Back to Journals » OncoTargets and Therapy » Volume 10
Combined detection of dickkopf-1 subtype classification autoantibodies as biomarkers for the diagnosis and prognosis of non-small cell lung cancer
Authors Shen L , Wu X, Tan J, Gu M, Teng Y , Wang Z, Yue W
Received 7 February 2017
Accepted for publication 21 April 2017
Published 18 July 2017 Volume 2017:10 Pages 3545—3556
DOI https://doi.org/10.2147/OTT.S134162
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Lei Shen,1,* Xiaoguang Wu,2,* Jinjing Tan,1 Meng Gu,1 Yu Teng,1 Zitong Wang,3 Wentao Yue1,4
1Department of Cellular and Molecular Biology, Beijing Tuberculosis and Thoracic Tumor Research Institute, 2Department of Ward 2, 3Department of Thoracic Surgery, Beijing Chest Hospital, Beijing, 4Central Laboratory, Beijing Obstetrics and Gynecology Hospital, Capital Medical University, Chaoyang, Beijing, People’s Republic of China
*These authors contributed equally to this work
Purpose: This study aims to identify the clinical significance of serum autoantibodies against dickkopf-1 (DKK1) and evaluate their feasibility in the immunodiagnosis and prognosis of non-small cell lung cancer (NSCLC).
Experimental design: Epitope mapping by peptide microarray-based serum screening of NSCLC patients (n=72) and healthy controls (n=16) was performed. Indirect ELISA with peptides was used to measure the serum levels of autoantibodies in 206 NSCLC patients and 99 healthy controls. A 3-year follow-up was monitored to evaluate the correlation between serological levels of autoantibodies and overall survival (OS) and progression-free survival (PFS).
Results: Four highly reactive epitopes were identified, which included peptides 67–84 (Pep A), 37–54 (Pep B), 145–156 (Pep C) and 247–261 (Pep D). The autoantibodies levels were considerably higher in sera of NSCLC patients compared with controls (P<0.001), and a highly significant correlation with distant metastases was observed (Pep A: P=0.09, Pep B: P<0.01, Pep C: P<0.01 and Pep D: P<0.01). High levels of antibody subtype to Pep B were remarkably associated with better OS (P=0.004) and PFS (P=0.006). Subsequent Cox regression analysis disclosed that antibody to Pep B was an independent prognostic factor for NSCLC (OS: P=0.008, HR =0.435, 95% CI 0.236–0.802; PFS: P=0.032, HR =0.533, 95% CI 0.322–0.950).
Conclusion: Identified linear epitopes of antigens by peptide microarray are easily available, and subtype classification of DKK1 autoantibodies as novel biomarkers for the diagnosis and prognosis of NSCLC. Our results also highlight the antibody subtype to Pep B as the most valuable biomarker for favorable prognosis of NSCLC.
Keywords: dickkopf-1, DKK1, autoantibodies, NSCLC, tumor immunity, antigenic sites
Introduction
Lung cancer has been the most common malignant cancer in terms of both morbidity and mortality worldwide. Non-small cell lung cancer (NSCLC) accounts for ~80% of all patients with lung cancer.1 Due to the paucity of clinically specific symptoms of early disease and effective screening methods, 80% of lung cancer patients were diagnosed with late-stage disease.2 Because of distant metastases, 5-year survival rate following curative tumor resection of stage I was ~60% and for stage II was 40%.3 Therefore, identification of sensitive biomarkers and effective methods for early detection and prognosis evaluation are warranted.
Serum carcinoembryonic antigen, neuron-specific enolase and cytokeratin-19 fragments 21–1 were introduced as biomarkers for the detection of NSCLC. However, these were not considered as appropriate screening biomarkers due to the lack of adequate diagnostic values for NSCLC. In contrast to these antigens, autoantibodies reflect both the pathophysiology of the disease and the hosts’ immune response.4,5 The induction of autoantibodies represents amplification of the antigenic signal.6 High levels of autoantibodies were detected at 5 years before patients developed lung cancer.7 Several studies have demonstrated that circulating autoantibodies banding to tumor-associated antigens may have the potential to serve as biomarkers in the diagnosis and prognosis of malignant diseases.8–10 However, the efforts to diagnose cancer and evaluate the prognosis by using autoantibodies were less successful. Furthermore, most of the studies to date analyzing the antibodies have used enzyme-linked immunosorbent assay (ELISA) for detecting all immunoglobulin antibodies with the antigens.
