Back to Journals » Open Access Emergency Medicine » Volume 13
Clinical Profile and Treatment Outcome of Aluminum Phosphide Poisoning in Felege Hiwot Referral Hospital, Northwest Ethiopia: A Retrospective Study
Authors Bogale DE, Ejigu BD, Muche TA
Received 28 March 2021
Accepted for publication 25 May 2021
Published 16 June 2021 Volume 2021:13 Pages 239—248
DOI https://doi.org/10.2147/OAEM.S313181
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Hans-Christoph Pape
Dereje Endeshaw Bogale,1 Birtukan Demilew Ejigu,2 Tsigereda Amsalu Muche3
1Private Practice, Addis Ababa, Ethiopia; 2Felege Hiwot Referral Hospital, Bahir Dar, Ethiopia; 3Amhara Public health Institute, Bahir Dar, Ethiopia
Correspondence: Dereje Endeshaw Bogale Email [email protected]
Background: Aluminum phosphide (AlP) is an effective fumigant and rodenticide which is a commonly used agent for self-poisoning in parts of Ethiopia. AlP poisoning results in serious manifestations involving many vital organs and it has high mortality. Despite its high incidence and mortality, studies on AlP poisoning in Ethiopia are lacking. Our objective was to study the clinical profile and treatment outcome of AlP poisoning in Felege Hiwot Referral Hospital (FHRH), a major referral hospital in Ethiopia.
Methods: It is a retrospective study conducted over all consecutive AlP poisoning cases who presented to the emergency department (ED) of the hospital from March 2018 to August 2020. A questionnaire was used to collect data from patient medical records. All data were analyzed using SPSS 25.
Results: A total of 125 patients were studied. Females were 57.6% and males were 42.4%. Age of patients ranged from 12 to 60 with mean age 28.5 years. The average number of AlP tablets taken was 1.2 (3.6 gm) and the average time of arrival to hospital after AlP ingestion was 4.8 hours. Nausea and vomiting were the commonest presenting features seen in 74.4% of the cases followed by hypotension which was seen in half of the cases. A wide range of laboratory findings and complications were also observed. Cases of AlP poisoning with hypotension were managed in the ICU with dopamine infusion, magnesium sulphate, hydrocortisone, and calcium gluconate in addition to gastric lavage and fluid administration. Those cases without hypotension were managed with gastric lavage and maintenance fluid only. Overall mortality from AlP poisoning was 31.2%.
Conclusion: With a treatment protocol used in FHRH, mortality from AlP poisoning was 31.2%. Using this protocol in resource limited settings might give opportunities to reduce mortality from AlP poisoning.
Keywords: aluminum phosphide, poisoning, magnesium sulphate, protocol, hypotension
Background
Aluminum phosphide (AlP) is a cheap and commonly used rodenticide. It is also an effective solid fumigant that is frequently used for grain preservation. AlP is marketed as dark grey 3-gram tablet and the common brand names are Celphos, Alphos, Synfume, Phostek, Phostoxin, Phosfume and Quickphos. Because it is freely available in the market and accessibility is not controlled in developing countries, it is one of the commonly used agents for self-poisoning in different parts of the developing world.1,2
AlP liberates a highly toxic phosphine gas (PH3) when it comes in contact with water or moisture or with hydrochloric acid (HCL) in the stomach.1–3 Toxicity to humans is most commonly after ingestion of AlP although toxicity from inhalation and absorption from the skin are possible.2,3 When AlP is ingested, phosphine gas is released from the reaction of AlP with HCL and water in the stomach. This highly toxic phosphine gas (PH3) then diffuses through the gastrointestinal tract and distributed throughout the body resulting in systemic toxicity.2 Phosphine inhibits cytochrome c oxidase which leads to the inhibition of oxidative phosphorylation and cellular respiration by up to 70%. Overproduction of reactive oxygen species (ROS) with subsequent cellular damage leads to eventual cell death.4 Organs with higher oxygen demands such as the heart, lung, kidney, liver and brain are more sensitive to PH3-induced damage involving oxygen free-radicals production.5
The most frequently seen clinical features of AlP poisoning include nausea, vomiting, abdominal pain and hemodynamic instability.6,7 Other features may include restlessness, headache, diarrhea, fatigue, cough, dyspnea, cyanosis and central nervous system (CNS) involvement resulting in seizure and varying degrees of altered level of consciousness.7,8 Cardiovascular involvement results in tachycardia, palpitation, marked hypotension, and ultimately unresponsive shock. AlP poisoning may have complications such as hepatic failure, pancreatitis, pleural effusion, pulmonary edema, acute respiratory distress syndrome (ARDS), congestive heart failure, arrhythmia, acute renal failure, and disseminated intravascular coagulation.3,8,9 Several electrocardiogram (ECG) changes ranging from ST segment elevation/depression, T wave changes, supraventricular tachycardia, atrial flutter/fibrillation, QRS interval prolongation, variable degrees of heart block, to life threatening ventricular tachycardia and ventricular fibrillation were observed in different studies.10–12
