Back to Journals » Cancer Management and Research » Volume 12
Clinical Significance of Down-Regulated CD70 and CD27 Expression in Poor Prognosis of Esophageal Squamous Cell Carcinoma
Authors Shan T, Zhao X, Zhang Z, Wang J, Zhang Y, Yang Y, Zhao S
Received 6 December 2019
Accepted for publication 19 June 2020
Published 5 August 2020 Volume 2020:12 Pages 6909—6920
DOI https://doi.org/10.2147/CMAR.S241377
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
This paper has been retracted.
Ting-ting Shan, 1,* Xuan Zhao, 2,* Zhen Zhang, 2,* Jing-pu Wang, 1 Yi Zhang, 2 Yang Yang, 1 Song Zhao 1, 3
1Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 2Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou 450052, People’s Republic of China; 3School of Medical Sciences, The Key Laboratory of Thoracic Tumor of Zhengzhou City, Zhengzhou 450052, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Song Zhao
Department of Thoracic Surgery, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450052, People’s Republic of China
Tel +86 1 367 366 5008
Email [email protected]
Yi Zhang
Biotherapy Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan 450052, People’s Republic of China
Tel +86 1 513 892 8971
Email [email protected]
Introduction: CD27 is a co-stimulatory immune checkpoint molecule in the tumor necrosis factor receptor superfamily. CD27 regulates the generation and maintenance of T cell immunity by binding to CD70 and regulating B-cell activation and immunoglobulin synthesis.
Materials and Methods: CD27 and CD70 expression were assessed in esophageal squamous cell carcinoma (ESCC) compared to normal tissue samples in the GSE53625 dataset of 179 paired cases and in 153 Chinese cases using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and immunohistochemistry. The correlation was also investigated between CD27 and CD70 expression and immune-related pathways, including CD8+ T cell recruitment, function, and other inhibitory immune checkpoints.
Results: Levels of both CD27 and CD70 expression were down-regulated in ESCC compared to the paired normal tissues. CD27 and CD70 expression was mainly present in lymphocytes surrounding and infiltrating the tumor lesions but rarely expressed in tumor cells. Lost expression of CD27 and CD70 was associated with clinicopathological features, including depth of tumor invasion and better patient survival. Furthermore, CD27 expression was significantly associated with levels of CD8A, GZMB, IFNG, the CD8+ T cell recruitment-associated chemokines (CXCL9, CXCL10, and CXCL11), and CD8 receptors (CCR5, CXCR6, and CXCR3), while CD70 expression was inversely associated with levels of immunosuppressive checkpoints (PD-L1, PD-L2, and HHLA2).
Conclusion: Detection of CD70/CD27 expression could be further verified as a biomarker for ESCC early detection and prognosis prediction.
Keywords: esophageal cancer, CD27, CD70, biomarker, prognosis
Introduction
Esophageal cancer is the eighth most common cancer and the sixth leading cause of cancer-related deaths in the world.1,2 Histologically, esophageal cancer can be classified into adenocarcinoma and squamous cell carcinoma (SCC)3 with a 5-year survival rate between 15% and 25%.4 Esophageal SCC (ESCC) is a major subtype of esophageal cancer, with a very high incidence rate, especially in Eastern and Central Asia; approximately 90–95% of all esophageal cancers diagnosed in the world are ESCC.5 To date, treatment of ESCC is limited to surgery for early stage diseases and adjuvant or neoadjuvant chemotherapy and/or radiotherapy,6 and immune-targeted therapy is limited for ESCC patients.7 Thus, there is an urgent need to identify and evaluate novel ESCC diagnostic and prognostic biomarkers and therapeutic targets for early diagnosis, prognosis, and treatment options. In recent years, the discovery of the signal axis of immune checkpoints, like the CTLA4 and CD274 (PD-L1)/PDCD1 (PD-1) axes, has brought a new era for cancer immunotherapy. Evidence in anti-tumor activity of the immunological checkpoint inhibitors is also accumulating.8 In this regard, understanding the regulation of the CD27 and CD70 interaction could be a novel strategy for cancer immunotherapy.9
