Back to Journals » Clinical Ophthalmology » Volume 15
Clinicopathological Analysis and Demographic Features of Ocular Malignancies
Authors Al-Mujaini A , Maurya RP , Bosak S, Karan MK, Roy M, Singh VP, Singh MK, Kumar A, Singh S
Received 17 October 2020
Accepted for publication 14 December 2020
Published 29 January 2021 Volume 2021:15 Pages 357—365
DOI https://doi.org/10.2147/OPTH.S287087
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Abdullah Al-Mujaini,1 Rajendra Prakash Maurya,2 Sanjay Bosak,2 Manish Kumar Karan,2 Meghna Roy,2 Virendra Pratap Singh,2 Mahendra Kumar Singh,2 Ajay Kumar,3 Samer Singh4
1Department of Ophthalmology, College of Medicine and Health Sciences, Sultan Qaboos University Hospital, Sultan Qaboos University, Muscat, Oman; 2Regional Institute of Ophthalmology, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India; 3Department of Zoology, Institute of Science, Banaras Hindu University, Varanasi 221005, India; 4Centre for Experimental Medicine & Surgery, Institute of Medical Sciences, Banaras Hindu University, Varanasi 221005, India
Correspondence: Rajendra Prakash Maurya; Samer Singh Email [email protected]; [email protected]
Objective: This study aimed to evaluate the epidemiological and clinicopathological spectrum of ocular malignancies among patients presenting to a teaching hospital in Northern India.
Methods: A total of 246 histopathologically diagnosed patients with ocular malignancies were included in the study. Tumor type and size, primary origin and location of tumor, clinical staging, radiological findings, histopathological type, and treatment outcomes were assessed.
Results: Overall, males over 55 years of age were most commonly affected and the majority of cases were primary ocular or adnexal malignancies (n = 226; 91.87%). The eyelids and periocular structures (n = 92; 37.40%) were the most commonly involved site, followed by the orbit (n = 72; 29.27%), ocular surface (n = 46; 18.70%) and intraocular region (n = 36; 14.63%). The majority of the patients (n = 68; 27.64%) were managed by primary surgical excision and reconstruction. However, 46 patients (18.70%) with advanced lesions underwent neoadjuvant chemotherapy followed by surgical excision and more extensive orbital lesions were treated by exenteration followed by adjuvant chemotherapy (n=48; 19.51%), while patients with metastatic tumor were given palliative chemotherapy/external beam radiation therapy (n= 46; 18.70%). Overall, 45.12% of patients were cured completely, 15.45% showed a partial response to the treatment, 13.04% had progressive disease and 16.67% demonstrated disease recurrence.
Conclusion: A clinicopathological analysis of ocular malignancies at a teaching hospital in Northern India indicated the preponderance of primary ocular malignancies, with eyelid sebaceous gland carcinomas being the most common pathological diagnosis. Most of our patients had advanced and extensive disease among them majority belonged to the rural background and poor socio-economic status.
Keywords: ocular malignancy, tumors, diagnosis, primary cancer, sebaceous gland carcinoma, neoadjuvant chemotherapy
Introduction
Ocular malignancies comprise not only primary malignant tumors arising from the eyeball and its adnexa but also primary malignant lesions extending from neighboring structures such as the nose, paranasal sinuses, and cranial fossa, as well as secondary metastases in the orbit.1 Globally, retinoblastomas (RB) are the most common type of pediatric intraocular malignant tumor, while uveal melanomas are the most common among adults.2 However, in India, retinoblastomas are more common than melanomas.2 The condition is bilateral and hereditary in approximately 40% of patients worldwide.3
Eyelid tumors are also more prevalent in Asian countries like India.4 The most frequent types of malignant eyelid tumors are basal cell carcinomas (BCC), sebaceous gland carcinomas (SGC) and squamous cell carcinomas (SCC) with these tumors represent about 90% of all ocular malignancies.1 In contrast, lymphomas, rhabdomyosarcomas and lacrimal gland adenocarcinomas are common primary orbital malignancies.5 In adults, the most common type of orbital metastasis comprises carcinomas from the breast, prostate gland, kidney, testes and gastrointestinal tract.6 Orbital metastasis in children is rarer and mainly arises from adrenal neuroblastomas, Wilm’s tumors or Ewing’s sarcomas.7
The clinical presentation of ocular malignancies varies depending on a range of factors, including geographical location, ethnicity, literacy and socio-economic status. In developing countries, ocular malignancies are often diagnosed at an advanced stage when curative treatment is no longer possible. Moreover, the clinical presentation of ocular malignancies may simulate that of benign conditions, resulting in delays in diagnosis and tumor metastasis.8 The aim of this study was to assess the clinicopathological spectrum of ocular malignancies in a teaching hospital in northern India. These findings may be helpful in the diagnosis and management of affected patients.
