Back to Journals » OncoTargets and Therapy » Volume 11
Combination chemotherapy with Zyflamend reduced the acquired resistance of bladder cancer cells to cisplatin through inhibiting NFκB signaling pathway
Authors Xue Y, Yang L, Li J, Yan Y, Jiang Q, Shen L, Yang S, Shen B, Huang R, Yan J , Guo H
Received 11 January 2018
Accepted for publication 20 April 2018
Published 30 July 2018 Volume 2018:11 Pages 4413—4429
DOI https://doi.org/10.2147/OTT.S162255
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Video abstract presented by Xue et al.
Views: 755
Yanshi Xue,1,* Lin Yang,2 Junzun Li,3 Yilin Yan,4 Qinghui Jiang,5,6 Lan Shen,3 Shuai Yang,3 Bing Shen,4 Ruimin Huang,5,6 Jun Yan,3,7,* Hongqian Guo1
1Department of Urology, Nanjing Drum Tower Hospital, Clinical College of Nanjing Medical University, Nanjing, China; 2Department of Urology, Nanjing Drum Tower Hospital, Nanjing University Medical School, Institute of Urology, Nanjing University, Nanjing, China; 3MOE Key Laboratory of Model Animals for Disease Study and State Key Laboratory of Pharmaceutical Biotechnology, Model Animal Research Center of Nanjing University, Nanjing, China; 4Department of Urology, Shanghai General Hospital, Shanghai Jiaotong University, Shanghai, China; 5University of Chinese Academy of Sciences, Beijing, China; 6Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, China; 7Collaborative Innovation Center of Genetics and Development, Shanghai, China
*These authors equally contributed to this work
Background: Cisplatin-based chemotherapy is mainstay treatment in urinary bladder cancer (UBC). However, tumor recurrence frequently occurs with the acquisition of cisplatin resistance. We explored the potential effect of a polyherbal preparation, Zyflamend, on UBC cells resistant to cisplatin treatment.
Methods: To establish a cisplatin-resistant human bladder cancer cell line, T24 cells were cultured in increasing concentrations of cisplatin for more than 10 months. These cells (T24R) were then treated with different concentrations of Zyflamend, and both proliferation and activity of nuclear factor kappaB (NFκB) signaling pathway were examined. To test the synergistic effect between Zyflamend and cisplatin, we treated T24R cells either with Zyflamend or cisplatin alone, or in combination. Apoptotic effect was evaluated by Annexin V/propidium iodide double staining, and the levels of the proteins involved in cell cycle and anti-apoptosis were examined by Western blotting. Finally, mice with palpable xenograft were treated either with cisplatin and Zyflamend alone or in combination for 28 days before they were sacrificed for measuring the sizes and weights of the tumor tissues. In addition, proliferation and apoptosis markers were examined by immunohistochemistry.
Results: Comparing to that in the parental T24 cells, NFκB is constitutively active in cisplatin-resistant T24R cells. Zyflamend is capable of inhibiting the growth of T24, T24R, as well as another UBC cell line J82 in a concentration-dependent manner. Mechanistically, Zyflamend suppresses NFκB-mediated cell proliferation, survival, and invasion/angiogenesis and induces apoptosis. In addition, Zyflamend significantly increased the sensitivity of T24R and J82 cells to cisplatin treatment and these findings were confirmed in T24R xenograft model with reduced proliferation index and decreased expression of RelA and its downstream target MMP9.
Conclusion: Zyflamend is capable of counteracting bladder cancer resistance to cisplatin by repressing proliferation and inducing apoptosis through targeting NFκB signaling pathway.
Keywords: Zyflamend, cisplatin, bladder cancer, NFκB, acquired resistance
Introduction
Urinary bladder cancer (UBC) is one of the most malignant tumors in the USA, with 58,950 new cases and 11,820 UBC-related deaths in 2016.1 In China, UBC prevalence in the general population and people older than 65 years was ranked 9th and 2nd, respectively.2 At diagnosis, ~70% of UBC patients have non-muscle invasive bladder cancer, while the other 30% have muscle invasive bladder cancer (MIBC).3,4 Since radiotherapy has very limited effects for MIBC, chemotherapy is currently the mainstay of treatment for this disease. However, the mechanisms of acquired resistance to chemotherapy are still unclear.5 The mechanistic understanding may help to identify the novel drug combination strategies to overcome the acquired drug resistance in UBCs.
