorbidities were significantly more prevalent in DME cases versus non-DME controls (P<0.05). A significantly greater proportion of DME cases utilized eye care-related visits compared with non-DME controls (P<0.001). DME cases had almost twice the mean number of total health care visit days compared to non-DME controls (28.6 vs 16.9 days, P<0.001), with a minority of visit days being eye care-related (mean 5.1 vs 1.5 days, P<0.001). Similar trends were observed in pseudophakic cohorts.
Conclusion: This working-age DME population experienced a mean of 29 health care visit days per year. Eye care-related visit days were a minority of the overall visit burden (mean 5 days) emphasizing the trade-offs DME patients face between managing DME and their overall diabetic disease. Insights into the complex comorbidity profile and health care needs of diabetic patients with DME will better inform treatment decisions and help optimize disease management.
Keywords: health care resource utilization, diabetes, real-world evidence, pseudophakic, retinal disease
Introduction
The prevalence of diabetes mellitus (DM) in the US has increased substantially over the previous two decades, rising from 6.2% of US adults in 1994 (10.2 million) to 9.3% (29.1 million) in 2012.1,2 DM is linked to several comorbid conditions, including hypertension, dyslipidemia, and renal dysfunction, which, in turn, lead to further complications of the disease.2 Among these complications are diabetic retinopathy (DR) and diabetic macular edema (DME), both of which may cause visual impairment and, potentially, blindness. The complex comorbidity profile of diabetics translates into higher medical service utilization, health care costs, and lost productivity compared to nondiabetics.3 Although the overall economic impact of DM has been well documented ($176 billion direct medical costs and $69 billion indirect costs, 2012 USD),2 there is a paucity of evidence about the health care burden of diabetic patients whose disease is complicated with DME.4
Based on various population estimates, DME affects between 3% and 16% of all diabetic adults.5,6 DME is debilitating because it may lead to progressive vision loss, negatively affecting the patient’s quality of life.4,7 In the US, DM is the primary cause of blindness among adults aged 20–74.8 Although the prevalence of blindness specifically attributable to DME has not been quantified,4 the lifetime risk for diabetics to develop DME is nearly 10%.9
Patients with DME face additional economic burden in terms of health care resource utilization and associated expenditures compared to diabetics without DME, including more health care visits,10 diagnostic procedures, and treatments requiring frequent monitoring and injections.11 Ocular treatments for DME include antivascular endothelial growth factor antagonists (anti-VEGFs), focal laser photocoagulation, intravitreal injections (IVIs) of corticosteroids, and vitrectomy.12–15 These DME-specific treatments are associated with additional ophthalmologist visits, transportation assistance needs, absence from or conflict with daily commitments, and potential productivity loss. Although DME can affect adults of any age, its implications may be different among different age segments. Working-age adults with DME may suffer most due to loss of work productivity and employment stability,16 while elderly patients may have increased caregiver needs and social isolation due to DME.17 Underlying risks of associated diabetes-related comorbidities may also differ by age. Therefore, it is important to distinguish between younger and older patients when examining health care outcomes among DME populations.
Since the number of people with DM continues to rise as a result of population growth, aging, obesity, and sedentary lifestyles, the health care burden of diabetic complications, including DME, is also expected to increase.18 Understanding the complex comorbidity profile of patients with DME and their resulting health care needs is essential for optimizing their disease management. In light of the rapidly evolving therapeutic landscape of DME, with several new drug approvals between 2012 and 2014, more current research is necessary to understand the trends and consequences of the changing treatment paradigm. Since the implications of both the disease and its treatment may differ between age segments, the authors conducted two studies: the current research examines the impact of DME in working-age adults, and the second study investigates DME patients of retirement age. Thus, the objectives of this study were to examine the comorbidity profile and update estimates of health care resource utilization for commercially insured, working-age diabetic adults with DME compared to diabetic adults without DME. Additionally, study outcomes were examined among the subgroup of patients with an intraocular lens (IOL) implant. These pseudophakic patients have an altered risk profile that underscores different treatment options. The subanalysis was also warranted by the high proportion of patients with pseudophakia among the DME population (28.9%–30%) as evidenced in clinical trials.19,20
Methods
Data source and study timeframes
This was a retrospective matched-cohort study of incident diabetic patients with DME (DME cases) vs diabetic patients without DME (non-DME diabetic controls) using the Truven Health MarketScan® Commercial Claims and Encounters Database (CCAE). The CCAE contains longitudinal patient-level medical and prescription claims of active employees, early retirees, Consolidated Omnibus Budget Reconciliation Act utilizers, and their dependents insured by employer-sponsored plans. The database covers 170 million individuals from 50 US states and provides cost and utilization data for the inpatient and outpatient settings. Paid medical claims are linked to prescription drug claims and patient-level enrollment records through a unique enrollee identifier. Informed consent is not required to utilize these de-identified claims data. The MarketScan® Database used in this study is de-identified and fully compliant with the Health Insurance Portability and Accountability Act of 1996. Because this study did not involve the collection, use, or transmittal of individually identifiable data, Institutional Review Board review or approval was not required according to the US Department of Health & Human Services.