Patients and Methods
This retrospective study was conducted between March 2014 and February 2019 at the Regional Institute of Ophthalmology, Institute of Medical Sciences, Banaras Hindu University, Varanasi, Uttar Pradesh, India. A total of 246 cases of histopathologically confirmed malignant tumors of the eyeball and ocular adnexa (including the eyelid, conjunctiva, and orbit) were reviewed. Old cases treated elsewhere and cases with less than three months of follow-up were excluded from the study. Patients were managed by multidisciplinary approach; radio-imagings were done in the department of radiodiagnosis, FNAC/histopathological examinations were performed in the department of pathology and radiation was given in the department of radiotherapy.
This study was approved by the ethical committee of the Institute of Medical Sciences, Banaras Hindu University, Varanasi, and the methods adhered to the tenets of the Declaration of Helsinki. Prior informed consent was obtained from the patients regarding participation in a research study, review of their medical records and to have their data including clinical images published.
Various demographic and clinical variables were recorded for each patient, including their age, gender, residence, educational background, socio-economic status, tumor size and anatomical location, time of presentation, histological diagnosis, clinical staging, radiological findings, treatment procedure, and disease course. The extraocular extension was assessed by CT Scan and MRI imaging. The clinical diagnosis was confirmed by fine-needle aspiration cytology of lymph node (FNAC) or histopathological examination from the incisional or excisional biopsy. The eyelid and periocular carcinoma cases were treated with wide local excision with >5 mm safety margins from clinical borders and an appropriate reconstruction. Frozen-section examination of surgical margins was performed in larger tumors or recurrent cases to maintain clear margins. Tissue specimen was fixed in 10% neutral buffered formalin and frozen in liquid nitrogen, paraffin section of 5 µm prepared and stained with hematoxylin and eosin. Histopathological examination was done to identify the pathological type, clearance of surgical margin and degree of differentiation. According to the degree of differentiation, the tumors were graded as well, moderately or poorly differentiated type. Immunohistochemical staining (vimentin positivity) was done in cases of orbital rhabdomyosarcoma. Tumor staging was done according to the 8th edition of TNM staging system proposed by the American Joint Committee on Cancer. Patients were treated either by surgical excision, chemotherapy and/or radiotherapy. The mean (SD) follow-up period was of 18.75 (2.68) months (median; 16.0 months) with a range of 6–35 months. The clinical Follow-up data included the patient outcome and incidence of recurrence.
Statistical analysis was conducted using the Statistical Package for the Social Sciences (SPSS), Version 16 (IBM Corp., Armonk, New York, USA). Results were reported using descriptive statistics. A pP- value of <0.05 was considered statistically significant. Ethical clearance for this study was obtained from the local institutional ethical committee.
Results
A total of 246 patients were included in the study; of these, 138 (56.10%) were male and 108 (43.90%) were female (male-to-female ratio: 1.28:1). The mean age of patients with malignant ocular tumors was 41.5 ± 30.7. Over half (n = 133; 54.07%) were 55 years of age or older, while 21.54% were under 16 years. Almost two-thirds (n = 162; 65.85%) were from rural areas, while the rest lived in urban or semi-urban regions. Overall, 151 patients (61.38%) were illiterate and 40 (16.26%) had only been educated to a preschool level. The majority of the other patients had been educated to primary (n = 25; 10.16%), middle school (n = 11; 4.47%) or secondary (n = 10; 4.07%) level. However, four of the subjects were graduates and five were postgraduates (Table 1). The majority of patients (65.85%) had per capita income (PCI), 1000–5000 Indian rupees while 14.63% of patients had PCI < 1000 rupees. As socio-economic status-concerned patients belonged to upper class (8.13%), upper middle class (10.16%), middle class (26.2%), lower middle class (40.65%) and lower class (14.63%). The mean duration between lesion appearance and consultation was 26.00± 20.05 months. Most of the patients having late consultation belonged to the rural background and low education and income group.
