Back to Journals » International Journal of Nanomedicine » Volume 11
Coating with spermine-pullulan polymer enhances adenoviral transduction of mesenchymal stem cells
Authors Wan L, Yao X, Faiola F, Liu B, Zhang T, Tabata Y, Mizuguchi H, Nakagawa S, Gao JQ, Zhao RC
Received 5 April 2016
Accepted for publication 21 July 2016
Published 13 December 2016 Volume 2016:11 Pages 6763—6769
DOI https://doi.org/10.2147/IJN.S109897
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Linlin Sun
Li Wan,1,* Xinglei Yao,1–3,* Francesco Faiola,3 Bojun Liu,4 Tianyuan Zhang,2 Yasuhiko Tabata,5 Hiroyuki Mizuguchi,6 Shinsaku Nakagawa,7 Jian-Qing Gao,2 Robert Chunhua Zhao1
1Institute of Basic Medical Sciences, Chinese Academy of Medical Sciences, School of Basic Medicine Peking Union Medical College, Peking Union Medical College Hospital, Center of Excellence in Tissue Engineering Chinese Academy of Medical Sciences, Beijing, 2Institute of Pharmaceutics, Zhejiang University, Hangzhou, 3State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, 4YouAn Hospital, Capital Medical University, Beijing, People’s Republic of China; 5Department of Biomaterials, Field of Tissue Engineering, Institute for Frontier Medical Sciences, Kyoto University, Kyoto, 6Department of Biochemistry and Molecular Biology, Graduate School of Pharmaceutical Sciences, Osaka University, Osaka, 7Department of Biotechnology and Therapeutics, Graduate School of Pharmaceutical Sciences, Osaka University, Suita, Osaka, Japan
*These authors contributed equally to this work
Abstract: Mesenchymal stem cells (MSCs) are adult stem cells with multilineage potential, which makes them attractive tools for regenerative medicine applications. Efficient gene transfer into MSCs is essential not only for basic research in developmental biology but also for therapeutic applications involving gene-modification in regenerative medicine. Adenovirus vectors (Advs) can efficiently and transiently introduce an exogenous gene into many cell types via their primary receptors, the coxsackievirus and adenovirus receptors, but not into MSCs, which are deficient in coxsackievirus and adenovirus receptors expression. To overcome this problem, we developed an Adv coated with a spermine-pullulan (SP) cationic polymer and investigated its physicochemical properties and internalization mechanisms. We demonstrated that the SP coating could enhance adenoviral transduction of MSCs without detectable cytotoxicity or effects on differentiation. Our results argue in favor of the potentiality of the SP-coated Adv as a prototype vector for efficient and safe transduction of MSCs.
Keywords: mesenchymal stem cells, adenovirus vectors, spermine-pullulan, polymer, gene transduction
Introduction
Mesenchymal stem cells (MSCs) have recently generated high enthusiasm as a novel therapeutic paradigm for a variety of diseases.1–3 The clinical potential of MSCs is mainly attributed to the following important biological properties: the ability to differentiate into osteoblasts, adipocytes, and other cell types; the ability to home into sites of inflammation following tissue injury; the ability to secrete several bioactive molecules capable of recovering injured cells or inhibiting inflammation; and the ability to perform immunomodulatory functions. However, the therapeutic application of MSCs has been facing low success rates, and gene delivery into MSCs prior to engraftment has been proposed as a mechanism to augment their therapeutic potential.
Adenovirus vectors (Advs) are widely used vectors in gene transduction because of many useful features, such as high transduction efficiency, ease of production of high-titer stocks, and low risk of gene mutation.4,5 Advs can efficiently introduce exogenous genes into many cell types via their primary receptors, the coxsackievirus and adenovirus receptors (CARs).6 However, gene transfer with Advs is not very efficient in MSCs because of the scarcity of CARs on their cell surface.7,8 For this reason, the application of Advs as gene transfer vectors for MSC transduction has been limited.