Laboratory abnormalities associated with AlP poisoning include leucopenia, leukocytosis, hyperglycemia, increased creatinine and blood urea nitrogen (BUN), increased serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT), metabolic acidosis and electrolyte abnormalities of serum potassium and magnesium.9,13–16
Treatment of AlP poisoning is mainly supportive as there is no effective antidote. Many agents have been proposed and tried in experimental and clinical studies. Magnesium sulfate (MgSO4), as an antioxidant and cell membrane stabilizer, has been commonly used as therapeutic agent in AlP poisoning although contradictory results have been reported concerning its use. Some studies indicate that AlP poisoning causes hypomagnesemia supporting the mortality benefit of supplementing magnesium sulfate,15,16 while others showed AlP poisoning was not associated with hypomagnesemia and claimed magnesium sulfate did not improve survival.17,18
Other agents which may serve as antidotes for AlP poisoning include melatonin, coconut oil, N-acetylcysteine, sodium selenite, vitamin C and E, triiodothyronine, liothyronine, vasopressin, milrinone, Laurus nobilis L., 6-aminonicotinamide, boric acid and acetyl-L-carnitine.2,19–21
Mortality from AlP poisoning ranges from 37% to 100%. Its fatal dose for an adult human being ranges from 0.15 to 0.5 grams.8,9,14
Although published papers on AlP poisoning in Ethiopia are lacking, a study done in Northwest Ethiopia showed rodenticides were the commonest agents for self-poisoning and it was associated with high mortality.22 The aim of our study was to characterize the clinical profile of these patients and assess their treatment outcome.
Methods
Study Design and Period
This study is an institution-based retrospective study that was conducted over all consecutive cases of AlP poisoning who presented to Felege Hiwot Referral Hospital (FHRH) from March 2018 to August 2020 over a period of two and half years.
Study Setting
Felege Hiwot Referral Hospital is a major referral hospital in Bahir Dar city, Northwest Ethiopia with a catchment population of more than 5 million. All cases of AlP poisoning were first seen in the emergency department. Gastric lavage with normal saline (NS) was done on every patient. All patients were also administered intravenous (IV) fluids, but central venous pressure was not monitored. Patients were closely monitored for hemodynamic instability and other vital signs. All cases of AlP poisoning who had hypotension, defined as systolic blood pressure (SBP) less than 90mmHg, were managed in the intensive care unit (ICU) with dopamine infusion, magnesium sulphate, hydrocortisone IV injection, and calcium gluconate infusion. All these treatments were given to AlP poisoning cases with hypotension.
Supportive Treatment in the Emergency Department
-Gastric lavage with 1–2 liters of Normal Saline (NS) for all patients (Potassium permanganate (KMnO4) is not available).
-NS 2–3 liters to run fast for hypotensive patients.
-Maintenance fluid, NS/5%DW 1 liter every 8 hours for non-hypotensive patients.
Regimen in the ICU (for Hypotensive Patients)
-Dopamine 5µg/kg/minute infusion.
-Hydrocortisone 200mg IV QID for 48 hours.
-Calcium gluconate 1 vial (10mL of 10% solution) with 10mL of NS to run over 10 minutes slowly, QID for 48 hours.
-MgSO4 1gm (2mL of 50% solution) with 5mL of NS IV push over 2 minutes, and MgSO4 0.5 mg (1mL) with 1 mL of lidocaine IM on each buttock.
-Then MgSO4 1gm in 100mL of NS after 1 hour, 2 hours and 3 hours for consecutive 3 hours.
-Then MgSO4 1gm in 100mL of NS IV TID for 48 hours.
Data Collection
Case records of AlP poisoning were retrieved with their record numbers. Data were collected by using a structured questionnaire from the patient charts. Information about sociodemographic variables, number of AlP tablets ingested, time lag from ingestion to hospital arrival, clinical features, laboratory abnormalities, treatment administered, patient outcome and duration of hospital stay was collected. Patient outcome was categorized as improved, expired and patient went against medical advice. All the cases in this study were also categorized as hypotensive and non-hypotensive based on systolic blood pressure (SBP). Available ECG papers obtained from the medical records were interpreted by a cardiologist.
Statistical Analysis
Data completeness was checked manually and those with incomplete information were excluded from the study. The collected data were then transferred to SPSS version 25 statistical software and analyzed. Simple descriptive statistics such as frequencies, percentages, mean, and standard deviation were used to characterize the variables and comparison between two groups was made by independent t-test for quantitative data. A P-value less than 0.05 was considered statistically significant.
Ethical Considerations
Ethical clearance with reference number H/R/T/T/D/3/935 was obtained from Amhara Public Health Institute. Permission to conduct this study at Felege Hiwot Referral Hospital was confirmed by the hospital administration office. Patient informed consent was waived as it was a retrospective medical record review and patient data were maintained with confidentiality. The data were used only for the study purpose.