Dickkopf-1 (DKK1) is a secretory protein, which is known to be the antagonist that inhibits the canonical Wnt signaling pathway, and plays a key role in a variety of cellular processes, such as proliferation, differentiation, survival, apoptosis and cell motility.11,12 In recent years, studies have demonstrated the role of DKK1 in the diagnosis of malignancy and in cancer progression. Also, it is abundantly expressed in diverse carcinomas including multiple myeloma, liver carcinoma, breast cancer, pancreas cancer, lung cancer and so on.13–18 ELISA was developed using recombinant DKK1 proteins as antigens for the detection of autoantibodies in plasma. Previous studies showed that DKK1 autoantibodies could serve as diagnostic biomarkers in NSCLC and esophageal squamous cell carcinoma.19,20 However, the association between DKK1 autoantibodies and the prognosis in cancer is still unclear.
In present study, we utilized peptide microarray to identify DKK1 autoantibody epitopes and detect the antibodies that are linked to the epitopes resulting from DKK1. Then, we evaluated the clinical significance of DKK1 autoantibodies subtypes in NSCLC. Our result showed that the linear peptide antigens identified by peptide microarray are easily available. Furthermore, the antibody to Pep B, more than other DKK1 autoantibodies, was the most valuable biomarker for favorable prognosis of NSCLC.
Materials and methods
Patients and samples
Inclusion criteria for the study population were as follows: 1) patients confirmed with NSCLC by histopathological examination (such as bronchoscopy, biopsy or surgery) and 2) patients with no history of other cancers. Exclusion criteria were as follows: patients who received preoperative chemotherapy or radiation and patients without follow-up information. The present study was approved by the Research Ethics Committee in Beijing Chest Hospital, and written informed consent was obtained from each subject in advance for this study.
Serum samples were collected from 278 NSCLC patients and 115 healthy controls at the Beijing Chest Hospital (Beijing, People’s Republic of China) between January 2009 and March 2010 and were analyzed retrospectively. Tumor stage was determined according to the 7th edition of the tumor-node-metastasis (TNM) staging system for lung cancer.21 Of all the NSCLC patients, the follow-up period was limited to 3 years. We used overall survival (OS) as the primary end point and progression-free survival (PFS) as the secondary end point. Thirty-six patients were lost to follow-up at the end of the study. The details of demographics, clinical pathological characteristics and follow-up data of these patients were extracted from medical records. Detailed clinical pathological data were presented in Table 1. All the samples were stored frozen at −80°C.
Peptide microarray preparation
The DKK1 protein sequence is searched on the UniProt (UniProtKB-O94907, Figure 1C). The peptide libraries were synthesized by an automated SPOT synthesizer (ASP SL, Intavis, Köln, Germany), according to the manufacturer’s instructions. The protein sequence was translated into 12-mer peptides with a peptide–peptide overlap of 9 amino acids. According to the SPOT-synthesis technique, fmoc-protected and activated amino acids were spotted onto the cellulose membranes to form a 4×22 array. Each subarray has two flags (MDYKDDDDK), one at the beginning and one at the terminal, which were used as positive controls (Figure S1 and Table S1). A commercial monoclonal anti-DKK1 antibody (ab109416, Abcam, Cambridge, UK) was used to validate the DKK1 microarrays.
Arrays for serological screening of NSCLC patients and healthy controls
First, 95% trifluoroacetic acid, 3% triisobutylsilane and 2% water were used for side chain deprotection and then were incubated in blocking buffer (5% skim milk) at room temperature for 4 h. Next, the microarray was rinsed four times with phosphate-buffered saline with Tween 20 and the microarrays incubated with human pool serum (1:1,000 dilution) in 5% skim milk overnight at 4°C. Following incubation with serum, we stained the microarrays with horseradish peroxidase (HRP)-conjugated goat anti-Human immunoglobulin G (IgG; 628420, 1:2,000 dilution; Thermo Fisher Scientific, MA, USA) and Anti-DDDDK tag antibody (ab49763, 1:2,000 dilution; Abcam) in 5% skim milk for another 4 h and then they were washed as described before. The microarrays were added with electrochemical luminescence reagent, and chemiluminescence signals were acquired with an AlphaView Imaging System (Fluor Chem SP; Alpha Innotech, San Leandro, CA, USA). Each peptide spotted on the microarray was processed using TotalLab TL100 software to calculate the chemiluminescence intensity. Positive seroreactivity was defined as an intensity value greater than half of the positive controls.