The nuclear factor kappaB (NFκB)/Rel family is composed of five members in mammals, RelA/p65, c-Rel, RelB, NFκB1 (p105/p50), and NFκB2 (p100/p52).6,7 Rel proteins bind to p50 and p52, which are proteolytically processed by proteasome from p105 and p100, and form heterodimer. Once the stimuli exist, cytosolic-localized NFκB complex is released from the tether of NFκB inhibitor α (IκBα) protein and translocates into nucleus, where it binds to its cognate DNA motif and transcriptionally regulates the downstream targets including Bcl2, cyclin D1, vimentin, MMP9, and VEGFa.8,9 NFκB plays important roles in various cellular processes, including survival, inflammation, and angiogenesis.10,11 Therefore, dysregulation of NFκB signaling pathway has been implicated in both cancer development and chemoresistance. Meanwhile, NFκB also serves as a promising drug target in cancer therapy.12–14
As a formulated dietary supplement, polyherbal Zyflamend contains 10 standardized herbal extracts: holy basil, turmeric, ginger, green tea, rosemary, Hu Zhang, barberry, oregano, Baikal skullcap, and Chinese goldthread. Others and we have reported that Zyflamend possesses anticancer property against prostate and pancreatic cancers either through its anti-inflammatory or anti-androgen receptor effects.15–18 However, whether Zyflamend has anticancer effect on UBC cells, particularly on the cisplatin-resistant UBC cells, has not been explored.
In this study, an acquired cisplatin-resistant UBC cells (T24R) was established using the human UBC cell line (T24) by repeated selection with elevated concentration of cisplatin for 10 months and NFκB/RelA activation was observed in these cells. Importantly, our in vitro and in vivo experiments showed that by inhibiting NFκB signaling pathway Zyflamend is capable of counteracting acquired cisplatin-resistance.
Materials and methods
Human UBC cell lines and reagents
Human UBC cell lines T24 and J82 were obtained from the Cell Bank of Type Culture Collection, Chinese Academy of Science (Shanghai, China) and maintained in RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) with 10% fetal bovine serum (Hyclone Laboratories, South Logan, UT, USA). To generate cisplatin-resistant T24 (T24R) cells, T24 cells were initially exposed to 0.01 μg/mL cisplatin and the dose was gradually increased over 10 months. Finally, we obtained T24R cells viable in the presence of 2 μg/mL cisplatin. Cells were incubated at 37°C in an atmosphere of 5% CO2 and 95% air. Zyflamend (New Chapter, Brattleboro, VT, USA) was dissolved in DMSO at a stock concentration of 10 mg/mL and stored at −80°C in separate aliquots. Cisplatin (EY0024; Shanghai Amquar Biological Technology, Shanghai, China) was dissolved in 0.9% NaCl solution at a stock concentration of 2 mg/mL and stored at −20°C in aliquots.
Cell viability assay
T24, T24R, and J82 cells were seeded into 96-well plates at 2,000 cells per well. Then, 1 day after cell inoculation, Zyflamend (0–1,200 μg/mL) and cisplatin (0–16 μg/mL) were added, respectively. After cells were treated with Zyflamend and cisplatin individually or combined for 72 h, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; 5 mg/mL) was added for 2 h at 37°C. Then, the culture medium was removed and 100 μL DMSO was added to dissolve formazan crystals. Absorbance at 490 nm was examined using a microplate reader (BioTek Instruments, Winooski, VT, USA). Each treatment was performed in triplicates, and experiments were repeated at least 3 times. IC50 of each cell line to cisplatin or Zyflamend was calculated by Graphpad Prism software (Graphpad Software, Inc, La Jolla, CA, USA).
Combination index analysis
The anti-proliferative effects of the interaction between Zyflamend and cisplatin were assessed by measuring the combination index (CI).19 T24, T24R, and J82 cells were cultured in 96-well plates at a density of 2,000 cells/well overnight, respectively. Cells were treated with individual or combined Zyflamend (0–1,200 μg/mL) and cisplatin (0–8 μg/mL) at various concentrations as indicated. Then, 72 h later, cells were stained with 5 mg/mL MTT. DMSO was used to dissolve formazan crystal. The CI values by Chou-Talalay method were assessed using CompuSyn 1.0 software (ComboSyn, Paramus, NJ, USA). CI value <1, =1, and >1 indicates synergism, additive, and antagonism effects, respectively.
Colony formation assay
T24R and J82 cells were seeded in 6-well plates at a density of 1,000 cells/well. After 2 days, cells were treated with 100 μg/mL Zyflamend and/or 1 μg/mL cisplatin. After 48 h, the media were changed to regular culture media. After 7 days, cells were fixed with 7:1 methanol:acetic acid, and stained with 0.5% crystal violet (Sigma-Aldrich Co, St Louis, MO, USA). Only colonies with >50 cells were counted.