Results
During the 30 months study period from March 2018 to August 2020, 141 case records of AlP poisoning were traced. Patient records with incomplete documentation, ambiguous history and/or poisoning involving multiple agents were excluded and a total of 125 case records were included in this study.
There were 72 (57.6%) females and 53 (42.4%) males with a female to male ratio of 1.4. Age of patients ranged from 12 to 60 with mean age 28.5 years and standard deviation of 10.8. Most cases were in the age group of 11 to 30 years. Fifty-three cases (42.4%) were in the age group 21–30 years and 36 cases (28.8%) were in the age group 11–20 years (Figure 1).
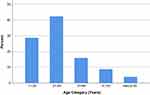 |
Figure 1 Percentage distribution of cases by age category. |
Ninety-seven (77.6%) cases were from rural areas and nearby small towns and only 28 (22.4%) cases were from Bahir Dar city. All cases were suicidal and the two most commonly reported reasons for taking the poison were disagreement with family members (11.2%) and quarrel with opposite sex partner (10.4%). Other reasons were documented in 4% of the cases. In the majority of cases (74.4%), the reason for taking the poison was unknown.
The amount of AlP tablets taken ranged from one-fourth of a tablet to 6 tablets. Nearly half (51.2%) of the cases took 1 full tablet only, and the majority of the rest took either half tablet or two tablets. The average number of AlP tablets taken was 1.2 (3.6 gm) in this study. The average number of tablets for the improved and expired group was 1.0 tablet and 1.6 tablet respectively. This difference was found statistically significant (P=0.006).
The time lag between ingestion of AlP tablet and arrival to the hospital ranged from as early as 20 minutes to 24 hours. The majority of patients arrived within 4 hours (61%), and nearly 90% arrived within 7 hours. Only 6 patients arrived after 12 hours. The mean time lag to hospital arrival was 4.8 hours with standard deviation of 3.67 hrs. The average time lag for the improved and expired group of patients was 5.0 hours and 4.8 hours respectively and the difference was not statistically significant (P=0.82).
The most common clinical presentations observed were nausea and vomiting seen in 93 patients (74.4%). Two patients had bloody vomiting. Epigastric pain was seen in 42 patients (33.6%). Sixty-three patients (50.4%) were hypotensive. Fifty-three patients (42.4%) were hypotensive at the time of admission and additional 10 patients (8%) developed hypotension after they were admitted. Pulse was not palpable in 34 patients due to severe hypotension. The mean pulse rate for the remaining 91 patients was 96/min. Other less commonly observed presenting features include headache, fatigue, diarrhea, restlessness, and change in mentation ranging from confusion to deep coma.
Observed laboratory abnormalities include leukocytosis, leukopenia, anemia, hyperglycemia, elevated BUN and creatinine levels. Liver enzyme abnormalities seen include increased levels of SGOT up to seven times upper normal level (UNL) and increased SGPT up to four times UNL. Slight elevation in serum alkaline phosphatase and bilirubin levels, hypokalemia, hyperkalemia and hyperchloremia were noted in few patients (Table 1).
 |
Table 1 Observed Laboratory Results |
There were two cases who developed severe anemia in this study. Both took one tablet of AlP, and they had unrecordable blood pressure at admission. Hemoglobin done on second day of admission was 3.8 g/dl and 5.3 g/dl. Both had elevated creatinine (4.15 mg/dl and 3.26 mg/dl) and low WBC count (1.6×103/mm3 and 1.83×103/mm3). Peripheral morphology and bilirubin levels were not done in these patients. The first one died on the 2nd day of admission and the other (HIV patient on antiretroviral therapy, regimen unknown) improved after transfusion and discharged after 11 days of stay in the hospital.
Electrocardiographic changes observed in our study include sinus tachycardia, short PR interval, ST segment depression, ST segment elevation, peaked T waves, T wave inversions, 2° AV block and incomplete LBBB.
Complications which patients developed during their course in the hospital include congestive heart failure, pleural effusion, pulmonary edema, ARDS, acute renal failure and acute hepatic failure.
Out of the total 125 patients included in this study, 39 (31.2%) patients expired, 78 (62.4%) improved, and 8 patients (6.4%) went against medical advice. On sub-group analysis, all the recorded deaths were in the hypotensive group. Out of the 63 patients with hypotension (SBP<90mmHg), 9 patients expired on arrival or shortly after admission before treatment with MgSO4, calcium gluconate, hydrocortisone and dopamine infusion was started in the ICU. All the remaining 54 cases in the hypotensive group were treated with MgSO4, IV Calcium gluconate, IV hydrocortisone and dopamine infusion. Mortality in this treated group (hypotensive patients treated with the above regimen) was 55.6% (30/54). On the other hand, all the cases in the non-hypotensive group were managed with gastric lavage and maintenance fluid in the emergency department but they were not treated with the above regimen. There was no mortality in this group (Table 2).
 |
Table 2 Sub-Group Analysis |
The average duration of hospital stay was 2 days and the maximum duration of stay, recorded in one patient, was 11 days.