Indirect peptide ELISA for DKK-1 autoantibodies
The optimal antigen coating concentration and the optimal serum dilution for the ELISA on autoantibody test were determined using a checkerboard titration in the preliminary studies. The peptide antigens were synthesized by GL Biochem (Shanghai, People’s Republic of China) with a purity of >95% and were verified by mass spectrometry. The synthetic peptides were dissolved in acetic acid to obtain a concentration of 1 mg/mL as stocking solution and were kept at 4°C. Wells were coated with 100 μL of peptides and were diluted in 0.8 ng/mL PBS-based coating buffer overnight at 4°C. The solution was removed, and the wells were washed three times with PBS-T. The wells were blocked with 300 μL of 5% skim milk for 1 h at 37°C and then 5% skim milk was removed. One hundred microliter plasma samples diluted at 1:100 in 5% skim milk and 100 μL 5% skim milk were added to the negative control wells and were then incubated overnight at 4°C. After washing three times, HRP-conjugated goat anti-Human IgG was used as secondary antibodies at a dilution of 1:2,000. After incubating at 37°C for 1.5 h, color development was initiated by adding 100 μL 3,3′,5,5′-tetramethylbenzidine solution (CW0050; CWBIO, Beijing, People’s Republic of China) and then was terminated after 25 min by adding 50 μL 2M H2SO4. Finally, the OD was measured on a microplate reader within 10 min at 450 nm with a reference wavelength of 620 nm. Each sample was conducted on duplicate wells.
To minimize an intra-assay deviation, the ratio of the difference between duplicated OD values to their sum was utilized for examining the precision for assay of each sample. If the ratio was >10%, the value of this sample was treated as being invalid and would not be used for data analysis. The inter-assay deviation was valued using a pooled serum sample, and the inter-assay coefficient of variation should be below 16%.
Competitive ELISA
Human pool serum obtained from two reactive patient serum samples was diluted 1:100 in PBS-T, and then pre-incubated with excess recombinant DKK1 protein, a scrambled control (bovine serum albumin) overnight at 4°C. The molar ratio of DKK1 and peptides was in 1:1, 3:1 and 10:1 ratios, respectively. Pep A, Pep B, Pep C, and Pep D were used to coat 96-well plates at 0.8 ng/μL. Following incubation, the samples were used to probe the plate in triplicate. HRP-conjugated goat anti-Human IgG was used as secondary reagent at the dilution of 1:2,000, and the OD was measured on a microplate reader.
Statistical analysis
All statistical analyses were carried out using SPSS software version 22.0 or Prism 6.0 software (GraphPad, La Jolla, CA, USA). The normality of the data was assessed by the Kolmogorov–Smirnov test. Kruskal–Wallis and Mann–Whitney U tests were used to evaluate differences between the NSCLC and healthy controls. A two-sided P-value <0.05 was deemed to be statistically significant. The concentrations of the serum autoantibodies of the two groups are presented as median and interquartile ranges. Receiver operating characteristic (ROC) analysis was performed to assess sensitivity, specificity and area under the ROC curve (AUC) with 95% CI. The optimal cut-off value was determined based on ROC analysis and the Youden index. PFS was defined as the time between the date of diagnosis and the date of disease progression, death or the last follow-up visit. OS was defined as the time between the date of diagnosis and date of death from any cause and the last follow-up visit. PFS and OS were established by Kaplan–Meier method and differences were calculated via the log-rank test. Cox’s proportional hazards model was applied to perform univariate and multivariate analyses while adjusting for other clinical covariates.