Western blotting
Cells were lysed in RIPA buffer containing phosphatase inhibitor cocktail I (Sigma-Aldrich Co) and protease inhibitor cocktail mini-tablet (Roche Diagnostics, Indianapolis, IN, USA). The supernatants were collected after centrifugation at 12,000 g at 4°C for 20 min. Then, 20 μg of protein lysates was separated by SDS-PAGE and transferred onto polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). After blocking with 5% non-fat milk in PBST, the primary antibodies for RelA (8242, dilution ratio: 1:1,000; Cell Signaling Technologies, Danvers, MA, USA), pRelA (S536) (3033, dilution ratio: 1:1,000; Cell Signaling Technologies), MMP2 (13132, dilution ratio: 1:1,000; Cell Signaling Technologies), MMP9 (13667, dilution ratio: 1:1,000; Cell Signaling Technologies), VEGFa (BS6496, dilution ratio: 1:1,000; Nanjing Bioworld Biotech Co. Ltd, Nanjing, China), c-Myc (BS4126, dilution ratio: 1:1,000, Bioworld), cyclin D1 (16663, dilution ratio: 1:1,000; Abcam, Cambridge, UK), vimentin (10366-1-AP, dilution ratio: 1:800, Proteintech, Chicago, IL, USA), cleaved caspase 3 (9661, dilution ratio: 1:1,000; Cell Signaling Technologies), PARP (9542, dilution ratio: 1:1,000; Cell Signaling Technologies), and actin (A1978, dilution ratio: 1:2,000; Sigma-Aldrich Co). The membranes were then washed with PBST 3 times and incubated with horseradish peroxidase-conjugated secondary antibody (dilution ratio: 1:2,000). The blots were visualized using the enhanced chemiluminescence reagents (WBKLS0100; EMD Millipore).
Cell death assay
Cell death rates were detected by Annexin V/propidium iodide (PI) staining kit (Cat # A211-01; Vazyme, Nanjing, China) according to manufacturer’s manual. Briefly, T24R or J82 cells were incubated in RPMI 1640 either with cisplatin or Zyflamend for 24 h. After cells were harvested and resuspended in 100 μL binding buffer, they were stained with 5 μL PI and 5 μL Annexin V at 4°C for 10 min in the dark. Cells were analyzed by flow cytometry within 60 min, and Annexin V-positive cells were defined as the dead cells.
Animal study
The experimental animal protocol was approved by the Institutional Animal Care and Use Committee of the Model Animal Research Center at Nanjing University and conducted in the animal facility of the Model Animal Research Center according to the animal experimental ethics committee guidelines of the Model Animal Research Center at Nanjing University. In total, 5×106 T24R cells were suspended in 50% Matrigel (BD Bioscience, San Jose, CA, USA) and subcutaneously inoculated into the dorsal flank of athymic nu/nu male mice (6-week-old). Then 1 week later, mice with palpable xenograft were randomly divided into 4 groups: 1) vehicle control (olive oil, 100 μL by gavage, daily); 2) cisplatin (5 mg/kg, once a week, intraperitoneal [ip] injection); 3) Zyflamend (1 g/kg, daily, per os [po]); 4) combined Zyflamend (1 g/kg, daily, po) and cisplatin (5 mg/kg, once a week, ip). Body weights and tumor dimensions were monitored every 3 days. Tumor volumes were calculated using the equation V =0.524 × width2 × length. The animals were sacrificed after drug treatment for 4 weeks.
Histological and immunohistochemistry (IHC) staining
For histological staining, 5 μm thick sections were deparaffinized and stained with hematoxylin and eosin. For IHC staining, sections were stained by the antibodies against Ki-67 (M7240, 1:1,000; DAKO), cleaved caspase 3 (9661, 1:1,000; Cell Signaling Technologies), RelA (8242, 1:1,000; Cell Signaling Technologies), and MMP9 (13667, 1:1,000; Cell Signaling Technologies) following the standard protocol.20
Statistical analysis
Each experiment was repeated 3 times. The mean values with SE from the triplicates were plotted. Statistical analysis was carried out using GraphPad Prism Software. Comparisons between groups were performed with 2-tailed Student’s t-test, and differences were considered statistically significant with P<0.05.
Results
Establishment of cisplatin-resistant UBC cell T24R
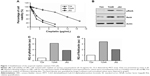
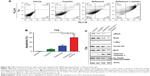
First, we successfully established a cisplatin-resistant cell line (T24R) from the parental T24 UBC cell line by culturing the cells with increasing concentrations of cisplatin over 10 months. Then, we examined the effect of cisplatin on these cells by measuring IC50 of different cells. As shown in Figure 1A, T24R cells were the most resistant cells to cisplatin with an IC50 of 11.53 μg/mL, which was 7.2-fold higher than that of the parental T24 cells (IC50=1.61 μg/mL). In addition, IC50 for J82 cells to cisplatin was 2.40 μg/mL, right between T24 and T24R cells.