Previously, some groups, including ours, reported the physical coating and chemical conjugation of Advs as alternative approaches for efficient adenoviral gene therapies.9–15 Thus, we aimed at modifying an Adv with another suitable polymer to overcome the obstacle of MSC low transduction efficiency. Spermine–pullulan (SP), one kind of cationic polymers prepared by the conjugation of pullulan and spermine, was demonstrated to be able to efficiently transfect plasmids for in vitro gene expression in various cell types, including MSCs.16–20 Pullulan is a water-soluble polysaccharide with a repeated unit of maltotriose connected through an α-1,6 bond and known to be a safe material for oral health care and pharmaceutical coating applications. This polysaccharide-based carrier was proven to be internalized by different cells through a sugar-recognition receptor on the cell surface. In this study, we hypothesized that SP could help Advs enter into MSCs by bypassing CAR-mediated endocytosis and consequently yield efficient transgene expression. Thus, we coated Advs with different SP coating combinations and compared their transgene expression and cytotoxicity in MSCs. We also investigated their physicochemical properties, internalization mechanism and effects on MSCs’ differentiation, and demonstrated that SP coating significantly increased the safe expression of transgenes in MSCs.
Materials and methods
Animals and cell lines
Three-week-old male Sprague-Dawley (SD) rats (50–60 g) were used for the isolation of MSCs. All the experimental procedures conducted on animals were approved and performed in accordance with the Guidelines for the Welfare and Ethics of Animals of Chinese Academy of Medical Sciences and Zhejiang University (Zju2012-0052).
MSCs were isolated from the bone shaft of femurs of 3-week-old male SD rats. Briefly, both ends of rat femurs were cut away from the epiphysis, and the bone marrow was flushed out using a syringe (21-gauge needle) with 1 mL low-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), L-glutamine (2 mM), penicillin (50 U/mL), and streptomycin (50 U/mL). The cell suspension was placed into two 25 cm2 flasks and cultured at 37°C in 5% CO2. The medium was changed on day 4 of the culture and every 3 days thereafter. Once subconfluence was reached, the cells were detached from the flask using trypsin/ethylenediaminetetraacetic acid (EDTA; 0.25/0.02%). Third or fourth passage cells at subconfluence were used for all the experiments. HEK293 cells were purchased from China Infrastructure of Cell Line Resources and were maintained with minimum essential medium supplemented with 10% FBS. HEK293 cells are only used for Adv amplification and titer check.
Viral vector construction
The E1/E3-deleted type 5 Adv, expressing firefly luciferase under the control of the cytomegalovirus promoter, was constructed with an improved in vitro ligation method, as previously reported.21–24 The Adv was amplified in HEK293 cells using established methods,25 and purified by cesium chloride step-gradient ultracentrifugation. The virus particles (vp) and biological titer were determined via spectrophotometry26 and the Adeno-X Rapid Titer protocol (Clontech Laboratories, Mountain View, CA, USA), respectively. The vp-to-titer ratio was around 100 (between 50 and 200).
Preparation of SP-coated Advs
SP was prepared using an N,N′-carbonyldiimidazole activation method as previously reported.27 The molar extent of spermine introduced into the hydroxyl groups of pullulan was 12.3%. An SP stock concentration of 4 mg/mL was used in all the experiments. To find the optimal coating ratio, we constructed SP-coated Advs using different concentrations of the SP solution. A total of 2×109 vp/mL Advs were simply mixed with 2, 4, 8, 16, 32, 64, or 128 μg/mL SP solutions at room temperature for 15 minutes because SP, as a cationic polymer, was readily attracted to the negatively charged surface of the Adv. We labeled the resulting Advs as SP-Adv2, SP-Adv4, SP-Adv8, SP-Adv16, SP-Adv32, SP-Adv64, and SP-Adv128, respectively. The SP to Adv molar ratios were 2.4×104, 4.8×104, 9.6×104, 1.9×105, 3.8×105, 7.7×105, and 1.5×105, respectively. SP-Adv2 morphology was observed under a transmission electron microscope (TEM, H-9500, Hitachi, Tokyo, Japan).
In vitro gene transduction
MSCs (2×104 cells/well) derived from rat bone marrow (shortly written as MSCs in the following study) were seeded into 48-well plates. The following day, each well was treated with 104 vp/cell of luciferase-encoding Adv (~100 multiplicity of infection) or SP-coated Adv. After 24 hours, luciferase activity was determined using a luciferase assay system (Promega Corporation, Fitchburg, WI, USA), in accordance with the manufacturers’ instructions, and the amount of proteins was measured with the bicinchoninic acid (BCA) assay. Relative luciferase activity was calculated as relative light units/mg protein.