Data from July 1, 2008 to June 30, 2013, were available for analysis, and an 18-month enrollment window (January 1, 2011 to June 30, 2012) was used for patient identification (Figure 1). The enrollment window was chosen to allow patients to be followed from 30 months prior to their index date through 12 months post-index. The 30-month pre-index period was used to assess study eligibility and to identify patients with pseudophakia; this relatively long look-back period was chosen to be able to obtain sufficient group sizes, capture incident patients at the time of the DME treatment decision, and help identify pseudophakic patients. A shorter 6-month pre-index period was used to assess baseline characteristics. A 12-month post-index period was used to evaluate outcomes.
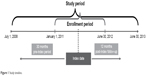  | Figure 1 Study timeline. |
Patient selection and matching
Patients were classified into two cohorts (DME and non-DME) and then matched in a ratio of one case to three controls. DME cases were required to have at least one nondiagnostic medical claim with a DME diagnosis (International Classification of Diseases, Ninth Revision [ICD-9] code 362.07) during the enrollment window, and their first date with a DME diagnosis was designated as the index date. Non-DME diabetic controls were required to have at least two nondiagnostic medical claims with a diagnosis of DM (ICD-9 250.xx) during the enrollment window and at least one claim for DM or one prescription fill for an antidiabetic medication during the 30-month pre-index period. The index date for non-DME controls was a randomly selected DM claim during the enrollment window.
All patients had to be between 18 and 63 years old on their index date and continuously enrolled with medical/prescription benefits from 30 months pre-index through 12 months post-index. DME cases were excluded if they had a diagnosis for cystoid macular edema (ICD-9 code 362.53), retinal edema (ICD-9 code 362.83), or DME at any time during the 30-month pre-index period. Non-DME diabetic controls were excluded if they had these during the pre-index or the enrollment periods, or a proliferative DR (ICD-9 code 362.02) or nonproliferative DR (ICD-9 codes 362.03–362.06) diagnosis during the pre-index period. To be included in the pseudophakic subcohort, a patient was required to have at least one medical claim for an IOL implant with or without cataract surgery (ICD-9 code V43.1 and/or Current Procedural Terminology code 66982 or 66984) in the 30-month pre-index period.
DME cases were matched in a ratio of 1:3 to the non-DME diabetic controls using individual matching based on the greedy matching algorithm without replacement.21 The matching variables included age at index with a maximum of 2 years difference between matched pairs, gender, US region, index year, and presence of a pseudophakic eye.
Baseline characteristics
Baseline patient characteristics of interest that were available in the data included age, gender, and US region at index; Charlson comorbidity index (CCI) score; and diabetes-related and ocular comorbidities measured over the 6-month pre-index period. Diabetes-related comorbidities included myocardial infarction (MI), congestive heart failure (CHF), peripheral vascular disease (PVD), cerebrovascular disease (CVD), stroke, renal disease, and lower limb amputation (Table 1).2 Ocular comorbidities included nonproliferative and proliferative DR, cataract, glaucoma, retinal vein occlusion (RVO) (central RVO and branch RVO), atrophic age-related macular degeneration (AMD), and exudative AMD (Table 1).