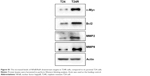
Constitutive activation of NFκB has been implicated in carcinogenesis and drug resistance in many cancer types.21–23 Therefore, we examined whether this pathway was altered in T24 and T24R cells. Western blotting data revealed that the levels of NFκB/RelA, also known as p65, and its phosphorylated form pRelA (S536) were elevated remarkably in T24R cells, compared to T24 cells. Consistent with IC50, the cisplatin-resistant J82 cells showed moderate level of RelA and phosphorylated RelA (Figure 1B–D). Besides, we also observed elevated levels of NFκB/RelA downstream targets in T24R cells (Figure S1). These data showed a clear correlation between the activation of NFκB and the resistance to cisplatin treatment in UBC cells.
Zyflamend is capable of inhibiting NFκB signaling pathway
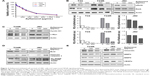
Since Zyflamend has been reported to possess anticancer property through targeting NFκB pathway, we next examined whether Zyflamend could inhibit cell proliferation by inactivating the NFκB signaling pathway in UBC calls. First, we found that Zyflamend indeed suppressed cell proliferation in a concentration-dependent manner for T24, J82, and T24R cells with respective IC50 values of 379.5, 310.3, and 296.4 μg/mL (Figure 2A). To examine whether Zyflamend inhibits NFκB signaling pathway, we performed Western blotting assays to assess the major NFκB transcription factor (RelA) and its downstream targets, including cyclin D1, c-Myc, and Bcl2, in T24, T24R, and J82 cells. As shown in Figure 2B, Zyflamend reduced the levels of both total and phosphorylated RelA in a dose-dependent manner. In line with this finding, levels of proliferation-associated proteins (cyclin D1 and c-Myc) and anti-apoptotic protein (Bcl2) were gradually reduced with Zyflamend treatment (Figure 2C and D and Figure S2A and B). In addition, Zyflamend treatment also led to increased levels of cleaved caspase 3 and PARP (Figures 2D and S2B). Furthermore, the downstream targets of NFκB such as vimentin, MMP2, MMP9, and VEGFa were all downregulated by Zyflamend in a dose-dependent manner (Figures 2E and S2C). These data indicate that Zyflamend is capable of inhibiting the NFκB pathway in UBC cells.
The synergistic effect of Zyflamend and cisplatin in inhibiting survival and inducing apoptosis of cisplatin-resistant UBC cells
To test whether Zyflamend can overcome UBC cell resistance to cisplatin, we treated T24R and J82 cells with either Zyflamend (50 μg/mL) and cisplatin (1 μg/mL) alone, or in combination for 72 h. We found that compared to single treatment, the combination of Zyflamend with cisplatin reduced the viability of both UBC cell lines (P<0.001; Figure 3A and B). To test the synergetic effect between Zyflamend and cisplatin, T24R and J82 cells were exposed to different concentrations of Zyflamend (0–1,200 μg/mL) and cisplatin (0–8 μg/mL) alone or in combination for 72 h followed by MTT assay. CI between Zyflamend and cisplatin was calculated using Chou-Talalay method. As shown in Figure 3C and D, the CI values of the combined treatment were all <1 with the mean CI values of 0.41 and 0.50 for T24R and J82 cells, respectively. To test whether Zyflamend can also enhance parental T24 cells to cisplatin, we treated them with either Zyflamend (50 μg/mL) and cisplatin (0.25 μg/mL) alone, or in combination for 72 h. The results on the synergistic assays revealed that Zyflamend can also enhance T24 cells to a low concentration of cisplatin treatment (Figure S3A). Chou-Talalay method also confirmed that low concentration of Zyflamend and cisplatin showed synergism on cytotoxicity (Figure S3B). Overall, these results indicate a synergistic interaction between Zyflamend and cisplatin on the growth inhibition of both UBC cells. Consistently, co-treatment with Zyflamend and cisplatin had a stronger inhibitory effect on the colony-formation capability of T24R and J82 cells (P<0.001; Figure 3E–G).