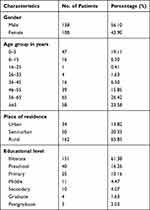 |
Table 1 Demographic Characteristics of Patients with Ocular Malignancies (N = 246) |
Unilateral ocular malignancies were noted in 232 patients (94.31%), with the right and left eyes affected in 68.29% and 26.02% of cases, respectively. The remaining 14 subjects (5.69%) had bilateral involvement. The eyelids and periocular structures were the most common site of involvement (n = 92; 37.40%), followed by the orbit (n = 72; 29.27%), ocular surface (n = 46; 18.70%) and intraocular region (n = 36; 14.63%). The most frequent type of malignancy was a primary ocular and adnexal tumor (91.87%). However, 13 patients (5.28%) had secondary invasion from adjacent structures, including 10 cases (4.07%) of metastasis from the paranasal sinus and nasopharynx and three (1.22%) from intracranial tumors. The remaining seven patients (2.85%) had secondary metastases from carcinomas in other regions of the body, including the breasts (n = 3; 1.22%), lungs (n = 2; 0.81%), testes (n = 1; 0.41%) and prostate (n = 1; 0.41%). Lymph node metastasis was reported in 43 patients (17.48%) and distant metastasis was observed in 11 patients (4.47%) (Table 2).
 |
Table 2 Clinicopathological Characteristics of Patients (N = 246) |
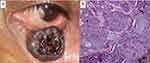
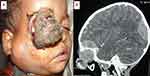
Among patients with eyelid or periocular tumors, the most common pathological diagnosis was SGC (n = 42; 17.07%) (Figure 1), followed by BCC (n = 26; 10.57%) (Figure 2), SCC (n =18; 7.32%), and melanoma (n = 6; 2.44%). For orbital malignancies, the three most common diagnoses were RB (n = 23; 9.35%) (Figure 3), rhabdomyosarcoma (n = 16; 6.50%) (Figure 4), and lacrimal gland adenocystic carcinoma (n = 10; 4.07%). Other diagnoses included osteosarcoma (n = 3; 1.22%), secondary extension from the adjacent structures (n = 13; 5.28%) and metastatic disease (n = 7; 2.85%). RB was the most common intraocular malignancy (n = 24; 5.69%), with other intraocular tumors diagnosed as uveal melanoma (n = 12; 4.88%). Ocular surface squamous neoplasia (Figure 5) accounted for most of the ocular surface tumors (n = 40; 16.26%), followed by melanoma (n = 4; 1.63%) and angiosarcoma (n = 2; 0.81%) (Table 3).
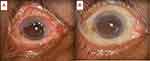 |
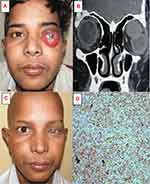
Figure 5 (A) Clinical photograph of a giant ocular surface squamous neoplasia (about 250°) in the right eye. (B) Clinical photograph after four cycles of topical 5FU drops. |
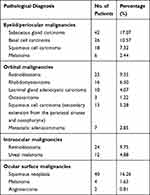 |
Table 3 Pathological Diagnoses According to Malignancy Type (N = 246) |
Upon histopathological examination, over one-third of the eyelid malignancies were well-differentiated (n = 34; 36.96%). However, 30 cases (32.61%) were moderately differentiated and 28 (30.43%) were poorly differentiated or undifferentiated (Table 4). Those with poor differentiation had a poor prognosis.