  | Table 1 Coding definitions |
Outcomes
Outcomes were assessed over the 12-month post-index period and included the same diabetes-related and ocular comorbidities that were measured at baseline and health care resource utilization measures. Health care resource utilization was captured as the number and proportion of patients with particular visit types and mean number of days/visits among utilizing patients (contingent means) in the following categories: any health care visit days, outpatient visit days, inpatient visit days, emergency room (ER) visits, eye care-related visit days (Table 1), and number of unique outpatient medications. The number and proportion of patients with cataract and glaucoma surgeries were also calculated (Table 1). Among DME cases, DME-related ocular diagnostic and therapeutic procedures were measured (as number and proportion of patients with each procedure and contingent mean number of procedures) for the following: optical coherence tomography (OCT), fluorescein angiography (FA), laser photocoagulation, IVI, and vitrectomy (Table 1). Outcomes were studied first in the full matched cohorts and then in the subgroup of matched pseudophakic patients.
Statistical analyses
Descriptive statistics (frequency, proportion, mean, standard deviation [SD]) were used to describe baseline characteristics and outcomes. The statistical significance of between-group differences was assessed with chi-square tests for categorical variables and Wilcoxon rank sum tests for continuous variables. All tests were based on a two-sided hypothesis of no difference between the matched cohorts, and a P-value of 0.05 or less was considered statistically significant.
Results
The final study cohorts included 4,006 DME cases matched with 12,018 non-DME diabetic controls. Within these cohorts, 483 pseudophakic DME cases were matched to 1,449 pseudophakic non-DME controls. Age, gender, and region were matching variables and therefore nearly identical between cases and controls. The average age for both the DME cases and non-DME controls was 54.4 years (SD, 7.5 for cases and 7.6 for controls), 54.1% of patients were male, and the South (47.2%) dominated in the regional distribution (Table 2). Pseudophakic cases and their controls were slightly older, with a mean age of 57.6 years (SD 5.1), 49.7% being male, and 45.1% from the South (Table 2).
Comorbidities
Working-age diabetic patients with DME had a significantly higher diabetes-related comorbidity burden (P<0.001 for all comorbidities) than matched non-DME diabetics both during the 6-month pre-index period (Table 2) and the 12-month post-index follow-up (Figure 2). Renal disease, CHF, and CVD were the dominant comorbid conditions. The pre-index CCI score in the DME cohort was twice as high as in the non-DME cohort (P<0.001; Table 2). Consistent with the older age, the CCI score and rates of diabetes-related comorbidities were generally higher in both the DME and non-DME pseudophakic cohorts than in the full matched cohorts (Table 2). Among pseudophakic DME patients, during the 6-month pre-index period, a significantly greater proportion had CHF (P<0.001), renal disease (P<0.001), and lower limb amputation (P<0.05) than the pseudophakic non-DME controls (Table 2). During follow-up, the prevalence of almost all diabetes-related comorbidities was significantly higher in the pseudophakic DME cases compared to the controls (P<0.05; Figure 3), with the exception of CVD.
Ocular comorbidities were also more prevalent in DME cases compared to the non-DME controls. In the pre-index period, all ocular comorbidities with the exception of atrophic AMD were significantly higher for DME compared to non-DME patients (P<0.001; Table 2). During the 12-month follow-up period, all ocular comorbidities were significantly more prevalent in DME patients vs non-DME controls (P<0.05; Figure 2). In the subcohorts of pseudophakic patients, during the pre-index period, the DME cases had significantly higher prevalence of cataract, glaucoma, and RVO (P<0.05; Table 2). During follow-up, the pseudophakic DME cases exhibited significantly higher prevalence of all ocular comorbidities (P<0.05; Figure 3), again with the exception of atrophic AMD.