To determine the mechanism for the synergistic effect between Zyflamend and cisplatin, we performed apoptosis assay with Annexin V/PI double staining. Flow cytometry analyses showed that ~48% T24R cells underwent apoptosis when treated with Zyflamend (50 μg/mL) and cisplatin (1 μg/mL) together, whereas only 23.1% and 9.21% T24R cells were undergoing apoptosis when they were treated with Zyflamend (50 μg/mL; P<0.01) and cisplatin (1 μg/mL; P<0.01), respectively (Figure 4A and B). Similar results were obtained when J82 cells were treated (Figure 4A and C). In addition, co-treatment reduced both total and phosphorylated RelA (S536) with reduced levels of proteins involved in cell cycle progression (c-Myc and Cyclin D1) and survival (Bcl-2). The co-treatment strikingly induced cleavages of caspase 3 and PARP in both T24R and J82 cells (Figure 4D and E). As for parental T24 cells, Zyflamend (50 μg/mL) and low concentration of cisplatin (0.25 μg/mL) synergistically induced apoptotic rate and the cleavages of caspase 3 and PARP, and reduced the NFκB downstream targets (Figure S4). These results demonstrated that combination of cisplatin and Zyflamend has a strong cytotoxic effect on UBC cells by inhibiting NFκB signaling pathway.
The synergistic effect of Zyflamend and cisplatin in inhibiting survival and inducing apoptosis of cisplatin-resistant UBC cells in vivo
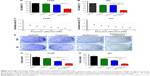
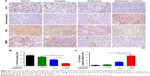
The synergistic effect of Zyflamend and cisplatin was further tested with the cisplatin-resistant T24R xenograft animal model. Then, 6-week old male nude mice were subcutaneously inoculated with T24R cells. After 1 week, palpable tumor-bearing mice were randomly divided into four groups and treated with either vehicle, Zyflamend (1 g/kg) or cisplatin (5 mg/kg) alone, or in combination (Figure 5A) for 4 weeks. Then, mice were sacrificed and tumors were collected for further analysis (Figure 5B). As shown in Figure 5C and D, tumor volumes and tumor weights in the cisplatin group did not show significant difference from those of the vehicle group, whereas Zyflamend treatment alone significantly reduced both tumor volume and weight. Of note, combined treatment showed the highest suppression on tumor growth among all groups (P<0.01). The body weights of the mice among different treatment groups were comparable (Figure 5E). In addition, there were no obvious morphological and weight changes of liver, spleen, lung, kidney, and heart among four groups (Figure S5A and B), suggesting that Zyflamend treatment either alone or in combination with cisplatin has no significant toxicity to the animals.
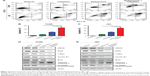
To understand the underlying mechanism, we examined the levels of the cell proliferation marker (Ki-67) and apoptotic marker (cleaved caspase 3) in xenografts (Figure 6A). As shown in Figure 6B and C, Zyflamend and cisplatin significantly decreased the Ki-67-positive cancer cells and increased the cleaved caspase 3-positive cells in xenografts compared with the single treatments (P<0.01 versus cisplatin group; P<0.05 versus Zyflamend group). IHC results showed that the xenografts from the co-treatment group expressed the least levels of RelA and MMP9 (Figure 6A, the 3rd row), and contained the most cleaved Caspase 3-positive cells compared with the single treatments (P<0.01 versus cisplatin group; P<0.05 versus Zyflamend group). IHC results showed that the xenografts from the co-treatment group had the lowest levels of RelA and MMP9 (Figure 6A, the 3rd row), which indicated that the xenografts decreased NFκB signaling under co-treatment with Zyflamend and cisplatin. As one of NFκB targets, MMP9 protein levels were also reduced in Zyflamend-treated xenografts, compared to vehicle group. Such effect was further enhanced in tissues treated with combination of cisplatin (Figure 6A, 4th row). The data demonstrated that the NFκB signaling pathway is effectively repressed by the co-treatment.
Discussion
Due to its high morbidity and recurrent rates, UBC is the costliest cancer on a per-patient basis.24 Cisplatin-based chemotherapy is currently the mainstay treatment for UBC, but chemoresistance inevitably develops. As a polyherbal supplement, Zyflamend is relatively pharmacologically safe and affordable to be used for the prevention/treatment of various chronic diseases. Since treating UBC cells with cisplatin leads to NFκB activation and Zyflamend is known to target the proinflammatory NFκB pathway,17 we focused the effect of Zyflamend on proliferation of UBC cells, especially the cisplatin-resistant UBC cells. We found that Zyflamend is capable of suppressing NFκB signaling pathway in a dose-dependent manner for both J82 and T24R cells. More importantly, Zyflamend synergistically inhibits cell proliferation of T24R and J82 cells with cisplatin both in vitro and in vivo.