Cytotoxicity assay
MSCs (1×104 cells/well) were seeded into 96-well plates. The following day, the control group was treated with phosphate-buffered saline (PBS), Adv group was treated with 104 vp/cell of luciferase-encoding Advs (~100 MOI), uncoated or SP-coated. After 24 hours, cell viability was determined using an MTT assay system (Sigma-Aldrich Co., St Louis, MO, USA) in accordance with the general protocol. Finally, we set control group as 100% and calculated other groups as % compared with control group.
Endocytosis-dependent cellular uptake
MSCs (2×104 cells/well) were seeded into 48-well plates. The following day, the control group was pretreated with PBS, others were pretreated with one of the following endocytosis inhibitors for 1 hour: 6.65 mg/mL methyl-β-cyclodextrin (MBCD) (Kaiyang Bio Co, Shanghai, People’s Republic of China), 10 μg/mL chlorpromazine (CPA) (Kaiyang Bio Co) or 2.5 mM amiloride HCl hydrate (Sigma-Aldrich Co.). Each well was treated with 2×108 vp (104 vp/cell) of uncoated or SP-coated Advs encoding luciferase (~100 MOI). After 24 hours of culture, luciferase activity was determined as described earlier. Finally, we set each control group of uncoated or SP-coated Advs as 100%, and calculated other groups as % compared with their control group.
Differentiation of Adv-transduced MSCs
MSCs (3×105 cells/well) were seeded into six-well plates. The following day, the control group was treated with PBS, Adv group was treated with 104 vp/cell of luciferase-encoding Advs (~100 MOI), uncoated or SP-coated. Twenty-four hours later, culture media were removed, and cells were washed with PBS and then incubated with adipogenesis induction medium (low-glucose DMEM supplemented with 10% FBS, 1 μM dexamethasone, 0.5 mM isobutylmethylxanthine, and 1 mM ascorbic acid) or ostogenesis induction medium (low-glucose DMEM supplemented with 10% FBS, 10 mM β-glycerophosphate, 10 nM dexamethasone, and 0.2 mM ascorbic acid). All reagents used in the osteogenic and adipogenic differentiations were from Sigma-Aldrich Co. Induction mediums were changed every other day for approximately 1 week. The expression of adipogenic and osteogenic markers was evaluated by reverse transcription quantitative polymerase chain reaction (RT-qPCR). Briefly, total RNA was extracted using Trizol® reagent (Thermo Fisher Scientific, Waltham, MA, USA) and purified according to the manufacturer’s instructions. RT-qPCR was carried out to with SYBR Green I (TaKaRa, Japan). The expression level of genes was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH). All experiments were performed in triplicates and data were calculated with the comparative Ct (ΔΔCt) method. Primers used for amplification were as follows: peroxisome proliferator-activated receptor-γ (PPARγ), (F) GGACGCTGAAGAAGAGACCTG and (R) AAGTTGGTGGGCCAGAATGG; LPL, (F) CCAGCTGGGCCTAACTTTGA and (R) GGAAAGTGCCTCCATTGGGA; AP2, (F) AGAAGTGGGAGTTGGCTTCG and (R) ACTCTCTGACCGGATGACGA; RUNX2, (F) CCATAACGGTCTTCACAAATCCT and (R) TCTGTCTGTGCCTTCTTGGTTC; ALP, (F) CATCGGACCCTGCCTTACC and (R) GGAGACGCCCATACCATCTC; glyceraldehyde 3-phosphate dehydrogenase, (F) CCATGTTCGTCATGGGTGTGAACCA and (R) GCCAGTAGAGGCAGGGATGATGTTC.
Statistical analysis
All results were repeated with at least three independent biological replicates that showed similar results. Finally, the most representative data were shown here and expressed as the mean ± standard deviation (sd) with six (n=6) or three (n=3) technical replicates. Differences were compared using Student’s t-test with two-tailed P-value or one-way analysis of variance.