 |
Table 4 Histopathological Differentiation of Eyelid Malignancies (N= 92) |
With regards to management, the majority of patients (n = 68; 27.64%) underwent primary surgical excision and reconstruction. However, 46 patients (18.70%) with advanced lesions underwent neoadjuvant chemotherapy followed by surgical excision. Neoadjuvant chemotherapy regimen used for SGC & SCC of eyelid is cisplatin 100 mg/m2 on day 1 and 5, fluorouracil (5FU) 1000 mg/m2 on day 1–5 and repeated at every 21 days cycle and for RB 3 weekly cycle of etoposide 150 mg/m2 on day 1 and 2, vincristine 1.5 mg/m2 on day 1 and carboplatin 560 mg/m2 on day 1. Orbital rhabdomyosarcoma was treated by vincristine, adriamycin and cyclophosphamide (VAC) regimen. A total of 38 patients (15.45%) with ocular surface tumors received either topical chemotherapy; 5FU 1% four times daily for 1 week and repeated at 1-week interval (Figure 5B) or immunotherapy in the form of interferon-alpha 2 b (INFαb) 5 MIU/0.5cc perilesional once a week until resolution. More advanced orbital, periorbital, intraocular, and ocular surface tumors were treated by exenteration or enucleation followed by adjuvant chemotherapy (n = 48; 19.51%) (Figure 4B). Patients who were not amenable to surgery were given palliative chemotherapy, along with external beam radiation therapy (n = 46; 18.70%) (Table 5).
 |
Table 5 Distribution of Cases According to Treatment Procedures Undertaken by Patients (N= 246) |
All patients were followed up during treatment and after the completion of treatment to look for a response of chemotherapy, drug toxicities and any recurrence. The clinical response of chemotherapy was assessed as a complete response (disappearance of all known disease, confirmed at ≥4 weeks), partial response (≥50% decrease in tumor size confirmed at ≥4 weeks), no response (<50% decrease or <25% increase in tumor size) and Pprogressive disease (≥25% increase in tumor size or appearance of new lesions). The overall response to neoadjuvant chemotherapy was seen in 69.57% (32/46) cases. There were mild gastrointestinal and hematological toxicities related to chemotherapy. A total of 123 patients (50.0%) were cured, while 38 (15.45%) showed a partial response to the treatment. However, disease progression and recurrence were noted in 32 (13.04%) and 41 (16.67%) patients, respectively (Table 6). Overall, 12 patients (4.88%) died.
 |
Table 6 Distribution of Study Subjects According to Treatment Outcomes After Six Months (N = 246) |
Discussion
The current study investigated the spectrum of ocular malignancies observed over a five-year period at a teaching hospital in Northern India. There was a slight male predominance, with 56.10% of the total cohort being male. A comparable study by Mishra et al conducted at a rural tertiary care center in central India noted a similar percentage of male patients (53.26%) among 92 cases of primary ocular and periorbital malignancies.9 Similarly, another study from Singapore reported that 53.60% of 125 patients with eye cancer were male.10 However, a study conducted in Nepal reported a greater frequency of malignant tumors of the eye and adnexa among female patients (54.20%); this may be because of differing environmental conditions (ie increased pollution and exposure to ultraviolet [UV] radiation) and the greater frequency with which Nepali women take part in outdoor activities.11
Half of the patients (n = 123; 50.07%) in the present study were over 55 years of age. However, there was a bimodal distribution of patients according to age category, with one peak at 15 years and another at 55 years. Comparable findings have been reported in Nepal and Central India.9,11 In addition, the majority of patients (65.85%) in the current study were from a rural background. This reflects the fact that most of the Indian national populations reside in rural areas. Unfortunately, the illiteracy rate was fairly high (57.32%); this is likely due to a lack of access to educational facilities in rural areas. Only nine subjects were graduates or postgraduates, clearly showing that the burden of disease is linked to a lack of awareness and education. Indeed, the majority of patients living in rural areas presented at an advanced stage.