Activation of NFκB has been implicated in chemoresistance of various cancer types,21–23 and cytokine secretion from apoptotic and/or necrotic cancer cells can activate NFκB signaling pathway through paracrine effect.25–27 In addition, cancer cells may also activate NFκB signaling through autocrine secretion of IL-1B.28,29 Activated NFκB pathway increases cell proliferation and survival, as well as cancer stemness.30 We found that activation of NFκB/RelA pathway was not only due to increased phosphorylation of Ser536 on RelA, but also due to the total RelA. This suggests that there may exist other mechanism responsible for NFκB activation. Recent study found that proinflammatory stimulus lymphotoxin α1β2 specifically induces RelA, instead of NFκB/RelB, at mRNA level, through activating its cognate receptor lymphotoxin β receptor in 5637 UBC cells.31 Upregulated RelA in cisplatin-resistant cancer could be the result of enhanced transcription or increased mRNA stability/translation regulated by miRNA. For instance, in acute lymphoblastic leukemia, oncoprotein MDM2 directly regulates RelA through the Sp1 site of the RelA promoter.32 Recent study revealed that miR-138 directly regulated NFκB/RelA expression through binding to its 3′UTR and further mediated drug efflux pump P-glycoprotein expression to induce chemoresistance.33 Therefore, molecular mechanisms for NFκB/RelA upregulation in cisplatin-resistant UBC cells is not clear yet.
Zyflamend showed anti-inflammatory and anticancer effects via decreasing the activities of several key enzymes involved in inflammation including COX-1/2, 5-LOX, and 12-LOX.34,35 Some of these effectors are regulated by inflammation-associated transcription factors NFκB and STAT3, which are also suppressed by Zyflamend in pancreatic and prostate cancer cells.17,35 Moreover, in an obesity-induced mammary gland inflammation mouse model, Zyflamend inhibited proinflammatory cytokines expression through suppression of AKT/NFκB pathway, which was confirmed in THP-1 human macrophages.36 In line with these findings, our data revealed that Zyflamend decreased the levels of total and phosphorylated RelA in UBC cells. Interestingly, Zyflamend was found to inhibit invasion of osteoclast and suppress osteoclastogenesis through downregulation of NFκB pathway.37 These evidences suggest that Zyflamend might exert its anticancer effects not only on cancer cells, but also affects tumor microenvironment in primary and metastatic sites.
Zyflamend is a dietary polyherbal preparation with 10 herbal extracts, which contains polyphenols, such as carnosol, epigallocatechin gallate, curcumin, and resveratrol.36,38–40 In the current study, we found that Zyflamend suppressed NFκB activation and the expression of its downstream targets associated with cell proliferation (cyclin D1 and c-Myc), survival (Bcl-2), and cell invasion/angiogenesis (vimentin, MMP2, and MMP9) in the cisplatin-resistant UBC cells (Figure 7). These findings are in consistence with that polyphenols possess antitumor properties through inhibition of NFκB activation.41–44 Moreover, several studies including our previous work demonstrated that Zyflamend can enhance apoptosis of bicalutamide-treated prostate cancer cells and TRAIL-induced colorectal cells.15,18 The current study demonstrated that Zyflamend can enhance apoptosis of cisplatin-treated UBC cells.
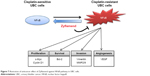  | Figure 7 Illustration of anticancer effect of Zyflamend against NFκB pathway in UBC cells. |
Conclusion
We have demonstrated for the first time that Zyflamend can sensitize UBC cells to the chemotherapeutic agent cisplatin in vitro and in vivo by inhibiting the NFκB signaling pathway. Given that Zyflamend is well tolerant and safe,45 cisplatin and Zyflamend combination could be a promising therapeutic approach for cisplatin-resistant UBC.
Acknowledgments
The authors would like to thank Xiaojing Huang and Jiakuan Liu for their helpful discussion and technical supports. We also appreciate the assistance of staff from the Institutional Technology Service Center of Shanghai Institute of Materia Medica for their technical support. This work was supported in part by the National Natural Science Foundation of China (81372168, 81470116, 81672873, and 81572519), Wu Jieping Medical Foundation (320.6750.16051), the Fundamental Research Funds for the Central Universities (090314380004), One Hundred Talent Program of Chinese Academy of Sciences, and the Research Fund of Institutes for Drug Discovery and Development, Chinese Academy of Sciences (CASIMM0120164001). This work was supported in part by New Chapter Incorporated, but the founding sponsor had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the paper; and in the decision to publish the results.