Results and discussion
Evaluation of transgene expression and cytotoxicity of SP-coated Advs
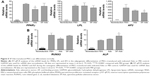
First, we compared the transgene expression of luciferase-encoding Advs coated with different SP ratios in MSCs derived from the bone marrow of SD rats. The relative luciferase expressions for SP-Adv2, 4, 8, 16, 32, 64, and 128 were 16.9, 18.0, 15.4, 25.3, 22.8, 10.0, and 28.9-fold higher than the one of the uncoated Adv, respectively (Figure 1A). Although the SP coating increased Adv transgene expression in MSCs, this positive effect was independent of the SP ratios used. We next evaluated the cytotoxicity in MSCs infected with uncoated and SP-coated Advs, relative to untreated control cells. An MTT cell viability assay was performed 24 hours after transduction (Figure 1B). Cell viability of SP-Adv32, SP-Adv64, and SP-Adv128 infected cells was notably lower than control. Based on the results in Figure 1, a wide range (from 2 to 16 μg/mL) of SP could be used for Adv coating, and in the following experiments we chose 2 μg/mL SP as the optimal coating concentration for 2×109 vps/mL Adv, with a final molar ratio of SP to Adv of 2.4×104. These conditions facilitated the highest transgene expression and lowest cytotoxicity and will hereafter be referred to as SP-Adv. In short, SP-Adv2 was chosen and used as SP-Adv in the following part of this study.
Determination of physicochemical properties of SP-Advs
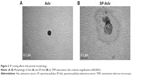
After coating optimization, we investigated why SP coating could enhance SP-Adv transduction in MSCs. First, we evaluated the physicochemical differences between Adv and SP-Adv by TEM. We observed that SP-Adv (Figure 2B) looked slightly larger than Adv (Figure 2A). Moreover, Adv particles appeared clear, whereas SP-Adv particles were blurry and dispersed. These results proved that SP could completely surround the adenoviral particles and form a hydration layer outside, suggesting a possible bypass of Adv-related CAR-mediated endocytosis.
Evaluation of the internalization mechanisms of SP-Advs
To further investigate how SP altered the pathway of adenoviral internalization into MSCs, we analyzed SP-Adv endocytosis in the presence of endocytosis inhibitors. As shown in Figure 3, Adv transduction was inhibited by the clathrin-mediated endocytosis inhibitor CPA and the lipid-raft inhibitor MBCD, but not by the macropinocytosis-mediated endocytosis inhibitor amiloride. However, SP-Adv transduction was only inhibited by MBCD, a chemical that can selectively extract cholesterol to organize sphingolipid rafts, and is related to caveolae-mediated and macropinocytosis-mediated endocytosis.28 The transduction of both uncoated and SP-coated Advs was inhibited by MBCD, but not completely by amiloride. These results indicated that both the Adv and SP-Adv were allowed to enter into the cells through caveolae-mediated endocytosis. In contrast, the clathrin-mediated endocytosis inhibitor CPA, which primarily affects receptor-mediated endocytosis,29 could only inhibit Adv transduction. This suggested that SP coating helped Adv enter into MSCs without the requirement for CAR receptors, which are only minimally expressed in MSCs, and could also explain the enhanced transgene expression with SP-Advs.
Analysis of the serum effects on SP-Adv expression
Generally, the serum in culture media affects the transgene expression from cationic-polymer coated plasmids, such as when the lipofectamine 2000 reagent is used. To investigate whether serum would affect transgene expression of cationic SP-coated Advs, we compared the following three methods for transduction of Advs or SP-Advs (Figure 3). In one case, MSCs were seeded overnight in culture medium containing FBS and then infected with Adv or SP-Adv particles for 24 hours. Alternatively, MSCs seeded overnight were only infected with Advs or SP-Advs for 1 hour in the presence of serum and cultured for additional 23 hours in FBS-containing medium. In a third case, MSCs were seeded overnight in the presence of serum; then cells were infected with Adv or SP-Adv particles in culture medium without FBS for 1 hour; finally, after infection, serum was added in the culture medium for additional 23 hours. As depicted in Figure 4, the three conditions tested did not affect the transgene expressions of both Advs and SP-Advs, indicating that serum can be present or absent during infection. This will not preclude the use of SP-Advs when medium conditions containing serum must be used.