CD27 is a co-stimulatory immune checkpoint molecule in the tumor necrosis factor receptor superfamily and functions to generate and maintain T cell immunity. Specifically, upon binding to CD70, CD27 can regulate B cell activation and immunoglobulin synthesis.10 After binding to CD70, CD27 can promote cell survival, enhance T cell and B cell receptor-mediated proliferation signals, and increase effector function.10 In addition, CD27 signaling can increase production of the T cell growth/survival factor IL-2,11,12 leading to either improved T cell function or dysfunction, depending on CD27 expression level, duration, and ability to bind to CD70.13–15 Thus, CD70 expression is strictly regulated and CD70 transient expression occurs only in activated T cells and B cells, or in antigen presenting dendritic cells (DC) and natural killer (NK) subpopulations.10 However, compared to very limited CD70 expression in normal cells, CD70 was reported to be up-regulated in human cancers16,17 or in chronic viral infections.13 Accumulating evidence supports the notion that the CD27–CD70 interaction enhances anti-tumor immunity.18
In this study, we first analyzed CD70 and CD27 expression in ESCC and para-cancerous tissues using the online GSE53625 dataset and then associated their expression with clinicopathological features and outcomes in ESCC patients. We then confirmed these data using our cohort of patients.
Materials and Methods
GSE53625 Dataset
We first retrieved the GSE53625 dataset of esophageal cancer from the GEO website (www.ncbi.nlm.nih.gov/gds/), which included differentially expressed genes in esophageal cancer compared to normal tissues, as well as clinicopathological parameters, like age, sex, pathological staging, T staging, N staging, and M staging. This dataset contains two cohorts of normal and cancerous esophageal tissue samples, for a total of 179 cases; however, this dataset did not specify ESCC localizations. We collected and analyzed data on CD27 and CD70 mRNA levels (see below).
Tissue Samples
The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) and written informed consent was received before each patient according to the Declaration of Helsinki. We consecutively collected 153 patients into this study from the First Affiliated Hospital, Zhengzhou University between November 2013 and January 2015. The patients underwent thoracic surgery to remove ESCC that was diagnosed according to the tumor nodule metastasis (TNM) staging system.19 No patients received any preoperative chemotherapy or radiation therapy. Fresh normal mucosa and ESCC tissue samples were collected after their surgical resection for qRT-PCR analysis. In addition, we obtained paraffin blocks from 126 ESCC patients for immunohistochemical analysis.
Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
Both tumor and normal esophageal tissue specimens were acquired following surgery, transferred to the lab, and immediately washed with phosphate-buffered saline (PBS) twice. Total cellular RNA was isolated using the TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA) according to the manufacturer’s instructions. cDNA was then synthesized using the reduction-assisted first-chain c-DNA synthesis kit (Symbid Fisher Scientific, Pittsburgh, PA, USA) according to the manufacturer’s protocol. The upper limit mRNA level of repeated use was quantified using Stratagene Mx3005P (Agilent Technologies, Santa Clara, CA, USA). qPCR was amplified using a premixed Tap Kit (Takara Bio, Inc., Otsu, Japan) according to the manufacturer’s instructions and GAPDH mRNA was used as the loading control. The qPCR conditions were an initial 95°C for 2 min followed by 40 cycles of 95°C for 15 s and 60°C for 1 min using the Primescript TM RT reagent kit (Takara, Primescript TM RT reagent Kit, Beijing, China). Each sample was amplified in triplicate and repeated at least once. The data were quantified and normalized to the control using the 2ΔΔCq method.20 The primer sequences were CD70, 5ʹ-CGTCCCACCCAAGTGACTC-3ʹ and 5ʹ-GCTTTGGTCCCATTGGTCG-3ʹ; CD27, 5ʹ-CGGTATGCAAGGATCACACTG-3ʹ and 5ʹ-CAGAGAGGCACTACTGGGCT-3ʹ; GAPDH, 5ʹ-GGAGGAGATCCCTCCAAAT-3ʹ and GGCTGTTGTCTACTTCTGG-3ʹ, which were synthesized by Sangon Biotech (Shanghai, China).