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no other conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Zheng R, Zeng H, Zhang S, Chen T, Chen W. National estimates of cancer prevalence in China, 2011. Cancer Lett. 2016;370(1):33–38. | ||
Kirkali Z, Chan T, Manoharan M, et al. Bladder cancer: epidemiology, staging and grading, and diagnosis. Urology. 2005;66(6 Suppl 1):4–34. | ||
Sonpavde G, Sternberg CN. Neoadjuvant chemotherapy for invasive bladder cancer. Curr Urol Rep. 2012;13(2):136–146. | ||
Housman G, Byler S, Heerboth S, et al. Drug resistance in cancer: an overview. Cancers (Basel). 2014;6(3):1769–1792. | ||
Mitchell S, Vargas J, Hoffmann A. Signaling via the NFκB system. Wiley Interdiscip Rev Syst Biol Med. 2016;8(3):227–241. | ||
Ghosh S, Hayden MS. Celebrating 25 years of NF-κB research. Immunol Rev. 2012;246(1):5–13. | ||
Hayden MS, Ghosh S. NF-κB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 2012;26(3):203–234. | ||
Chang C, Zhao W, Xie B, et al. Pao pereira extract suppresses castration-resistant prostate cancer cell growth, survival, and invasion through inhibition of NFκB signaling. Integr Cancer Ther. 2014;13(3):249–258. | ||
Sun SC, Ley SC. New insights into NF-kappaB regulation and function. Trends Immunol. 2008;29(10):469–478. | ||
Ghosh G, Wang VY, Huang DB, Fusco A. NF-κB regulation: lessons from structures. Immunol Rev. 2012;246(1):36–58. | ||
Li F, Zhang J, Arfuso F, et al. NF-κB in cancer therapy. Arch Toxicol. 2015;89(5):711–731. | ||
Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. | ||
Mukherjee N, Houston TJ, Cardenas E, Ghosh R. To be an ally or an adversary in bladder cancer: the NF-κB story has not unfolded. Carcinogenesis. 2015;36(3):299–306. | ||
Yan J, Xie B, Capodice JL, Katz AE. Zyflamend inhibits the expression and function of androgen receptor and acts synergistically with bicalutimide to inhibit prostate cancer cell growth. Prostate. 2012;72(3):244–252. | ||
Huang EC, Chen G, Baek SJ, et al. Zyflamend reduces the expression of androgen receptor in a model of castrate-resistant prostate cancer. Nutr Cancer. 2011;63(8):1287–1296. | ||
Kunnumakkara AB, Sung B, Ravindran J, et al. Zyflamend suppresses growth and sensitizes human pancreatic tumors to gemcitabine in an orthotopic mouse model through modulation of multiple targets. Int J Cancer. 2012;131(3):E292–E303. | ||
Kim JH, Park B, Gupta SC, Kannappan R, Sung B, Aggarwal BB. Zyflamend sensitizes tumor cells to TRAIL-induced apoptosis through up-regulation of death receptors and down-regulation of survival proteins: role of ROS-dependent CCAAT/enhancer-binding protein-homologous protein pathway. Antioxid Redox Signal. 2012;16(5):413–427. | ||
Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. | ||
Zhang Q, Zhao W, Ye C, et al. Honokiol inhibits bladder tumor growth by suppressing EZH2/miR-143 axis. Oncotarget. 2015;6(35):37335–37348. | ||
Heavey S, Godwin P, Baird AM, et al. Strategic targeting of the PI3K-NFκB axis in cisplatin-resistant NSCLC. Cancer Biol Ther. 2014;15(10):1367–1377. | ||
Almeida LO, Abrahao AC, Rosselli-Murai LK, et al. NFκB mediates cisplatin resistance through histone modifications in head and neck squamous cell carcinoma (HNSCC). FEBS Open Bio. 2013;4:96–104. | ||
Lau MC, Ng KY, Wong TL, et al. FSTL1 promotes metastasis and chemoresistance in esophageal squamous cell carcinoma through NFκB-BMP signaling cross-talk. Cancer Res. 2017;77(21):5886–5899. | ||
Yeung C, Dinh T, Lee J. The health economics of bladder cancer: an updated review of the published literature. Pharmacoeconomics. 2014;32(11):1093–1104. | ||
Butera G, Pacchiana R, Donadelli M. Autocrine mechanisms of cancer chemoresistance. Semin Cell Dev Biol. 2018;78:3–12. | ||
Völp K, Brezniceanu ML, Bösser S, et al. Increased expression of high mobility group box 1 (HMGB1) is associated with an elevated level of the antiapoptotic c-IAP2 protein in human colon carcinomas. Gut. 2006;55(2):234–242. | ||
Zhou J, Chen X, Gilvary DL, et al. HMGB1 induction of clusterin creates a chemoresistant niche in human prostate tumor cells. Sci Rep. 2015;5:15085. | ||