Discussion
Self-poisoning with pesticides is common in Ethiopia. Commonly used pesticides used for poisoning vary from place to place. In some places, organophosphates are the most common poisoning agents,23–25 whereas in other places including areas of Northwestern Ethiopia, rodenticides, which include aluminum phosphide, are the most frequently used agents.22 In fact, there are limited studies in Ethiopia on AlP poisoning to know how common it is in different areas. We found only one study, a case series done on 7 patients in Addis Ababa, Central Ethiopia.26
Aluminum phosphide is a commonly available fumigant and rodenticide. In Northwest Ethiopia, AlP is marketed as dark grey 3-gram tablets and the common brand names are Celphos, Alphos and Quickphos. The tablet is composed of about 44% inert ingredients (ammonium carbonate) to prevent decomposition of the tablet, while aluminum phosphide is about 56% of the tablet. On coming in contact with water or moisture or OH radical or hydrochloric acid in the stomach, a 3 g tablet of AlP releases 1 g of phosphine gas, the active ingredient of AlP, which has fishy or garlic odor.1,9 AlP toxicity is mediated by phosphine gas which is thought to inhibit mitochondrial cytochrome oxidase and cellular oxygen utilization. In vitro studies of phosphine have shown that it acts on the complex IV cytochrome oxidase to inhibit the electron transport chain, but studies have shown that the inhibition of cytochrome oxidase by phosphine in vivo is much less. This inhibits oxidative respiration by 70% and ultimately results in a marked decrease in mitochondrial membrane potential.27,28 AlP also exerts its toxicity by generating disproportionate cellular oxidative stress through increased reactive oxygen species (ROS) and compromising the cellular antioxidant protection mechanism. It was noted that there is an increase in the activity of superoxide dismutase and an inhibition of catalase resulting in excessive hydrogen peroxide (H2O2) load and significantly higher levels of malonyl dialdehyde (MDA) indicating enhanced lipid peroxidation in patients with AlP poisoning.29,30 This PH3-induced damage involving oxygen free-radicals production and lipid peroxidation results in eventual cell death.
Some studies showed that the time elapsed from ingestion to hospital arrival and number of tablets ingested are important determinants of mortality. In our study, the majority of patients were females (57.6%) and most of the cases (71.2%) were in the age group between 10 and 30 years. The mean time of presentation was 4.8 hr. All of these findings agree with studies done on self-poisoning in similar geographic areas.22,31 More than half of the studied patients (51.2%) took only one full tablet while 76% of our cases took between half tablet and 2 tablets. The average number of tablets taken was 1.2 (3.6 gm). We found a statistically significant difference in the amount of AlP tablets consumed between the improved group and the expired group, a finding which agrees with other studies.13,32
In many studies, gastrointestinal symptoms including nausea, vomiting and epigastric pain are the most common presenting symptoms of AlP poisoning.6,7,33 In the present study, nausea and vomiting were observed in 74.4% of the cases and epigastric pain was observed in 33.6% which is in agreement with these studies. Life threatening upper gastrointestinal hemorrhage after ingestion of AlP has been reported, and it is thought to occur due to the corrosive effects of phosphides and phosphine on body tissues.34,35 There were two patients who had bloody vomiting in our study.
Hypotension, which is the major lethal consequence of AlP ingestion, is the cardinal feature of acute AlP toxicity. It is usually refractory to treatment and does not respond to massive fluid administration. The cause of hypotension in AlP poisoning is thought to be due to the direct toxic effects of phosphine on cardiac myocytes, fluid loss and adrenal gland damage.28 The frequency of hypotension reported in different studies is different.33,35 In our study, a total of 63 patients (50.4%) had hypotension. Cardiac arrhythmias are another common cardiovascular manifestation of AlP poisoning. Siwach et al reported electrocardiographic changes in 30 patients and supraventricular tachycardia, atrial flutter/fibrillation, ST-T changes, variable degrees of heart block and life-threatening ventricular tachycardia and ventricular fibrillation, which seem to be due to phosphine induced toxic myocarditis were observed.12 It was not possible to know the frequency of these arrhythmias in our study, but the observed electrocardiographic changes include sinus tachycardia, ST segment depression, ST segment elevation, peaked T waves, T wave inversions, 2° AV block and incomplete LBBB which agrees with the above study by Siwach et al.
Laboratory abnormalities associated with AlP poisoning, some of which may have prognostic value, include leukopenia, leukocytosis, hyperglycemia, increased liver enzymes, increased serum creatinine, changes in serum levels of magnesium, potassium and metabolic acidosis.9,36,37 Due to non-uniformly done laboratory investigations on the studied patients, we were not able to assess these criteria in terms of prognosis in this study. Rare case reports have been published on intravascular hemolysis after ingestion of aluminum phosphide. Some of these cases were reported in glucose-6-phosphate dehydrogenase (G6PD) deficient patients while others were in patients with normal levels of glucose-6-phosphate dehydrogenase.38–40 We found two cases of AlP poisoning who developed severe anemia in our study which might be due to intravascular hemolysis.