Results
Epitope mapping by peptide array-based immunological screening
To identify autoantibodies linked with epitopes within the DKK1, we probed DKK1 arrays with pooled sera from patients with NSCLC (n=72) and healthy controls (n=16). As shown in Figure 1A, the spots A1 and D22 are the positive controls. Serological screening showed distinct binding patterns between NSCLC patient samples and healthy controls. Most peptides were strongly reactivated in NSCLC patients but weakly in healthy controls. The results were verified by DKK1 microarrays using a commercial monoclonal antibody which recognized the regions of DKK1 that are represented on the microarrays. As shown in Figure 1A, the result indicated that DKK1 monoclonal antibody responded to the spots D16–D19 (amino acids 241–261), which were located in the epitope of the antibody (amino acids 150–266). Figure 1B showed the coverage plot of the response of 86 DKK1 peptides in NSCLC and healthy serum samples. This plot has provided a comprehensive overview of diverse binding patterns between NSCLC patients, with outcomes of multiple epitopes with high coverage (Figure 1C).
Subgroup analysis of DKK1 autoantibodies in NSCLC patients and healthy controls
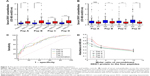
DKK1 autoantibodies were tested with four linear peptides in 206 NSCLC patients and 99 healthy controls. As shown in Figure 2A, compared with control group, the levels of IgG antibodies in response to the four peptides were considerably higher in patients with NSCLC (P<0.001). To further understand the relationship between the levels of IgG antibodies in response to the peptides and tumor progression, the patients were subdivided into M0 and M1 groups. The levels of IgG antibodies to Pep B, Pep C and Pep D were tightly focused to be higher in M0 patients than in M1 patients (P<0.01, Figure 2B), implying that the serum IgG antibodies to Pep B, Pep C and Pep D are predictors of tumor aggressiveness. Furthermore, as indicated in Table 1, the serum levels of IgG antibodies to Pep B, Pep C and Pep D were significantly correlated with the clinical pathological variables, especially with the clinical stages and distant metastasis.
Furthermore, we investigated the specificity of DKK1 autoantibodies detection in ELISA. The patient serum samples were estimated to have positive reactivity in response to the four peptides and were preincubated with an excess amount of recombinant DKK1 protein, and at OD450, reduction in a dose-dependent manner was observed (Figure 2D). However, OD450 was not changed in scrambled control (Figure S2). These results suggested that the indirect peptide ELISA we developed specifically detects DKK1 autoantibodies.
Subtypes of DKK1 autoantibodies are associated with better progression in NSCLC patients
We subsequently investigated whether the serum levels of DKK1 autoantibodies could be of any prognostic relevance in NSCLC. Kaplan–Meier curves were used to compare OS and PFS between NSCLC with DKK1 antibody positive and antibody negative. As shown in Figure 3, better OS and PFS were noted following positive antibody to Pep B compared with negative antibody of Pep B in NSCLC (P=0.004 and P=0.006, respectively). The overall median survival time was 32 months (95% CI 22.2–41.8 months) for the group with positive antibody to Pep B and 17 months (95% CI 9.1–24.9 months) for the group with negative antibody to Pep B. The median PFS time was 16 months (95% CI 12.1–19.9 months) for the group with positive antibody to Pep B and 8 months (95% CI 5.3–10.7 months) for the group with negative antibody to Pep B. However, no statistical significances in OS and PFS were found in the other three peptides. A Cox proportional hazards analysis was used to further evaluate the potential of serum DKK1 autoantibodies as independent prognostic biomarkers. The multivariate Cox proportional hazards analysis (shown in Table 2) suggested that patients with positive antibody to Pep B had a decreased risk of death compared with patients with negative antibody to Pep B (P=0.001, HR =0.471, 95% CI 0.298–0.743). Furthermore, positive antibody to Pep B was independently associated with prolonged PFS time (P=0.033, HR =0.647, 95% CI 0.433–0.965). However, autoantibody subtype analysis did not reveal any association of antibody to Pep A, C and D levels with OS and PFS.