Chen T, Li J, Xu M, et al. PKCε phosphorylates MIIP and promotes colorectal cancer metastasis through inhibition of RelA deacetylation. Nat Commun. 2017;8(1):939. | ||
Ko SC, Huang CR, Shieh JM, Yang JH, Chang WC, Chen BK. Epidermal growth factor protects squamous cell carcinoma against cisplatin-induced cytotoxicity through increased interleukin-1β expression. PLoS One. 2013;8(2):e55795. | ||
Sun Y, Guan Z, Liang L, et al. NF-κB signaling plays irreplaceable roles in cisplatin-induced bladder cancer chemoresistance and tumor progression. Int J Oncol. 2016;48(1):225–234. | ||
Shen M, Zhou L, Zhou P, Zhou W, Lin X. Lymphotoxin β receptor activation promotes mRNA expression of RelA and pro-inflammatory cytokines TNFα and IL-1β in bladder cancer cells. Mol Med Rep. 2017;16(1):937–942. | ||
Gu L, Findley HW, Zhou M. MDM2 induces NF-kappaB/p65 expression transcriptionally through Sp1-binding sites: a novel, p53-independent role of MDM2 in doxorubicin resistance in acute lymphoblastic leukemia. Blood. 2002;99(9):3367–3375. | ||
Requenez-Contreras JL, López-Castillejos ES, Hernández-Flores R, et al. MiR-138 indirectly regulates the MDR1 promoter by NF-κB/p65 silencing. Biochem Biophys Res Commun. 2017;484(3):648–655. | ||
Yang P, Cartwright C, Chan D, Vijjeswarapu M, Ding J, Newman RA. Zyflamend-mediated inhibition of human prostate cancer PC3 cell proliferation: effects on 12-LOX and Rb protein phosphorylation. Cancer Biol Ther. 2007;6(2):228–236. | ||
Bemis DL, Capodice JL, Anastasiadis AG, Katz AE, Buttyan R. Zyflamend, a unique herbal preparation with nonselective COX inhibitory activity, induces apoptosis of prostate cancer cells that lack COX-2 expression. Nutr Cancer. 2005;52(2):202–212. | ||
Subbaramaiah K, Sue E, Bhardwaj P, et al. Dietary polyphenols suppress elevated levels of proinflammatory mediators and aromatase in the mammary gland of obese mice. Cancer Prev Res (Phila). 2013;6(9):886–897. | ||
Sandur SK, Ahn KS, Ichikawa H, et al. Zyflamend, a polyherbal preparation, inhibits invasion, suppresses osteoclastogenesis, and potentiates apoptosis through down-regulation of NF-kappa B activation and NF-kappa B-regulated gene products. Nutr Cancer. 2007;57(1):78–87. | ||
Mohebati A, Guttenplan JB, Kochhar A, et al. Carnosol, a constituent of Zyflamend, inhibits aryl hydrocarbon receptor-mediated activation of CYP1A1 and CYP1B1 transcription and mutagenesis. Cancer Prev Res (Phila). 2012;5(4):593–602. | ||
Ekmekcioglu S, Chattopadhyay C, Akar U, Gabisi A Jr, Newman RA, Grimm EA. Zyflamend mediates therapeutic induction of autophagy to apoptosis in melanoma cells. Nutr Cancer. 2011;63(6):940–949. | ||
Huang EC, McEntee MF, Whelan J. Zyflamend, a combination of herbal extracts, attenuates tumor growth in murine xenograft models of prostate cancer. Nutr Cancer. 2012;64(5):749–760. | ||
Yao H, Chen Y, Zhang L, et al. Carnosol inhibits cell adhesion molecules and chemokine expression by tumor necrosis factor-α in human umbilical vein endothelial cells through the nuclear factor-κB and mitogen-activated protein kinase pathways. Mol Med Rep. 2014;9(2):476–480. | ||
Li YJ, Wu SL, Lu SM, et al. (-)-Epigallocatechin-3-gallate inhibits nasopharyngeal cancer stem cell self-renewal and migration and reverses the epithelial-mesenchymal transition via NF-κB p65 inactivation. Tumour Biol. 2015;36(4):2747–2761. | ||
Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett. 2016;12(5):4139–4146. | ||
Karthikeyan S, Hoti SL, Prasad NR. Resveratrol loaded gelatin nanoparticles synergistically inhibits cell cycle progression and constitutive NF-kappaB activation, and induces apoptosis in non-small cell lung cancer cells. Biomed Pharmacother. 2015;70:274–282. | ||
Capodice JL, Gorroochurn P, Cammack AS, et al. Zyflamend in men with high-grade prostatic intraepithelial neoplasia: results of a phase I clinical trial. J Soc Integr Oncol. 2009;7(2):43–51. |
Supplementary materials
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.