Immunohistochemistry
Paraffin blocks were retrieved from the Pathology Department and sectioned into 4-µm thick sections for immunohistochemistry. In brief, the tissue sections were dewaxed and re-hydrated and then, incubated with an antigen retrieval solution. The tissue sections were then treated with 3% H2O2 and 5% goat serum. Next, the sections were incubated with an anti-CD27 or anti-CD70 antibody [CD27 Clone EPR8569, Abcam (Cambridge, UK); CD70 Clone #301731, R&D Systems/Thermo Fisher Scientific (Waltham, MA)] at 4°C overnight. After rewarming to room temperature for 1 h, the sections were incubated with a secondary biotinylated antibody, and diaminobenzidine was used as a chromogen. Hematoxylin was used for nuclear counterstaining. The sections were observed using light microscopy and all images were acquired at 200× magnification and assessed by two pathologists independently using a semi-quantitative immunoreactivity score (IRS). According to the proportion of positive tumor cells examined, the tissues were scored based on percent positive staining as follows: 0 (0%), 1 (0–25%), 2 (26 −50%), 3 (51 −75%), and 4 (76–100%). Staining intensity was scored as 0 (no staining), 1 (week staining), 2 (intermediate staining), or 3 (strong staining). IRS was calculated as staining intensity × proportion of positive tumor cells, ranging between 0 and 12. Patients with a total score < 4 were considered low expressing and those with a total score ≥ 4 were considered high expressing. Accordingly, we divided patients into two groups for each marker: high CD27 group (n = 46) and low CD27 group (n = 80), and high CD70 group (n = 40) and low CD70 group (n = 86).
Statistical Analysis
Data were analyzed using the SPSS 22.0 software (IBM, Armonk, NY, USA) and GraphPad Prism 7.0 software (GraphPad software, Inc., La Jolla, CA, USA). The paired t test was used to compare expression of CD27 and CD70 between tumor and adjacent normal tissues, while CD70 and CD27 expression in each group were compared using the Mann–Whitney U-test and association of their expression with clinicopathological parameters from patients was analyzed using the chi-square test. The Kaplan–Meier curves and the Log-rank test were used to determine associations between CD27 and CD70 expression with overall survival of patients. A P value < 0.05 was considered statistically significant.
Results
Association of CD27 and CD70 mRNA Levels with Clinicopathological Parameters from ESCC Patients in the GSE53625 Dataset
We first assessed CD27 and CD70 expression and their association with clinicopathological parameters from ESCC patients using the GSE53625 dataset (www.ncbi.nlm.nih.gov/gds/). This dataset contains two cohorts of ESCC patients: 119 and 60 pairs of ESCC and normal esophageal tissues. Our analysis showed that levels of CD27 and CD70 mRNA were lower in ESCC tissues than in the paired normal tissues, while CD27 expression was inversely associated with depth of tumor invasion (P = 0.026), but CD27 and CD70 expression were not associated with other clinicopathological parameters (Table 1).
 |
Table 1 Association of CD27 and CD70 Expression with Clinicopathological Data from the GSE53626 Dataset |
Association of CD27 and CD70 Expression with Prognosis of ESCC Patients
We then analyzed CD27 and CD70 levels in our cohort of 153 ESCC patients and found that both CD27 and CD70 mRNA levels were significantly lower in ESCC tissues compared to para-cancer normal tissues (P < 0.0043 and P < 0.0029, respectively; Figure 1A and B). Our Kaplan–Meier curves and Log-rank test showed that both CD27 and CD70 expression were significantly associated with better overall survival of ESCC patients (P = 0.0027 and P = 0.045; Figure 1C and D).