Potential of DKK1 autoantibodies as immunobiomarkers for lung cancer detection
To assess the predictive value of the four peptides, a number of ROC analyses were performed (Figure 2C). The results suggested that DKK1 autoantibodies may serve as potential biomarkers for the immunodiagnosis of NSCLC. We also assessed the diagnostic performance when combined with four peptides. This combined diagnostic model increased the AUC to 0.821 (95% CI 0.764–0.868), with an overall sensitivity of 58.11% and a specificity of 85.53%. In addition, there were relatively higher serum DKK1 autoantibodies levels in patients with early-stage NSCLC (stages I/II) than those in healthy controls (P<0.001). To further investigate whether DKK1 autoantibodies possess early diagnostic values for NSCLC, the cases were subdivided into early-stage (I and II) and advanced-stage (III and IV) lung cancer according to the clinical stage. We observed similar diagnostic performances of DKK1 autoantibodies in the early-stage NSCLC patients (Table 3).
  | Table 3 Results for measurement of autoantibodies against the four peptides, or all the four peptides in the diagnosis of NSCLC patients |
Discussion
In the current study, we conducted a peptide microarray to detect the circulating antibodies to DKK1 epitopes. Four highly responsive epitopes have been identified. Autoantibodies to DKK1-derived peptides were grouped into four subtypes and the corresponding antibodies were then characterized. Autoantibody induction to Pep B was observed to be significant with a reduced risk of metastases and increased OS, suggesting that autoantibodies to Pep B may play a role in the development and progression of NSCLC.
No prognostic studies of DKK1 autoantibodies have been published to date, and there were limited studies published regarding the analysis of diagnostic values. Furthermore, most of the studies have detected all immunoglobulin antibodies to recombinant antigens and limited studies using antigen-derived peptides with limited sample sizes have been published.10,19,20,22,23 The correlation of autoantibodies with prognosis is rather contradictory across studies. For example, several studies have reported the antibody to tumor-suppressor antigen p53 to be correlated with worse prognosis in ovarian cancer,24,25 but showed a better prognosis in others.26,27 In the present study, we subdivided DKK1 autoantibodies into four IgG subsets according to the response to the four linear epitopes derived from DKK1. Our results showed that different IgG subsets may play a different role in NSCLC, which may exactly explain the association of autoantibodies with prognosis. The utility of peptides to detect autoantibodies and subtypes division could be more efficient to determine the prognostic significance. Importantly, this study was the first to identify epitopes recognized by DKK1 autoantibodies.
Our data (Table 1) showed that the presence of antibodies to Pep B, Pep C and Pep D in NSCLC patients was associated with TNM stage and distant metastasis. Also, we enrolled an unselected cohort of patients with distant metastases (stage M1) in the present study and consequently found significantly lower DKK1 autoantibodies serological levels compared with those in the non-metastatic cases (stage M0). These results indicated that these antibodies may be functional in suppressing the progression. DKK1 consists of conserved NH2-terminal (N1) and COOH-terminal (C1) cysteine-rich regions.28 N1 lacks the Wnt-antagonizing activity, and the C1 and N1 domains in DKK1 are well known for activating distinct signaling pathways. The study by Sato et al29 found that the function of NH2-terminal with 120 amino acids of DKK1 was involved in the enhanced invasive activity and antiapoptotic signaling pathway. In fact, anti-Pep B antibody was immunized against an NH2-terminal portion of DKK1 protein (amino acids 37–54). We speculate that anti-Pep B antibody could bind to N1 domains in DKK1 and neutralize its function. Further investigation of this interaction is required to elucidate the mechanism of DKK1 autoantibodies efficacy.
In Phase I clinical study, DKN-01, a monoclonal antibody that targets extracellular DKK1, has demonstrated clinical efficacy in patients with advanced NSCLC.30 This indicates that DKK1 may be an important therapeutic target in NSCLC. In addition, tumor vaccine therapy based on two DKK1 peptides showed that the DKK1 peptides which are effective immunogens could induce specific endogenous T cells from multiple myeloma patients.31 The two DKK1 peptides were predicted by a peptide-binding database32 and Pep A contained one of the peptides. However, antibodies to Pep A showed no effect on tumor progression in NSCLC. The protective effect of DKK1 autoantibodies may be mediated by antitumor immunity. A previous report has shown that autoantibodies may promote antibody-dependent cellular cytotoxicity and induce antitumor immunity.33 Thus, we hypothesized that such immune responses against DKK1 epitopes may also impart a survival benefit. The strategy of targeting DKK1 by tumor vaccine will become a new therapeutic method.