The predominant type of malignancy noted in the present study was a primary ocular or adnexal tumor (91.87%). Only 13 cases (5.28%) were due to secondary invasion from adjacent structures and there were seven patients (2.85%) with metastatic tumors. A study from Bulgaria by Parashkevova et al reported that 56% of patients with orbital malignancies were due to primary orbital tumours, 36% had secondary orbital invasion and 8% had metastatic orbital tumours.6 These variations in the findings may be due to the small sample size (n = 28).12 Regardless, both the present study and that of Parashkevova et al showed a predominance of primary ocular tumors. Other studies often exclude secondary and metastatic tumors from analysis, so further epidemiological research on such tumors is necessary.9,11
In terms of pathological diagnosis, most patients in the current study suffered from a sebaceous gland carcinoma (n= 42; 17.07%) and ocular surface squamous neoplasia (n = 40; 16.26%). Other eyelid malignancies were basal cell carcinoma (10.57%) and squamous cell carcinoma (7.32%). In contrast, other studies have reported a higher incidence of SCC compared to other diagnoses. In Central India, 38.04% of cases were found to have SCC, while a study conducted by Poso et al in Congo reported 33.50% of malignant tumors of the eye and adnexa to be SCC.9,13 Similar findings have also been reported in Nepal and Sudan.11,14 This might be due to patients in these countries having increased exposure to solar UV radiation while working outdoors, especially if employed as farmers or laborers. With regards to sebaceous gland carcinomas, previous studies have observed a wide variation in prevalence rates, ranging from 1.7% in Papua New Guinea to 3.26% in Central India and 31.7% in China.1,15,16 This seems to indicate that geographical factors may be involved in the etiology of this disease. Kass and Hornblass reported sebaceous gland carcinomas to comprise 1.0–5.5% of all malignancies involving the eyelid in the USA.17 High incidence of SGC in Asian countries may be because of the possible influence of racial factors/genetic constitution.18
Following sebaceous gland carcinomas, basal cell carcinomas were the second most frequent pathological diagnosis in the present study (10.57%). Studies from Sudan and Papua New Guinea have reported similar prevalence rates (6.1% and 9.1%, respectively).14,15 However, Misra et al observed a much high incidence of this disease in Central India (17.39%).9 This may be because patients in the latter study had fairer skin which is more at risk when exposed to sunlight. In the current study, the incidence of retinoblastoma was 15.04%; of these, 9.35% were orbital and the remaining 5.69% were intraocular. Wide variations in retinoblastoma prevalence rates have been reported, ranging from 19.8% in Switzerland to 32% in Sudan.14,19 As retinoblastomas are the most frequent ocular malignancy in children, it is likely that the majority of retinoblastoma cases in the study were pediatric in nature.20
The present study also highlighted the different treatment modalities used in the management of ocular malignancies in Northern India, with the mainstay of treatment being surgical excision followed by reconstruction (27.64%). However, neoadjuvant chemotherapy and palliative chemotherapy (18.70% each) were also popular therapeutic modalities. While treatment options for ocular malignancies include radiotherapy, chemotherapy, phototherapy and cryotherapy, surgical resection is the gold standard with success determined by the absence of local infiltration and distant metastasis.21,22 However, surgery may be contraindicated in certain patients due to their poor condition or the need for extensive surgery leading to disfigurement and functional loss.
Conclusion
Primary ocular malignancies are frequently observed in Northern India, with sebaceous gland carcinomas of the eyelid and periocular area being the most common type of malignancy. Unfortunately, late presentation and delays in diagnosis are frequent due to illiteracy, poor socio-economic status and lack of awareness and access to education in rural areas. Multimodal treatments are advocated in advanced cases and steps should be taken to increase education and awareness among the local population to ensure timely diagnosis and management.
Ethical Clearance
The study was approved by the “Ethical Committee” of the Institute of medical Sciences, Banaras Hindu University (EC Registration No. ECR/526/Inst/UP/2014 Dt. 31.1.14) with reference No. Dean/2015-16/EC/85, dated 28.05.2016.
Informed Consent
Informed consent was obtained from the patients/parents of the patients regarding participation in research study, review of their medical records and to have their data including clinical images published.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
No funding was received for this study.
Disclosure
The authors declare no potential conflicts of interest.
References
1. Maurya RP, Bhatia RP, Jain RK, Maurya OPS. Ocular manifestations of the tumors of the paranasal sinuses and nasopharynx. Ann Ophthalmol Glaucoma. 1996;28(4):254–257.