Analysis of adipogenesis and osteogenesis in SP-Adv-transduced MSCs
MSCs have the basic ability to readily differentiate into adipocytes and osteoblasts. To investigate whether SP-Adv transduction could perturb this basic MSCs ability, we employed RT-qPCR to evaluate the key markers for adipogenesis (Figure 5A, PPARγ, lipoprotein lipase [LPL] and adipocyte-specific fatty acid-binding protein [AP2]) and ostogenesis (Figure 5B, runt-related gene 2 [RUNX2] and alkaline phosphatase [ALP]) in transduced or control MSCs. In short, PPARγ is the central transcription factor in adipogenic differentiation, and the increased PPARγ expression promoted adipogenesis and inhibited osteogenesis of MSCs;29 LPL is a fat storage indicator and adipocyte-specific marker gene; AP2, also called fatty acid binding protein-4 (FABP4), is a specific adipocyte markers and upregulated PPARγ targets;30 RUNX2 is considered a master transcription factor in regulating osteogenic differentiation of MSCs, and upregulation of RUNX2 in MSCs promotes their differentiation potential into immature osteoblasts, while inhibiting their lineage commitment to the adipocytes;31,32 ALP is related to matrix mineralization and therefore can be used as an early osteogenic differentiation marker.30 As a result, compared to noninduction controls, the mRNAs for all the adipogenesis and ostogenesis markers analyzed showed significant upregulation upon induction of differentiation (Figure 5A and B, PBS vs noninduction). It demonstrated that we succeeded in setting up adipogenic and osteogenic model of MSC differentiation. Next, when comparing transduced cells with uninfected cells, no matter which of the two differentiations was induced, all the markers tested showed similar expression levels (Figure 5, Adv and SP-Adv vs PBS). Although the levels of PPARγ (69.4% of PBS, Figure 5A) and RUNX2 (73.9% of PBS, Figure 5B) in Adv-transduced MSCs, and AP2 (63.6% of PBS, Figure 5A) and ALP (147.4% of PBS, Figure 5B) in SP-Adv-transduced MSCs showed weakly significant differences, the increase/decrease ratios were no more than twofold and could be physiologically negligible as limitation of RT-qPCR experiment. Therefore, Advs or SP-Advs did not change the expression levels of adipogenesis and osteogenesis of MSCs and then demonstrated that uncoated or SP coated Advs did not impair the basic differentiation ability of MSCs. These results advocated for the safety of both Adv- and SP-Adv-transduced MSCs.
Conclusion
SP coating of the Adv to form a cationic particle enhances adenoviral transduction into MSCs independently of CAR receptors. These results demonstrate the potential of the SP-coated Adv as a prototype vector for efficient and safe transduction into MSCs. In fact, because of their enhanced but still safe transgene induction, when AP-Adv-transduced MSCs are employed in cell-replacement therapies, they may integrate into targeted tissues more quickly, differentiate into specified cell types more precisely, secrete specific bioactive molecules capable of inhibiting inflammation more rapidly, and perform immunomodulatory functions more efficiently. In conclusion, SP-coated adenoviral transduction makes MSCs more attractive in both clinical therapies as well as basic research.
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (81101719, 21577167, and 81273441), by Chinese Academy of Sciences (CAS) Strategic Leading Science & Technology Program grant (XDB14040301), the Hundred Talent Program of CAS at the Research Center for Eco-Environmental Sciences, CAS (121311ZXPP2014004), and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry ([2012]1707).
Disclosure
The authors report no conflicts of interest in this work.
References
Wang S, Qu X, Zhao R. Clinical applications of mesenchymal stem cells. J Hematol Oncol. 2012;5:19. | ||
Mendicino M, Bailey AM, Wonnacott K, Puri RK, Bauer SR. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell. 2014;14(2):141–145. | ||
Caplan AI. Adult mesenchymal stem cells: when, where, and how. Stem Cells Int. 2015;2015:628767. | ||
Mizuguchi H, Hayakawa T. Targeted adenovirus vectors. Hum Gene Ther. 2004;15(11):1034–1044. | ||
Yao XL, Nakagawa S, Gao JQ. Current targeting strategies for adenovirus vectors in cancer gene therapy. Curr cancer drug targets. 2011;11(7):810–825. | ||
Roelvink PW, Lizonova A, Lee JG, et al. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72(10):7909–7915. | ||