Health care utilization
Working-age adults with DME utilized more health care resources than matched diabetic patients without DME. Significantly greater proportions of DME cases utilized eye care-related visits compared with their matched non-DME controls in both the full matched study cohorts (86.4% vs 24.9%, P<0.001) and the pseudophakic subcohorts (92.6% vs 46.5%, P<0.001). DME cases had almost twice the mean number of total health care visit days compared to non-DME controls (28.6 vs 16.9 days, P<0.001), with a minority of visit days being eye care-related (5.1 vs 1.5 days, P<0.001) (Figure 4). This suggests an average of more than two monthly health care visits for DME patients, but only one ophthalmology-related visit every other month. Pseudophakic DME cases also had more average days/visits in every visit category than the pseudophakic non-DME controls (P<0.05; Figure 5) among those with at least one visit day; the mean total health care visit days were 33.2 for DME cases and 23.2 for non-DME controls (P<0.001). Both cataract surgery (7.8% vs 1.7%, P<0.001) and glaucoma surgery (1.2% vs 0.2%, P<0.001) were significantly more prevalent among the full matched DME cohort compared to the non-DME cohort, while only glaucoma surgery was significantly more prevalent among pseudophakic DME cases vs their controls (2.3% vs 0.6%, P<0.05).
Of the DME-related ocular diagnostic and therapeutic procedures studied in DME patients alone, 71% had FA and/or OCT (30% FA and 66% OCT) during follow-up, while the proportions of DME cases treated with IVI, laser photocoagulation, or vitrectomy were 35.5%, 47.4%, and 4%, respectively (61% combined for all three treatments). The mean number of IVI injections was 3.9 for patients who received at least one IVI. Among the pseudophakic DME cases, 77% had an FA and/or OCT procedure, while 61% had at least one of the three therapeutic procedures. Pseudophakic patients with an IVI received 3.7 injections, on average.
Discussion
This study adds to the published literature by examining the comorbidity profile and health care resource utilization in the working-age segment of DME patients based on recent claims data (2008 to 2013) from a nationally representative dataset of commercially insured adults. It also analyzes the same outcomes among the subcohort of DME patients with pseudophakia, as these patients have a potentially more severe disease with a different risk profile and treatment options than the general population of DME patients. The main findings of the study include a significantly higher prevalence of diabetes-related and ocular comorbidities and significantly higher health care resource utilization among DME patients compared to matched diabetic patients without DME, both in the full study population and in the pseudophakic subgroup.
The current findings are similar to the results reported in previous research.10,22 A prior study that used a comparable methodology also found that working-age DME patients had significantly more baseline and post-index comorbidities than non-DME patients.10 The prevalence of cardiovascular, cerebrovascular, and renal disease was high, considering that the average age was close to 50 years. The current study has substantiated these findings with more recent data and also reported consistent findings in the pseudophakic subpopulation. As various therapy choices pose different risks and benefits depending on the comorbidity profile and personal context of each patient, it is important to consider the baseline health and existing treatment burden of a diabetic patient newly diagnosed with DME. Thus, therapy should be selected based on the patient’s underlying conditions.
High health care resource utilization by DME patients has also been reported in prior research. A study of US employees aged 18–64 years with DR found that the subgroup who had a DME diagnosis cost their employers 75% more than the subgroup who did not have a DME diagnosis (annual direct health care plus indirect work loss costs of $28,606 vs $16,363 in 2005 USD, P<0.0001).23 High cost and resource use trends (with varying degrees of statistical significance) were also reported for DME in two parallel analyses of US commercial drivers and nondriver employees: drivers with DME and nondrivers with DME had higher costs (annual health care costs of $12,511 and $17,433 in 2012 USD, respectively) than their diabetic controls without DME ($8,785 and $10,926, respectively), missed more work days (27 and 14 days, respectively) than their diabetic controls without DME (15 and 9 days, respectively), and were more likely to use health benefits across a range of services (medical care, prescriptions, sick leave, disability, and workers’ compensation) than their respective control groups.16 In the current study, working-age diabetic patients with DME also utilized more health care resources in terms of both the proportions of patients utilizing services and the mean number of visit days by utilizers than the matched non-DME patients. Consistent with studies of anti-VEGF-treated DME patients,24–26 these incident DME patients exhibited a relatively low rate of eye care-related visit days, potentially constrained by the high overall treatment burden.