Magnesium levels and the benefit of magnesium sulphate in AlP poisoning has been a subject of much debate. In addition, there are not enough studies on the benefit of MgSO4 in this poisoning. Chugh et al41 studied 105 patients and observed hypomagnesemia consistently in AlP poisoning cases who were not treated with MgSO4, while magnesium levels rose immediately after parenteral MgSO4 administration and remained persistently above normal in the group treated with MgSO4. The same author, on another study involving 155 patients, observed the benefit of MgSO4 in reducing mortality. The efficacy of MgSO4 has been attributed to the rapid correction of magnesium levels and it was suggested that hypomagnesemia might be responsible for the high mortality of patients with AlP poisoning.15 Magnesium helps in scavenging free radicals hence acts as an antioxidant and it is a cell membrane stabilizer and antiarrhythmic agent. Magnesium sulphate therapy has been consistently used for AlP poisoning in many studies and claimed to reduce mortality by up to 50%.42–44 On the contrary, Siwach et al in the study involving 50 patients, found no significant difference in AlP-related mortality in patients treated with and without magnesium sulphate and found no evidence of hypomagnesemia in these patients.17
In our study, overall mortality was 31.2%. Reported mortality from AlP poisoning is generally high and it ranges from 37% to 100%.8 In a study on 418 cases by Chugh et al, the overall mortality from AlP poisoning was 77.2%.45 The relatively lower mortality may be due to exposed tablets taken by many patients in this study, as AlP liberates its toxic component (PH3) when exposed to air/moisture which results in reduced toxicity.
Since 2012, FHRH has been using a protocol for hypotensive AlP poisoning cases with magnesium sulfate, calcium gluconate, hydrocortisone and dopamine infusion, in addition to gastric lavage and fluid administration. In contrast, there is no standard treatment for AlP poisoning in Ethiopia and almost all other hospitals manage this poisoning with supportive treatment with gastric lavage, fluid administration and dopamine infusion.22 Before the above protocol was introduced to FHRH, hypotensive AlP poisoning cases used to be managed similarly and, mortality was almost 100%. Similar “almost 100%” mortality trends were also reported in other countries before interventions (with MgSO4) were introduced.35 Treatment of AlP poisoning cases with only gastric lavage and dopamine infusion were used in the earliest reported series of AlP poisoning cases, and in one study, out of 13 patients who had shock (SBP < 90mmHg), 11 patients died, and one of the two survivors improved after hemodialysis because the patient developed acute renal failure.46 In another earlier study involving 44 patients with AlP ingestion, where the median number of ingested AlP tablets was 1 tablet and the mean time interval to hospital arrival was approximately 2 hours, a total of 42 deaths occurred.30 In the present study, mortality among the hypotensive group treated according to the protocol with MgSO4, Ca gluconate, hydrocortisone and dopamine was 55.6%. Compared with the above figures, the 55.6% mortality in the treated hypotensive group in our study is low and the above-mentioned protocol might help lowering mortality.
Due to the risk of hypermagnesemia, the routine use of MgSO4 for all AlP poisoning cases is not practical and magnesium administration is recommended in patients with low serum magnesium levels.44,47 In this study, because serum magnesium levels could not be determined due to resource limitations, a cut off SBP of 90mmHg was selected and only patients with SBP<90mmHg were treated with the above-mentioned protocol.
Hypotension is an important early sign of toxicity48 and studies suggested low serum magnesium levels and arrhythmias are present in hypotensive patients but not in normotensive patients. Chugh et al reported that magnesium levels were normal in AlP poisoning patients without shock or cardiotoxicity and significant hypomagnesemia was observed in those with evidence of acute cardiotoxicity.49 Similarly, Morteza et al studied 77 patients with arrhythmia after AlP ingestion and all types of arrhythmia, except atrial fibrillation, were seen only in patients with initial SBP ≤ 90mmHg. 88.2% of these patients with SBP ≤ 90mmHg had arrhythmia. Among those with SBP > 90mmHg, only 2 patients had atrial fibrillation.50
Recent reports have emphasized the use of calcium in addition to magnesium in the treatment of AlP poisoning, and Ca gluconate is used to treat ECG changes related to AlP poisoning for its membrane stabilizing effect.44,47 As both hypomagnesemia and arrhythmias are found in hypotensive patients, and also because there is a direct relationship between hypomagnesemia and ECG changes in AlP poisoning,49 treatment of all patients in the hypotensive group with MgSO4 and Ca gluconate in our study is rational. The additional treatments in the protocol, dopamine and hydrocortisone, directly address the hypotension. Hydrocortisone combats shock; reduces the dose of dopamine; and it additionally checks capillary leakage in the lungs to prevent ARDS.1,4,9
ARDS develops lately, hours after patients develop shock,51,52 and pulmonary edema tends to develop 4–48 hours after AlP ingestion.48 In our study, there were 7 patients who had pulmonary edema or ARDS at or after admission and all had unrecordable blood pressure at admission except one patient with SBP of 80mmHg. In AlP poisoning, 95% of deaths occur within 24 hours of AlP ingestion due to shock and arrhythmia, and death after 24 hours is usually due to shock, metabolic acidosis, ARDS and cardiac dysrhythmia.9,50
In view of the points mentioned above, we suggest treating patients with SBP <90mmHg with the above-mentioned protocol or similar protocols is a simple and acceptable approach in resource limited settings where follow-up with serum magnesium levels, cardiac monitoring or blood gas analysis is not possible.