Association of CD27 and CD70 Expression with Clinicopathological Parameters from Our Cohort of ESCC Patients
Next, we determined the association of CD27 and CD70 expression with clinicopathological parameters from our patients and found that CD27 expression was inversely associated with tumor T stage and N stage, while CD70 expression was inversely associated with tumor N stage (Table 2).
 |
Table 2 Association of CD27 and CD70 mRNA Levels with Clinicopathological Data from 153 Patients |
Expression of CD27 and CD70 Proteins in ESCC Tissues and Association with Overall Survival
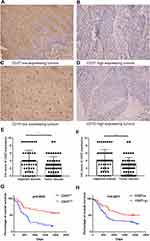
Since protein is the functional unit of any protein-coding gene, we measured CD27 and CD70 protein expression using immunohistochemistry in 126 paired surgical tissue samples from ESCC patients. We divided the patients into CD27 low-expressing tumors (n = 70; Figure 2A) and CD27 high expressing tumors (n = 56; Figure 2B), and CD70 low-expressing tumors (n = 86; Figure 2C) and CD70 high expressing tumors (n = 40; Figure 2D). We found that both CD27 and CD70 protein expression were significantly lower in ESCC tissues compared to normal esophageal tissues (P < 0.0001 and P < 0.05, respectively; Figure 2E and F). Moreover, CD27 and CD70 expression were significantly associated with better overall survival of patients (P = 0.0071 and P = 0.0022, respectively; Figure 2G and H).
Association of CD27 and CD70 Protein Expression with Clinicopathological Parameters from ESCC Patients
We associated CD27 and CD70 protein expression with clinicopathological parameters from ESCC patients and found that CD27 protein expression was inversely associated with tumor T and N stages, while CD70 protein expression was inversely associated with tumor T stage, clinical stage, and N stage (Table 3).
 |
Table 3 Association of CD27 and CD70 Protein Expression with Clinicopathological Data from 126 ESCC Patients |
CD27 and CD70 Expression and Association with Prognosis Using the GSE53625 Dataset
To confirm our current data, we searched, retrieved, and analyzed GSE53625 data for CD27 and CD70 mRNA levels in ESCC samples. We found that both CD27 and CD70 mRNA levels were significantly lower in ESCC tissues compared to para-cancer normal tissues (both P < 0.0001; Figure 3A and B), which was consistent with our cohort of Chinese patients. The Kaplan–Meier curves and the Log-rank test showed that CD70 mRNA levels were significantly associated with better overall survival of patients (P=0.041; Figure 3C. P = 0.047; Figure 3D).
Association of CD27 and CD70 Expression with the Recruitment, Function, and Inhibitory Immune Checkpoints of CD8T Cells
We further determined the association of CD27 and CD70 mRNA expression with the recruitment, function, and inhibitory immune checkpoints of CD8 T cells. Our data showed that CD27 expression was significantly associated with CD8A (Figure 4A), GZMB (Figure 4C), and IFNG (Figure 4B), as well as with CD8 T cell recruitment of related chemokines CXCL9 (Figure 4D), CXCL10 (Figure 4E), and CXCL11 (Figure 4F), and their receptors CCR5 (Figure 4G), CXCR6 (Figure 4H), and CXCR3 (Figure 4I). However, CD70 expression was inversely associated with levels of immunosuppression checkpoint markers PD-L1 (Figure 4J), PD-L2 (Figure 4K), and HHLA2 (Figure 4L).
Discussion
Immunotherapy has gained increasing attention in the field of cancer research. Specifically, monoclonal antibodies (mAbs)-targeting CTLA-4, PD-1, or PDL-1 have shown clinical potential for effectively controlling and treating human cancers.21 Abnormal expression of CD70 has also been documented in blood malignancies and solid tumors as a marker of adverse clinical outcomes,16,22-24 indicating a novel target for cancer treatment. ESCC is one of the most aggressive cancers in the world due to lack of early detection and effective treatment options.25 In the current study, we analyzed CD27 and CD70 expression in ESCC and found that their mRNA levels were frequently reduced in ESCC tissues compared to para-cancer tissues. Our immunohistochemical staining also confirmed that expression of CD27 and CD70 proteins was also decreased in ESCC, which was associated with poor overall survival of ESCC patients.