In recent years, autoantibodies to tumor-associated antigen have been widely studied for the early diagnosis as a complement to mammography. Autoantibodies have also been detected before the occurrence of symptomatic disease. This study develops a sensitive peptide using indirect ELISA, which has the same diagnostic power with the conventional ELISA using recombinant DKK1 protein. Whereas compared with recombinant protein, the linear peptide antigens had small molecular sizes and are easily synthesized. Meanwhile, they are completely exposed to antibodies and with low backgrounds to detect autoantibodies. In addition, patients with early-stage NSCLC (stage I/II) also had the high positivity for DKK1 autoantibodies (Table 2). The early diagnostic value of DKK1 autoantibodies has also been identified in esophageal squamous cell carcinoma.20 These observations suggest that DKK1 autoantibody may be a novel member of potential biomarkers in the early diagnosis of NSCLC. However, there were some limitations to the study. Due to its single-center retrospective nature, it is difficult to collect ideal control serum samples for use. The age demographics of healthy controls results in the mean age of these people being significantly younger than the NSCLC patients. The healthy control serum samples were collected at the same site and at the same time as the NSCLC patients, which thus reflects the same population in both cohorts. The insufficient sensitivity and specificity of the DKK1 peptide-based serological examinations should be improved. Recently established evidence indicates that using a panel of tumor-associated antigens could enhance the sensitivity and specificity of autoantibodies detection in cancer.34 Further studies are required to identify more highly specific autoantibodies and then develop a panel of biomarkers for early diagnosis of NSCLC.
In conclusion, our data demonstrate that patients have detectable tumor antigen-specific antibody immunity and that immunity to DKK1 epitopes may have different association with prognosis. Serological levels of DKK1 autoantibodies subtypes could provide precise evaluation for the prognosis of NSCLC. Furthermore, this study indicates that naturally occurring DKK1 autoantibodies may have protective effect on the development and metastasis of NSCLC. Induction of endogenous DKK1 autoantibodies may be of clinical benefit for NSCLC. Further study on such therapy is encouraged.
Acknowledgment
Wentao Yue was supported by The National Natural Science Foundation of China (No 81672838), The Capital Health Research and Development of Special (No 2014–2-1041), and The Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1):9–29. | ||
Parkin DM, Ferlay J, Curado MP, et al. Fifty years of cancer incidence: CI5 I-IX. Int J Cancer. 2010;127(12):2918–2927. | ||
Strauss GM. Adjuvant chemotherapy of lung cancer: methodologic issues and therapeutic advances. Hematol Oncol Clin North Am. 2005;19(2):263–281. | ||
Caron M, Choquet-Kastylevsky G, Joubert-Caron R. Cancer immunomics using autoantibody signatures for biomarker discovery. Mol Cell Proteomics. 2007;6(7):1115–1122. | ||
Finn OJ. Immune response as a biomarker for cancer detection and a lot more. N Engl J Med. 2005;353(12):1288–1290. | ||
Belousov P, Kuprash D, Nedospasov S, Shebzukhov Y. Autoantibodies to tumor-associated antigens as cancer biomarkers. Curr Mol Med. 2010;10(2):115–122. | ||
Zhong L, Coe SP, Stromberg AJ, Khattar NH, Jett JR, Hirschowitz EA. Profiling tumor-associated antibodies for early detection of non-small cell lung cancer. J Thorac Oncol. 2006;1(6):513–519. | ||
Ma L, Yue W, Teng Y, Zhang L, Gu M, Wang Y. Serum anti-CCNY autoantibody is an independent prognosis indicator for postoperative patients with early-stage nonsmall-cell lung carcinoma. Dis Markers. 2013;35(5):317–325. | ||
Blixt O, Bueti D, Burford B, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13(2):R25. | ||
Qi S, Huang M, Teng H, et al. Autoantibodies to chromogranin A are potential diagnostic biomarkers for non-small cell lung cancer. Tumour Biol. 2015;36(12):9979–9985. | ||