Finally, this study has its own limitations. As it is a retrospective study, certain clinical parameters and laboratory values were not complete due to documentation problems and limitations of the setup. Diagnosis was also based on history. The recommended gastric lavage with KMnO4 solution was not used in this study, because KMnO4 is not available in Ethiopia. Gastric lavage with normal saline, as used in this study, is found only in earlier reports.35,53 While recognizing the limitations of our study, we believe that this study can be a basis for future research. We recommend more studies on magnesium levels in AlP poisoning; the benefit of MgSO4; and preparation of a standard treatment for AlP poisoning.
Conclusion
With a treatment protocol which has been used for more than 9 years in this hospital, mortality from AlP poisoning was 31.2% in this study. We recommend the use of this protocol in resource limited settings, as it might give opportunities to reduce mortality from this poisoning. More studies need to be done and standardized treatment should also be available.
Abbreviations
AlP, aluminum phosphide; ARDS, acute respiratory distress syndrome; ARF, acute renal failure; BP, blood pressure; BUN, blood urea nitrogen; CNS, central nervous system; ECG, electrocardiogram; FHRH, Felege Hiwot Referral Hospital; HCL, hydrochloric acid; ICU, intensive care unit; IV, intravenous; KMnO4, potassium permanganate; MgSo4, magnesium sulfate; PH3, phosphine; SBP, systolic blood pressure; SGOT, serum glutamic oxaloacetic transaminase; SGPT, serum glutamic pyruvic transaminase; SPSS, Statistical Package for Social Sciences; SD, standard deviation; WBC, white blood cell; UNL, upper normal level.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
Ethical clearance with reference number H/R/T/T/D/3/935 was obtained from Amhara Public Health Institute. Permission to conduct this study at Felege Hiwot Referral Hospital was confirmed by the hospital administration office. Patient informed consent was waived as it was a retrospective medical record review and patient data were maintained with confidentiality. The data were used by the investigators only for the study purpose. This study used secondary data from medical records of patients and did not involve human participants.
Acknowledgment
The authors wish to acknowledge the staff of Felege Hiwot Referral Hospital for their cooperation.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Singh Y, Joshi SC, Satyawali V, Gupta A. Acute aluminium phosphide poisoning, what is new? Egypt J Intern Med. 2014;26(3):99–103. doi:10.4103/1110-7782.145298
2. Karimani A, Mohammadpour AH, Zirak MR, et al. Antidotes for aluminum phosphide poisoning - An update. Toxicol Rep. 2018;5:1053–1059. doi:10.1016/j.toxrep.2018.10.009
3. Gurjar M, Baronia AK, Azim A, Sharma K. Managing aluminum phosphide poisonings. J Emerg Trauma Shock. 2011;4(3):378–384. doi:10.4103/0974-2700.83868
4. Ghazi MA. “Wheat pill (aluminum phosphide) poisoning”; Commonly ignored dilemma. A comprehensive clinical review. Professional Med J. 2013;20(6):855–863.
5. Moazezi Z, Abedi SH. A successful management of aluminum phosphide intoxication. Caspian J Intern Med. 2011;2(3):286–288.
6. Alnasser S, Hussain SM, Kirdi TS, Ahmed A. Aluminum phosphide poisoning in Saudi Arabia over a nine-year period. Ann Saudi Med. 2018;38(4):277–283. doi:10.5144/0256-4947.2018.277
7. Louriz M, Dendane T, Abidi K, Madani N, Abouqal R, Zeggwagh AA. Prognostic factors of acute aluminum phosphide poisoning. Indian J Med Sci. 2009;63(6):227–234. doi:10.4103/0019-5359.53386
8. Goel A, Aggarwal P. Pesticide poisoning. Natl Med J India. 2007;20:182–191.
9. Moghadamnia AA. An update on toxicology of aluminum phosphide. Daru. 2012;20(1):25. doi:10.1186/2008-2231-20-25
10. Bogle RG. Aluminium phosphide poisoning. Emerg Med J. 2006;23(1):e3. doi:10.1136/emj.2004.015941
11. Jain D. Persisting ECG changes in Aluminium Phosphide poisoning-a dilemma. Asian J Med Clin Sci. 2013;2.
12. Siwach SB, Singh H, Jagdish KVK, Bhardwaj G. Cardiac arrhythmias in aluminium phosphide poisoning studied by on continuous holter and cardioscopic monitoring. J Assoc Physicians India. 1998;46(7):598–601.