CD27 signaling can inhibit tumor growth. For example, mice that overexpressed and constitutively expressed CD70 on CD11c-positive cells exhibited a stronger tumor-specific CD8+ T cell response and rejection of tumor cells compared to wild-type mice.26,27 Approximately one-third of patients with Hodgkin’s or diffuse large B cell lymphoma had CD27 or CD70 germline defects,28–30 and CD70 somatic mutations or deletions were common in diffuse large B cell lymphomas and Burkitt lymphomas,31,32 further suggesting that reduced activity of CD27 and CD70 signaling could contribute to development of these malignancies in humans. Other previous studies reported that a monoclonal antibody against CD27 helped to promote the rejection of CD8+ T-cell-dependent tumors in mice,33–35 which was consistent with CD40-mediated dendritic cell dependence and subsequent CD8+ T-cell initiation of CD70 signaling.36–38 In the tumor microenvironment, agonistic anti-CD27 antibodies were able to potently boost multiple aspects of endogenous responses and tumor immunity,35 while Varlilumab, a monoclonal antibody against human CD27, caused CD8+ T-cell-dependent tumor rejection in transgenic mice of human CD27 protein and enhanced human T cells in vitro.39,40 A previous study also showed that CD27 levels were lower in cancer patients compared to healthy donors,41 but there are no reports of CD70 expression in ESCC. Taken together, these studies all confirmed that CD27 and CD70 have anti-tumor properties in human cancers and in animal models. Our current data also showed that CD27 and CD70 expression was reduced in ESCC tissues, which was associated with unfavorable ESCC prognosis.
To better understand the association of CD27 and CD70 expression with ESCC prognosis, we confirmed our data using the GSE53625 dataset. Both our data and the dataset showed reduced CD27 and CD70 expression in ESCC and poor survival of ESCC patients. In our analysis, we first optimized our cut-off point for both CD27 and CD70 by dividing CD27 and CD70 expression using median criteria: median for high vs low level of CD27 and CD70 mRNA levels, and median for high vs low CD27 and CD70 protein expression. Although these criteria are arbitrary, they are frequently used in the literature for semi-quantifying RT-qPCR or immunohistochemical data. We found that loss of CD27 and CD70 expression was significantly associated with unfavorable ESCC prognosis. However, a previous study of serum CD27 levels in 96 lung cancer patients showed that high CD27 levels were associated with poorer lung cancer prognosis compared to those with low serum CD27 levels.41 However, screening of the Cancer Cell Line Encyclopedia (CCLE) showed that approximately 90% of cell lines had no or very low expression of CD27, CEACAM1, CTLA4, LRIG1, PDCD1LG2, or TNFRSF18, which was associated with poor survival phenotypes.42 Moreover, another previous study reported that CD70 expression in tumor-associated fibroblasts was associated with poor survival of colorectal cancer patients,43 while an additional study showed an association of CD70 expression with breast cancer to lung-specific metastasis, and that CD70+ cells (but not CD70− cells) possessed self-renewal and differentiation potential.44 To date, there are no publications listed in PubMed showing an association of CD27/CD70 expression in human esophageal cancer tissues, although a previous study reported dysregulated expression of immune checkpoints, including CD27, in T cells of esophageal cancer patients.45 Thus, our current study is novel and demonstrates an association of reduced CD27/Cd70 expression with poor survival of ESCC patients. We acknowledge that our data may be inconsistent with some previous studies of other solid tumors, and thus future studies with larger sample sizes from multiple institutions are needed to confirm our current data.