Knaan-Shanzer S, van de Watering MJ, van der Velde I, Goncalves MA, Valerio D, de Vries AA. Endowing human adenovirus serotype 5 vectors with fiber domains of species B greatly enhances gene transfer into human mesenchymal stem cells. Stem Cells. 2005;23(10):1598–1607. | ||
Janssen JM, Liu J, Skokan J, Goncalves MA, de Vries AA. Development of an AdEasy-based system to produce first- and second-generation adenoviral vectors with tropism for CAR- or CD46-positive cells. J Gene Med. 2013;15(1):1–11. | ||
Yao X, Yoshioka Y, Morishige T, et al. Adenovirus vector covalently conjugated to polyethylene glycol with a cancer-specific promoter suppresses the tumor growth through systemic administration. Biol Pharm Bull. 2010;33(6):1073–1076. | ||
Yao X, Yoshioka Y, Morishige T, et al. Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol Ther. 2011;19(9):1619–1625. | ||
Yao X-L, Yoshioka Y, Ruan G-X, et al. Optimization and internalization mechanisms of pegylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules. 2012;13(8):2402–2409. | ||
Yao X, Zhou N, Wan L, et al. Polyethyleneimine-coating enhances adenoviral transduction of mesenchymal stem cells. Biochem Biophys Res Commun. 2014;447(3):383–387. | ||
Kasman L, Barua S, Lu P, Rege K, Voelkel-Johnson C. Polymer-enhanced adenoviral transduction of CAR-negative bladder cancer cells. Mol Pharm. 2009;6(5):1612–1619. | ||
Han J, Zhao D, Zhong Z, Zhang Z, Gong T, Sun X. Combination of adenovirus and cross-linked low molecular weight PEI improves efficiency of gene transduction. Nanotechnology. 2010;21(10):105106. | ||
Singarapu K, Pal I, Ramsey JD. Polyethylene glycol-grafted polyethylenimine used to enhance adenovirus gene delivery. J Biomed Mater Res A. 2013;101(7):1857–1864. | ||
Okazaki A, Jo J, Tabata Y. A reverse transfection technology to genetically engineer adult stem cells. Tissue Eng. 2007;13(2):245–251. | ||
He CX, Li N, Hu YL, et al. Effective gene delivery to mesenchymal stem cells based on the reverse transfection and three-dimensional cell culture system. Pharm Res. 2011;28(7):1577–1590. | ||
Zhang TY, Huang B, Yuan ZY, Hu YL, Tabata Y, Gao JQ. Gene recombinant bone marrow mesenchymal stem cells as a tumor-targeted suicide gene delivery vehicle in pulmonary metastasis therapy using non-viral transfection. Nanomedicine. 2014;10(1):257–267. | ||
Zhang TY, Huang B, Wu HB, et al. Synergistic effects of co-administration of suicide gene expressing mesenchymal stem cells and prodrug-encapsulated liposome on aggressive lung melanoma metastases in mice. J Control Release. 2015;209:260–271. | ||
Hu YL, Miao PH, Huang B, et al. Reversal of tumor growth by gene modification of mesenchymal stem cells using spermine-pullulan/DNA nanoparticles. J Biomed Nanotechnol. 2014;10(2):299–308. | ||
Eto Y, Yoshioka Y, Mukai Y, Okada N, Nakagawa S. Development of PEGylated adenovirus vector with targeting ligand. Int J Pharm. 2008;354(1–2):3–8. | ||
Yao X, Yoshioka Y, Morishige T, et al. Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene Ther. 2009;16(12):1395–1404. | ||
Mizuguchi H, Kay MA. Efficient construction of a recombinant adenovirus vector by an improved in vitro ligation method. Hum Gene Ther. 1998;9(17):2577–2583. | ||
Mizuguchi H, Kay MA. A simple method for constructing E1- and E1/E4-deleted recombinant adenoviral vectors. Hum Gene Ther. 1999;10(12):2013–2017. | ||
Gao JQ, Eto Y, Yoshioka Y, et al. Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J Control Release. 2007;122(1):102–110. | ||
Maizel JV Jr, White DO, Scharff MD. The polypeptides of adenovirus. I. Evidence for multiple protein components in the virion and a comparison of types 2, 7A, and 12. Virology. 1968;36(1):115–125. | ||
Jo J, Ikai T, Okazaki A, et al. Expression profile of plasmid DNA obtained using spermine derivatives of pullulan with different molecular weights. J Biomater Sci Polym Ed. 2007;18(7):883–899. | ||
Imelli N, Meier O, Boucke K, Hemmi S, Greber UF. Cholesterol is required for endocytosis and endosomal escape of adenovirus type 2. J Virol. 2004;78(6):3089–3098. | ||
Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature. 2003;422(6927):37–44. | ||
Chen Q, Shou P, Zheng C, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts? Cell Death Differ. 2016;23(7):1128–1139. | ||
Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. | ||
Menssen A, Haupl T, Sittinger M, Delorme B, Charbord P, Ringe J. Differential gene expression profiling of human bone marrow-derived mesenchymal stem cells during adipogenic development. BMC Genomics. 2011;12:461. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.