13. Sharma T, Sharma A, Kapoor D. Profile of aluminum phosphide poisoning in a tertiary care institute in the sub-Himalayan region. J Family Med Prim Care. 2018;7(3):581–583. doi:10.4103/jfmpc.jfmpc_231_17
14. Mehrpour O, Alfred S, Shadnia S, et al. Hyperglycemia in acute aluminum phosphide poisoning as a potential prognostic factor. Hum Exp Toxicol. 2008;27(7):591–595. doi:10.1177/0960327108096382
15. Chugh SN, Kumar P, Aggarwal HK, Sharma A, Mahajan SK, Malhotra KC. Efficacy of magnesium sulphate in aluminium phosphide poisoning–comparison of two different dose schedules. J Assoc Physicians India. 1994;42(5):373–375.
16. Chugh SN, Kolley T, Kakkar R, Chugh K, Sharma A. A critical evaluation of anti-peroxidant effect of intravenous magnesium in acute aluminium phosphide poisoning. Magnes Res. 1997;10(3):225–230.
17. Siwach SB, Singh P, Ahlawat S, Dua A, Serum SD. tissue magnesium content in patients of aluminium phosphide poisoning and critical evaluation of high dose magnesium sulphate therapy in reducing mortality. J Assoc Physicians India. 1994;42(2):107–110.
18. Siwach SB, Dua A, Sharma R, Sharma D, Mehla RK. Tissue magnesium content and histopathological changes in non-survivors of aluminium phosphide poisoning. J Assoc Physicians India. 1995;43(10):676–678.
19. Taghaddosinejad F, Farzaneh E, Ghazanfari-Nasrabad M, Eizadi-Mood N, Hajihosseini M, Mehrpour O. The effect of N-acetyl cysteine (NAC) on aluminum phosphide poisoning inducing cardiovascular toxicity: a case-control study. Springerplus. 2016;5(1):1948. doi:10.1186/s40064-016-3630-2
20. Sweilum OAH, Shaban Kandeel F, Abdel Razik Noya D. Management of acute aluminum phosphide toxicity in rat model with a novel intervention, a trial of boric acid. Egypt J Forensic Sci Appl Toxicol. 2017;17(2):57–72. doi:10.21608/ejfsat.2017.46121
21. Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol. 2005;24(4):215–218. doi:10.1191/0960327105ht513oa
22. Endayehu Y, Shenkutie E. Magnitude of acute poisoning and associated factors in Debretabor General Hospital, Ethiopia. J Clin Toxicol. 2019;9:429.
23. Desalew M, Aklilu A, Amanuel A, Addisu M, Ethiopia T. Pattern of acute adult poisoning at Tikur Anbessa specialized teaching hospital, a retrospective study, Ethiopia. Hum Exp Toxicol. 2011;30(7):523–527. doi:10.1177/0960327110377520
24. Teklemariam E. Pattern of acute poisoning in Jimma University Specialized Hospital, South West Ethiopia. World J Emerg Med. 2016;7:290. doi:10.5847/wjem.j.1920-8642.2016.04.009
25. Adinew G, Belay A. Pattern of acute poisoning in teaching hospital, northwest Ethiopia. Int J Pharmacol Toxicol. 2016;4:47. doi:10.14419/ijpt.v4i1.5975
26. Mohammed MS, Negeri A, Tilahun K. A high case fatality report of aluminum phosphide poisoning: a case serious from Aabet hospital. Ethiop Med J. 2017;55(3):July issue.
27. Dua R, Gill KD. Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain. Biochim Biophys Acta. 2004;1674(1):4–11. doi:10.1016/j.bbagen.2004.05.003
28. Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin Toxicol (Phila). 2009;47(2):89–100. doi:10.1080/15563650802520675
29. Chugh SN, Arora V, Sharma A, Chugh K. Free radical scavengers & lipid peroxidation in acute aluminium phosphide poisoning. Indian J Med Res. 1996;104:190–193.
30. Kariman H, Heydari K, Fakhri M, et al. Aluminium phosphide poisoning and oxidative stress: serum biomarker assessment. J Med Toxicol. 2012;8(3):281–284. doi:10.1007/s13181-012-0219-1
31. Adinew GM, Asrie AB, Birru EM. Pattern of acute organophosphorus poisoning at University of Gondar Teaching Hospital, Northwest Ethiopia. BMC Res Notes. 2017;10:149. doi:10.1186/s13104-017-2464-5
32. Navabi SM, Navabi J, Aghaei A, Shaahmadi Z, Heydari R. Mortality from aluminum phosphide poisoning in Kermanshah Province, Iran: characteristics and predictive factors. Epidemiol Health. 2018;40:e2018022. doi:10.4178/epih.e2018022
33. Mathai A, Bhanu MS. Acute aluminium phosphide poisoning: can we predict mortality? Indian J Anaesth. 2010;54(4):302–307. doi:10.4103/0019-5049.68372
34. Hugar B, Praveen S, Sh H, Kainoor J, Shetty S, Raj A. Gastrointestinal Hemorrhage in Aluminum Phosphide Poisoning. J Forensic Sci. 2014;60. doi:10.1111/1556-4029.12588.