Furthermore, we speculated that the reduced level of CD27 and CD70 expression in ESCC could lead to inhibition of the effector T cells in the ESCC microenvironment. We, therefore, analyzed levels of CD8+ T lymphocyte recruitment and function, as well as inhibitory immune checkpoint-related proteins in ESCC tissues and found that CD27 levels were significantly associated with CD8A levels, functional molecules (GZMB and IFNG), CD8T cell recruitment related chemokines (CXCL9, CXCL10, and CXCL11), and receptors (CCR5, CXCR6, and CXCR3), whereas CD70 levels were inversely associated with levels of immunosuppression checkpoint markers PD-l1, PD-l2, and HHLA2. Thus, these results suggest that down-regulated CD27 and CD70 expression may be accompanied by decreased CD8 T cell recruitment, function, and inhibitory immune checkpoint markers, which in turn affects ESCC prognosis. Indeed, CD27 is a co-stimulatory molecule that is expressed on CD4+ and CD8+ T lymphocytes as an important factor for immune activation. A previous study using a novel CD27 agonist antibody (αhCD27) and peptide vaccine showed their efficacy on active cancer immunotherapy.46 Furthermore, PD-1 blockade and CD27 stimulation activated and synergized CD8+ T-cell-driven antitumor immunity in multiple tumor models.47 CD70–CD27 interactions are important for the regulation of adaptive immunity. However, further research is needed to confirm our current findings.
There are limitations to our current study. First, we used an ex vivo model and did not explore the association of CD27 and CD70 using functional experiments. Moreover, we only detected CD27/CD70 expression in ESCC and para-tumor tissues without assessing their expression in stromal cells. These deficiencies limit our data interpretation.
Conclusion
In our current study, we demonstrated that CD27 and CD70 mRNA levels and protein expression were down-regulated in ESCC tissues and that CD27 and CD70 expression were associated with better overall survival of ESCC patients. Thus, CD27 and CD70 may be useful as biomarkers for ESCC early detection and prognosis.
Abbreviations
ESCC, esophageal squamous cell carcinoma; DC, dendritic cell; GZMB, granzyme B; IFNG, interferon gamma.
Data Sharing Statement
The datasets generated and analyzed during the present study are available from the corresponding authors on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (Zhengzhou, China) and written informed consent was received before each patient according to the Declaration of Helsinki. All data published here are under the consent for publication.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li F, Zhu DY, Yang Y, Wu K, Zhao S. Overexpression of calcyphosine is associated with poor prognosis in esophageal squamous cell carcinoma. Oncol Lett. 2017;14(5):6231–6237. doi:10.3892/ol.2017.6973
2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–2150. doi:10.1200/JCO.2005.05.2308
3. Siewert JR, Ott K. Are squamous and adenocarcinomas of the esophagus the same disease? Semin Radiat Oncol. 2007;17(1):38–44. doi:10.1016/j.semradonc.2006.09.007
4. Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381(9864):400–412. doi:10.1016/S0140-6736(12)60643-6
5. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology. 2018;154(2):360–373. doi:10.1053/j.gastro.2017.08.023
6. Liu Y, Xiong Z, Beasley A, D’Amico T, Chen XL. Personalized and targeted therapy of esophageal squamous cell carcinoma: an update. Ann N Y Acad Sci. 2016;1381(1):66–73. doi:10.1111/nyas.13144
7. Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, et al. Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541(7636):169–175. doi:10.1038/nature20805
8. Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nat Rev Clin Oncol. 2017;14(8):463–482. doi:10.1038/nrclinonc.2017.43