Because diabetic patients with DME already have a considerable health care burden, as demonstrated in this study, it is important to consider the treatment intensity and monitoring requirements when deciding on DME therapy for this population. Any additional health care visits may not only affect the patient but also their caregivers and clinicians who are responsible for the coordination of multiple visits.15 Recent research has reported that patient compliance with anti-VEGF injections and monitoring may not be as frequent as mandated by treatment paradigms based on clinical trial data, negatively affecting outcomes with these medications.24–27
The study findings should be interpreted with limitations in mind that are typical of a retrospective cohort design. Information bias may have been introduced into the study by misclassifying patients to the cohorts due to coding errors or undercoding. For example, medical coding in claims is often influenced by implications for reimbursement; providers may therefore have undercoded the V-codes that were used to determine the pseudophakic subpopulation. To counter this bias, a longer-than-usual 30-month pre-index period was used to identify pseudophakic patients, and cataract procedure codes were included in the definition in addition to V-codes. Another limitation is the potential for unobservable cohort differences. For example, information about the duration and severity of DM (onset date, glycosylated hemoglobin A1c values), general health (body mass index, smoking), race, and socioeconomic status was not available in the data. Some of these variables may be confounders of the study findings. To mitigate potential confounding, matching was employed based on measurable characteristics such as age, gender, region, index year, and pseudophakic status. Still, residual confounding due to unavailable, unmeasured, or unsuspected factors may have distorted the outcome measures. Further investigation using prospective designs may overcome some of these limitations.
This study evaluated the health care resource utilization (eg, visits and procedures) of the population of interest and is not a comprehensive report of the burden of DME patients. It did not evaluate costs, productivity loss (absenteeism, disability), or the humanistic burden (health-related quality of life measures). In addition, resource use may have been underestimated due to a lack of information about the amount of time a visit/service took and the complexity of the visit. Different data sources may be utilized in future research to assess the full disease burden of DME patients.
The generalizability of this study is limited to individuals with employer-sponsored health coverage and may not be representative of other populations, particularly working-age adults without health insurance. In terms of timeframe, the estimates of eye care-related health care visits and procedures may not accurately reflect practice patterns outside of the study period due to the constantly changing treatment landscape for DME.
Conclusion
In this claims-based analysis, diabetic patients with DME had higher comorbidity burden and health care resource utilization than diabetic patients without DME. Similar trends were observed in the pseudophakic subcohorts. The high overall visit burden (~30 health care visit days per year, of which ~5 only were eye care-related) in this working-age population might represent a choice between seeking treatment and going to work or managing other personal and family commitments and may limit the capacity for DME-specific treatment. DME-related therapy choices that require frequent monitoring or treatment visits must be considered within the context of the already-burdened health care landscape of these patients. Insights into the complex comorbidity profile and health care needs of diabetic patients with DME will better inform treatment decisions and optimize disease management. For example, extended-duration IVI therapies may offer a solution to the high visit burden and limited capacity for intensive treatment. Further research should be aimed at investigating individual DME medications and their impact on health care costs and utilization or at specific segments of the diabetic population at increased risk for developing DME based on ethnicity, duration of DM, or severity of disease.28
Acknowledgment
This study was funded by Allergan.
Author contributions
HSC had full access to all the data in the study, participated in all aspects of the manuscript, and takes responsibility for the integrity of the data and the accuracy of the data analysis. The study was conducted and designed by SK, HSC, OEL, and PUD. Data acquisition/collection and management were conducted by ALC, OEL, and HSC. HSC, ALC, VDP, and OEL analyzed the data and interpreted the findings. SK, HSC, ALC, VDP, OEL, and PUD prepared, revised, and approved the manuscript.
Disclosure
SK reports consulting fees or honoraria, Alimera Sciences Inc., Allergan, Alcon, EyeTech, Genentech, Merge/OIS, Optos, Regeneron, and ThromboGenics; speakers bureaus, Alimera, Allergan, Genentech, Optos, Regeneron, and ThromboGenics; clinical research projects, Allergan, Genentech, Optos, and Regeneron; stock/stock options, Merge/OIS. HSC and VDP are employees of Allergan, and ALC was an employee of Allergan at the time of analysis. OEL is an employee of Xcenda. PUD is a consultant for and receives research grants from Allergan.
The authors report no other conflicts of interest in this work.