35. Bajwa SJ, Bajwa SK, Kaur J, Singh K, Panda A. Management of celphos poisoning with a novel intervention: a ray of hope in the darkest of clouds. Anesth Essays Res. 2010;4(1):20–24. doi:10.4103/0259-1162.69301
36. Pannu AK, Bhalla A, Sharma A, Sharma N. “PGI score”: a simplified three-point prognostic score for acute aluminum phosphide poisoning. Indian J Crit Care Med. 2020;24(9):790–793. doi:10.5005/jp-journals-10071-23555
37. Shadnia S, Mehrpour O, Soltaninejad K. A simplified acute physiology score in the prediction of acute aluminum phosphide poisoning outcome. Indian J Med Sci. 2010;64:532–539. doi:10.4103/0019-5359.75928
38. Sood AK, Mahajan A, Dua A. Intravascular haemolysis after aluminium phosphide ingestion. J R Soc Med. 1997;90(1):47–48. doi:10.1177/014107689709000116
39. Srinivas R, Agarwal R, Jairam A, Sakhuja V. Intravascular haemolysis due to glucose-6-phosphate dehydrogenase deficiency in a patient with aluminium phosphide poisoning. Emerg Med J. 2007;24(1):67–68. doi:10.1136/emj.2006.040097
40. Malakar S, Negi BD, Dutt K, Raina S. Intravascular hemolysis in aluminum phosphide poisoning. Indian J Crit Care Med. 2019;23(2):106–107. doi:10.5005/jp-journals-10071-23128
41. Chugh SN, Kamar P, Sharma A, Chugh K, Mittal A, Arora B. Magnesium status and parenteral magnesium sulphate therapy in acute aluminum phosphide intoxication. Magnes Res. 1994;7(3–4):289–294.
42. Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012;63(1):61–73. doi:10.2478/10004-1254-63-2012-2182
43. Vaidyanathan R, Hg A, Noor A, Adarsh S. Comparative study of management of aluminium phosphide poisoning -our experience. J Evid Based Med Healthcare. 2020;7:2349–2562. doi:10.18410/jebmh/2020/445
44. Navabi SJ, Reza HY. Comparison of the prognosis of the new and old therapeutic protocols in poisoning by phosphide compounds article info. J Kermanshah Univ Med Sci. 2017;21:23–26.
45. Chugh SN, Dushyant RS, Arora B, Malhotra KC. Incidence & outcome of aluminium phosphide poisoning in a hospital study. Indian J Med Res. 1991;94:232–235.
46. Singh S, Dilawari JB, Vashist R, Malhotra HS, Sharma BK. Aluminium phosphide ingestion. Br Med J (Clin Res Ed). 1985;290:1110–1111.
47. Hashemi-Domeneh B, Zamani N, Hassanian-Moghaddam H, et al. A review of aluminium phosphide poisoning and a flowchart to treat it. Arh Hig Rada Toksikol. 2016;67(3):183–193. doi:10.1515/aiht-2016-67-2784
48. Mostafazadeh B (2012). Aluminium Phosphide Poisoning, Toxicity and Drug Testing, Prof. Bill Acree (Ed.), ISBN: 978-953-51-0004-1, InTech. Available from: http://www.intechopen.com/books/toxicity-and-drugtesting/aluminium-phosphide-poisoning.
49. Chugh SN, Jaggal KL, Sharma A, Arora B, Malhotra KC. Magnesium levels in acute cardiotoxicity due to aluminium phosphide poisoning. Indian J Med Res. 1991;94:437–439.
50. Taromsari MR, Shad B, Nargesi DA, Akhoundzadeh N, Karkan MF. The study of various cardiac arrhythmias in patients poisoned with aluminum phosphide (Rice Tablet). Ind J Tox. 2011;5:1–2.
51. Chugh SN, Ram S, Mehta LK, Arora BB, Malhotra KC. Adult respiratory distress syndrome following aluminium phosphide ingestion. Report of 4 cases. J Assoc Physicians India. 1989;37(4):271–272.
52. Bhalla A, Mahi S, Sharma N, Singh S. Polyserositis: an unusual complication of aluminum phosphide poisoning. Asia Pac J Med Toxicol. 2012;1(1):14–17.
53. Agrawal A, Kaur H. Successful treatment of Aluminum phosphide poisoning with limited resources. J Clin Diagn Res. 2010;4:2316–2319.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