9. Riether C, Schurch C, Ochsenbein AF. Modulating CD27 signaling to treat cancer. Oncoimmunology. 2012;1(9):1604–1606. doi:10.4161/onci.21425
10. Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17(3):275–281. doi:10.1016/j.coi.2005.04.004
11. Matter M, Mumprecht S, Pinschewer DD, et al. Virus-induced polyclonal B cell activation improves protective CTL memory via retained CD27 expression on memory CTL. Eur J Immunol. 2005;35(11):3229–3239. doi:10.1002/eji.200535179
12. Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120(1):168–178. doi:10.1172/JCI40178
13. Matter M, Odermatt B, Yagita H, Nuoffer JM, Ochsenbein AF. Elimination of chronic viral infection by blocking CD27 signaling. J Exp Med. 2006;203(9):2145–2155. doi:10.1084/jem.20060651
14. Nolte MA, van Olffen RW, van Gisbergen KPJM, van Lier RAW. Timing and tuning of CD27-CD70 interactions: the impact of signal strength in setting the balance between adaptive responses and immunopathology. Immunol Rev. 2009;229:216–231. doi:10.1111/j.1600-065X.2009.00774.x
15. Penaloza-MacMaster P, Ur Rasheed A, Iyer SS, Yagita H, Blazar BR, Ahmed R. Opposing effects of CD70 costimulation during acute and chronic lymphocytic choriomeningitis virus infection of mice. J Virol. 2011;85(13):6168–6174. doi:10.1128/JVI.02205-10
16. Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. CD70+ non-Hodgkin lymphoma B cells induce Foxp3 expression and regulatory function in intratumoral CD4+CD25 T cells. Blood. 2007;110(7):2537–2544. doi:10.1182/blood-2007-03-082578
17. Jak M, Mous R, Remmerswaal EB, et al. Enhanced formation and survival of CD4+ CD25hi Foxp3+ T-cells in chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(5):788–801. doi:10.1080/10428190902803677
18. Lorenz MG, Kantor JA, Schlom J, Hodge JW. Anti-tumor immunity elicited by a recombinant vaccinia virus expressing CD70 (CD27L). Hum Gene Ther. 1999;10(7):1095–1103. doi:10.1089/10430349950018094
19. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol. 2010;17(7):1721–1724. doi:10.1245/s10434-010-1024-1
20. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
21. Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi:10.1038/nature10673
22. Bertrand P, Maingonnat C, Penther D, et al. The costimulatory molecule CD70 is regulated by distinct molecular mechanisms and is associated with overall survival in diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2013;52(8):764–774. doi:10.1002/gcc.22072
23. Wischhusen J, Jung G, Radovanovic I, et al. Identification of CD70-mediated apoptosis of immune effector cells as a novel immune escape pathway of human glioblastoma. Cancer Res. 2002;62(9):2592–2599.
24. Law CL, Gordon KA, Toki BE, et al. Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res. 2006;66(4):2328–2337. doi:10.1158/0008-5472.CAN-05-2883
25. Tentzeris V, Lake B, Cherian T, Milligan J, Sigurdsson A. Poor awareness of symptoms of oesophageal cancer. Interact Cardiovasc Thorac Surg. 2011;12(1):32–34. doi:10.1510/icvts.2010.247213
26. Arens R, Schepers K, Nolte MA, et al. Tumor rejection induced by CD70-mediated quantitative and qualitative effects on effector CD8+ T cell formation. J Exp Med. 2004;199(11):1595–1605. doi:10.1084/jem.20031111
27. Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29(6):934–946. doi:10.1016/j.immuni.2008.10.009
28. Alkhairy OK, Perez-Becker R, Driessen GJ, et al. Novel mutations in TNFRSF7/CD27: clinical, immunologic, and genetic characterization of human CD27 deficiency. J Allergy Clin Immunol. 2015;136(3):703–712 e710. doi:10.1016/j.jaci.2015.02.022
29. Izawa K, Martin E, Soudais C, et al. Inherited CD70 deficiency in humans reveals a critical role for the CD70-CD27 pathway in immunity to Epstein-Barr virus infection. J Exp Med. 2017;214(1):73–89. doi:10.1084/jem.20160784