2. Eagle RC
3. Aerts I, Lumbroso-le Rouic L, Gauthier-Villars M, Brisse H, Doz F, Desjardins L. Retinoblastoma. Orphanet J Rare Dis. 2006;1:31. doi:10.1186/1750-1172-1-31
4. Kaliki S, Bothra N, Bejjanki KM, et al. Malignant eyelid tumors in India: a study of 536 Asian Indian patients. Ocul Oncol Pathol. 2019;5(3):210–219. doi:10.1159/000491549
5. Koopman JH, van der Heiden-van der Loo M, van Dijk MR, Bijlsma WR. Incidence of primary malignant orbital tumours in the Netherlands. Eye (Lond). 2011;25(4):461–465. doi:10.1038/eye.2011.9
6. Shields CL, Shields JA, Peggs M. Tumors metastatic to the orbit. Ophthalmic Plast Reconst Surg. 1988;4(2):73–80. doi:10.1097/00002341-198804020-00003
7. Yildiz D, Maden G, Özturan SG, et al. A metastatic tumor of Ewing’s sarcoma in the orbit. Trends Ophthalmol. 2018;1(5). doi:10.32474/TOOAJ.2018.01.000123
8. Al-Mujaini A, Sabt B, Al-Hamdani A. Ocular sebaceous carcinoma: the great masquerader. Oman Med J. 2009;24(3):226–227. doi:10.5001/omj.2009.46
9. Misra S, Patil K, Misra N, Tandon A. Epidemiological study and treatment outcome of primary ocular and adnexal malignancies in a rural Indian tertiary eye care center. Niger J Ophthalmol. 2016;24(2):67–70. doi:10.4103/0189-9171.195197
10. Lee SB, Au Eong KG, Saw SM, Chan TK, Lee HP. Eye cancer incidence in Singapore. Br J Ophthalmol. 2000;84(7):767–770. doi:10.1136/bjo.84.7.767
11. Thakur SK, Sah SP, Lakhey M, Badhu BP. Primary malignant tumours of eye and adnexa in Eastern Nepal. Clin Exp Ophthalmol. 2003;31(5):415–417. doi:10.1046/j.1442-9071.2003.00688.x
12. Parashkevova B, Balabanov C, Stateva D. Orbital tumors: clinical cases presentation. J Int Med Assoc Bulgaria. 2007;13(1):47–50.
13. Poso MY, Mwanza JC, Kayembe DL. Malignant tumors of the eye and adnexa in Congo-Kinshasa. J Fr Ophtalmol. 2000;23(4):327–332.
14. Malik MO, El Sheikh EH. Tumors of the eye and adnexa in the Sudan. Cancer. 1979;44(1):293–303. doi:10.1002/1097-0142(197907)44:1<293::aid-cncr2820440150>3.0.co;2-r
15. Verma N, Murthy DP, Kerek A. Orbital malignancy in Papua New Guinea: A 21 year review. Aust N Z J Ophthalmol. 1999;27(1):27–31. doi:10.1046/j.1440-1606.1999.00156.x
16. Ni Z. Histopathological classification of 3510 cases with eyelid tumor. Zhonghua Yan Ke Za Zhi. 1996;32(6):435–437.
17. Kass LG, Hornblass A. Sebaceous carcinoma of the ocular adnexa. Surv Ophthalmol. 1989;33(6):477–490. doi:10.1016/0039-6257(89)90049-0
18. Zurher M, Hintschich C, Gamer A, Bunce C, Collin J. Sebaceous carcinoma of the eyelid: a clinicopathological stydy. Br J Ophthalmol. 1998;82(9):1049–1055. doi:10.1136/bjo.82.9.1049
19. Deprez M, Uffer S. Clinicopathological features of eyelid skin tumors: a retrospective study of 5504 cases and review of literature. Am J Dermatopathol. 2009;31(3):256–262. doi:10.1097/DAD.0b013e3181961861
20. Marshall EC. Epidemiology of tumors affecting the visual system. Optom Clin. 1993;3(3):1–16.
21. Maurya RP, Bhushan P, Singh VP, et al. Clinico-pathological response of neoadjuvant chemotherapy in advance ocular carcinoma. World J Surg Med Radiat Oncolol. 2012;1:25–31.
22. Wali U, Al-Mujaini A. Sebaceous gland carcinoma of the eyelid. Oman J Ophthalmol. 2010;3(3):117–121. doi:10.4103/0974-620X.71885
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.