References
Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–525. | ||
Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA: US Department of Health and Human Services; 2014. | ||
Ramsey S, Summers KH, Leong SA, Birnbaum HG, Kemner JE, Greenberg P. Productivity and medical costs of diabetes in a large employer population. Diabetes Care. 2002;25(1):23–29. | ||
Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin. 2010;26(7):1587–1597. | ||
Ding J, Wong TY. Current epidemiology of diabetic retinopathy and diabetic macular edema. Curr Diab Rep. 2012;12(4):346–354. | ||
Petrella RJ, Blouin J, Davies B, Barbeau M. Prevalence, demographics, and treatment characteristics of visual impairment due to diabetic macular edema in a representative Canadian cohort. J Ophthalmol. 2012;2012:159167. | ||
Gonder JR, Walker VM, Barbeau M, et al. Costs and quality of life in diabetic macular edema: Canadian burden of diabetic macular edema observational study (C-REALITY). J Ophthalmol. 2014;2014:939315. | ||
Resnikoff S, Pascolini D, Etya’ale D, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82(11):844–851. | ||
Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care. 2003;26(9):2653–2664. | ||
Wallick CJ, Hansen RN, Campbell J, Kiss S, Kowalski JW, Sullivan SD. Comorbidity and health care resource use among commercially insured non-elderly patients with diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina. 2015;46(7):744–751. | ||
Shea AM, Curtis LH, Hammill BG, et al. Resource use and costs associated with diabetic macular edema in elderly persons. Arch Ophthalmol. 2008;126(12):1748–1754. | ||
Kulkarni AD, Ip MS. Diabetic macular edema: therapeutic options. Diabetes Ther. 2012;3(1):1–14. | ||
Witkin AJ, Brown GC. Update on nonsurgical therapy for diabetic macular edema. Curr Opin Ophthalmol. 2011;22(3):185–189. | ||
Grover D, Li T, Chong C. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev. 2008;(1):CD005656. | ||
Jain A, Varshney N, Smith C. The evolving treatment options for diabetic macular edema. Int J Inflamm. 2013;2013:689276. | ||
Brook RA, Kleinman NL, Patel S, Smeeding JE, Beren IA, Turpcu A. United States comparative costs and absenteeism of diabetic ophthalmic conditions. Postgrad Med. 2015;127(5):455–462. | ||
Cardarelli WJ, Smith RA. Managed care implications of age-related ocular conditions. Am J Manag Care. 2013;19(5 Suppl):S85–S91. | ||
Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond). 2004;18(10):963–983. | ||
Boyer DS, Yooh YH, Belfort R Jr, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014;121(10):1904–1914. | ||
Diabetic Retinopathy Clinical Research Network; Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077. | ||
Bergstralh EJ, Kosanke JL. Computerized Matching of Cases to Controls. Technical Report Series No. 56. Rochester, NY: Department of Health Science Research, Mayo Clinic; 1995. | ||
Nguyen-Khoa BA, Goehring EL, Werther W, et al. Hospitalized cardiovascular events in patients with diabetic macular edema. BMC Ophthalmol. 2012;12(1):11. | ||
Lee LJ, Yu AP, Cahill KE, et al. Direct and indirect costs among employees with diabetic retinopathy in the United States. Curr Med Res Opin. 2008;24(5):1549–1559. | ||
Kiss S, Campbell J, Almony A, et al. Real-world outcomes in diabetic macular edema treated with anti-vascular endothelial growth factors: an analysis of EMR data from a large integrated US health system. Presented at: American Academy of Ophthalmology Annual Meeting; October 18–21; 2014; Chicago, IL, USA. | ||
Kiss S, Chandwani H, Almony A, et al. Clinical utilization of anti-VEGFs in the treatment of diabetic macular edema: a claims-based analysis. Presented at: Academy of Managed Care Pharmacy 2014 Nexus Meeting; October 7–10; 2014; Boston, MA, USA. | ||
Kiss S, Liu Y, Brown J, et al. Clinical monitoring of patients with age-related macular degeneration treated with intravitreal bevacizumab or ranibizumab. Ophthalmic Surg Lasers Imaging Retina. 2014;45(4):285–291. | ||
Holekamp NM, Liu Y, Yeh WS, et al. Clinical utilization of anti-VEGF agents and disease monitoring in neovascular age-related macular degeneration. Am J Ophthalmol. 2014;157(4):825–833. | ||
Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–1340. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