30. Abolhassani H, Edwards ES, Ikinciogullari A, et al. Combined immunodeficiency and Epstein-Barr virus-induced B cell malignancy in humans with inherited CD70 deficiency. J Exp Med. 2017;214(1):91–106. doi:10.1084/jem.20160849
31. de Miranda NF, Georgiou K, Chen L, et al. Exome sequencing reveals novel mutation targets in diffuse large B-cell lymphomas derived from Chinese patients. Blood. 2014;124(16):2544–2553. doi:10.1182/blood-2013-12-546309
32. Scholtysik R, Nagel I, Kreuz M, et al. Recurrent deletions of the TNFSF7 and TNFSF9 genes in 19p13.3 in diffuse large B-cell and Burkitt lymphomas. Int J Cancer. 2012;131(5):E830–E835. doi:10.1002/ijc.27416
33. French RR, Taraban VY, Crowther GR, et al. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109(11):4810–4815. doi:10.1182/blood-2006-11-057216
34. Ahrends T, Babala N, Xiao Y, Yagita H, van Eenennaam H, Borst J. CD27 agonism plus PD-1 blockade recapitulates CD4+ T-cell help in therapeutic anticancer vaccination. Cancer Res. 2016;76(10):2921–2931. doi:10.1158/0008-5472.CAN-15-3130
35. Roberts DJ, Franklin NA, Kingeter LM, et al. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33(8):769–779. doi:10.1097/CJI.0b013e3181ee238f
36. Taraban VY, Rowley TF, Al-Shamkhani A. Cutting edge: a critical role for CD70 in CD8 T cell priming by CD40-licensed APCs. J Immunol. 2004;173(11):6542–6546. doi:10.4049/jimmunol.173.11.6542
37. Feau S, Garcia Z, Arens R, Yagita H, Borst J, Schoenberger SP. The CD4(+) T-cell help signal is transmitted from APC to CD8(+) T-cells via CD27-CD70 interactions. Nat Commun. 2012;3:1–9.
38. Taraban VY, Rowley TF, Tough DF, Al-Shamkhani A. Requirement for CD70 in CD4+ Th cell-dependent and innate receptor-mediated CD8+ T cell priming. J Immunol. 2006;177(5):2969–2975. doi:10.4049/jimmunol.177.5.2969
39. Ramakrishna V, Sundarapandiyan K, Zhao B, Bylesjo M, Marsh HC, Keler T. Characterization of the human T cell response to in vitro CD27 costimulation with varlilumab. J Immunother Cancer. 2015;3:37. doi:10.1186/s40425-015-0080-2
40. He LZ, Prostak N, Thomas LJ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191(8):4174–4183. doi:10.4049/jimmunol.1300409
41. Kashima J, Okuma Y, Hosomi Y, Hishima T. High serum soluble CD27 level correlates with poor performance status and reduced survival in patients with advanced lung cancer. Oncology. 2019;97(6):365–372.
42. Xiong D, Wang Y, You M. Tumor intrinsic immunity related proteins may be novel tumor suppressors in some types of cancer. Sci Rep. 2019;9(1):10918. doi:10.1038/s41598-019-47382-3
43. Inoue S, Ito H, Tsunoda T, et al. CD70 expression in tumor-associated fibroblasts predicts worse survival in colorectal cancer patients. Virchows Archiv. 2019;475(4):425–434. doi:10.1007/s00428-019-02565-1
44. Liu L, Yin B, Yi Z, et al. Breast cancer stem cells characterized by CD70 expression preferentially metastasize to the lungs. Breast Cancer. 2018;25(6):706–716. doi:10.1007/s12282-018-0880-6
45. Xie J, Wang J, Cheng S, et al. Expression of immune checkpoints in T cells of esophageal cancer patients. Oncotarget. 2016;7(39):63669–63678. doi:10.18632/oncotarget.11611
46. Riccione KA, He LZ, Fecci PE, et al. CD27 stimulation unveils the efficacy of linked class I/II peptide vaccines in poorly immunogenic tumors by orchestrating a coordinated CD4/CD8 T cell response. Oncoimmunology. 2018;7(12):e1502904. doi:10.1080/2162402X.2018.1502904
47. Buchan SL, Fallatah M, Thirdborough SM, et al. PD-1 blockade and CD27 stimulation activate distinct transcriptional programs that synergize for CD8(+) T-cell-driven antitumor immunity. Clin Cancer Res. 2018;24(10):2383–2394. doi:10.1158/1078-0432.CCR-17-3057
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.