Niehrs C. Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene. 2006;25(57):7469–7481. | ||
Niida A, Hiroko T, Kasai M, et al. DKK1, a negative regulator of Wnt signaling, is a target of the beta-catenin/TCF pathway. Oncogene. 2004;23(52):8520–8526. | ||
Tian E, Zhan F, Walker R, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med. 2003;349(26):2483–2494. | ||
Li S, Qin X, Guo X, et al. Dickkopf-1 is oncogenic and involved in invasive growth in non small cell lung cancer. PLoS One. 2013;8(12):e84944. | ||
Rachner TD, Thiele S, Gobel A, et al. High serum levels of Dickkopf-1 are associated with a poor prognosis in prostate cancer patients. BMC Cancer. 2014;14:649. | ||
Huang Y, Yang X, Zhao F, et al. Overexpression of Dickkopf-1 predicts poor prognosis for patients with hepatocellular carcinoma after orthotopic liver transplantation by promoting cancer metastasis and recurrence. Med Oncol. 2014;31(7):966. | ||
Han SX, Zhou X, Sui X, et al. Serum dickkopf-1 is a novel serological biomarker for the diagnosis and prognosis of pancreatic cancer. Oncotarget. 2015;6(23):19907–19917. | ||
Voorzanger-Rousselot N, Goehrig D, Journe F, et al. Increased Dickkopf-1 expression in breast cancer bone metastases. Br J Cancer. 2007;97(7):964–970. | ||
Yao X, Jiang H, Zhang C, et al. Dickkopf-1 autoantibody is a novel serological biomarker for non-small cell lung cancer. Biomarkers. 2010;15(2):128–134. | ||
Peng YH, Xu YW, Guo H, et al. Combined detection of serum Dickkopf-1 and its autoantibodies to diagnose esophageal squamous cell carcinoma. Cancer Med. 2016;5(7):1388–1396. | ||
Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15(1):4–9. | ||
Li P, Shi JX, Dai LP, et al. Serum anti-MDM2 and anti-c-Myc autoantibodies as biomarkers in the early detection of lung cancer. Oncoimmunology. 2016;5(5):e1138200. | ||
Zhang C, Ye L, Guan S, et al. Autoantibodies against p16 protein-derived peptides may be a potential biomarker for non-small cell lung cancer. Tumour Biol. 2014;35(3):2047–2051. | ||
Vogl FD, Frey M, Kreienberg R, Runnebaum IB. Autoimmunity against p53 predicts invasive cancer with poor survival in patients with an ovarian mass. Br J Cancer. 2000;83(10):1338–1343. | ||
Mayerhofer K, Tempfer C, Kucera E, et al. Humoral p53 antibody response is a prognostic parameter in ovarian cancer. Anticancer Res. 1999;19(1B):875–878. | ||
Anderson KS, Wong J, Vitonis A, et al. p53 autoantibodies as potential detection and prognostic biomarkers in serous ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2010;19(3):859–868. | ||
Goodell V, Salazar LG, Urban N, et al. Antibody immunity to the p53 oncogenic protein is a prognostic indicator in ovarian cancer. J Clin Oncol. 2006;24(5):762–768. | ||
Glinka A, Wu W, Delius H, Monaghan AP, Blumenstock C, Niehrs C. Dickkopf-1 is a member of a new family of secreted proteins and functions in head induction. Nature. 1998;391(6665):357–362. | ||
Sato N, Yamabuki T, Takano A, et al. Wnt inhibitor Dickkopf-1 as a target for passive cancer immunotherapy. Cancer Res. 2010;70(13):5326–5336. | ||
Edenfield WJ, Richards DA, Vukelja SJ, et al. A phase 1 study evaluating the safety and efficacy of DKN-01, an investigational monoclonal antibody (Mab) in patients (pts) with advanced non-small cell lung cancer. J Clin Oncol. 2014;32(15_suppl):8068–8068. | ||
Qian J, Zheng Y, Zheng C, et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood. 2012;119(1):161–169. | ||
Qian J, Xie J, Hong S, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood. 2007;110(5):1587–1594. | ||
Tabuchi Y, Shimoda M, Kagara N, et al. Protective effect of naturally occurring anti-HER2 autoantibodies on breast cancer. Breast Cancer Res Treat. 2016;157(1):55–63. | ||
Chapman CJ, Healey GF, Murray A, et al. EarlyCDT(R)-Lung test: improved clinical utility through additional autoantibody assays. Tumour Biol. 2012;33(5):1319–1326. |
Supplementary materials
  | Table S1 Peptide sequences corresponding to each dot on the microarray |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.