Back to Journals » Clinical Ophthalmology » Volume 14
Clinical Outcomes After Topography-Guided Refractive Surgery in Eyes with Myopia and Astigmatism – Comparing Results with New Planning Software to Those Obtained Using the Manifest Refraction
Authors Brunson PB, Mann PM II, Mann PM, Potvin R
Received 8 September 2020
Accepted for publication 29 October 2020
Published 17 November 2020 Volume 2020:14 Pages 3975—3982
DOI https://doi.org/10.2147/OPTH.S280959
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Phillip B Brunson, 1 Paul M Mann II, 1 Paul Michael Mann, 1 Richard Potvin 2
1Mann Eye Institute and Laser Centers, Houston, TX, USA; 2Science in Vision, Akron, NY, USA
Correspondence: Phillip B Brunson
Mann Eye Institute and Laser Centers, 5115 Main Street, Suite #300, Houston, TX 77002, USA
Email [email protected]
Purpose: To compare clinical outcomes from topography-guided laser refractive surgery based on new planning software to outcomes based on using the manifest refraction.
Design: Single site, two-arm, retrospective chart review.
Methods: Clinical outcomes at a single site after topography-guided LASIK using the Wavelight excimer laser were evaluated, with a target postoperative follow-up time of 90 days. Eligible eyes were those that received on-label topography-guided treatment of myopia or myopic astigmatism with correction based on either the manifest refraction or results from the Phorcides Analytical Engine (PAE). Measures analyzed included the uncorrected (UDVA) and best-corrected (CDVA) distance visual acuity, the magnitude of refractive cylinder after surgery, the refractive error and changes from preoperative CDVA.
Results: The study included 115 eyes in the PAE group and 133 eyes in the Manifest group. Significantly more eyes in the PAE group had a CDVA of 20/15 or better (p = 0.05) and a UDVA of 20/15 or better (p = 0.05). Significantly more eyes in the Manifest group had a UDVA of 20/25 or worse (13/133 vs 1/115 in the PAE group, p = 0.002). There were significantly more eyes in the PAE group with no postoperative refractive cylinder (90% vs 77% in the Manifest group, p = 0.004). No eye in either group had a postoperative CDVA a line or worse than their preoperative CDVA. Three eyes in the Manifest group and no PAE eyes have had subsequent enhancement surgery.
Conclusion: Mean results for postoperative refractive astigmatism, CDVA and UDVA were similar between the groups, but the clinical outcomes for the PAE group appeared less variable, with more eyes having no refractive astigmatism and a higher percentage of eyes having 20/15 or better CDVA and UCVA. The objective nature of the PAE is an advantage.
Keywords: LASIK, laser refractive surgery, topography-guided LASIK, Phorcides, Contoura
A Letter to the Editor has been published for this article.
A Response to Letter has been published for this article.
Plain Language Summary
Laser eye surgery is one of the most common surgeries in the world. Advancements in laser technology, imaging technology and analysis methods have resulted in continuous improvements in visual outcomes. One recent advancement is the ability to smooth the front of the eye with the laser, which can improve optical quality – it is called topography-guided surgery. Results are excellent, but it requires a bit more planning by the eye surgeon and there can be some judgement involved. In this study, we examined whether a new software method to assist with this had any clinical advantages over just using the patient’s prescription from their glasses. The software uses methods originally developed to evaluate contour maps of the Earth to analyze the irregular surfaces on the eye, then automatically incorporates those findings into the surgery plan. We found both methods produced excellent results, but the results using the new software were a bit less variable, and the objective nature of the process is likely to appeal to surgeons.
Introduction
Laser in situ keratomileusis (LASIK) involves reshaping the cornea to reduce or eliminate refractive error in the eye using an excimer laser. First described by Pallikaris et al in 1990, early ablation algorithms treated only the manifest refractive sphere and cylinder of the eye.1 Later efforts included treating all measured aberrations in the eye, both the lower order (sphere and cylinder) and higher order.2 This was met with mixed success, though appropriate management of spherical aberration was identified as important to the quality of vision after surgery. Modern ablation patterns incorporate spherical aberration correction; these wavefront-optimized (WFO) algorithms have become, perhaps, the most common LASIK treatment profiles for myopia and myopic astigmatism.
More recently, attention has been paid to the optical quality of the corneal surface. Development of algorithms designed to improve corneal surface regularity was driven primarily by the desire to treat highly aberrated eyes with significant irregular astigmatism; these eyes were not amenable to treatment based on the measured refraction.3 One resulting algorithm has now been successfully incorporated into treatments for normal eyes – the Contoura® Vision (Alcon, Fort Worth, Texas, USA) topography-guided LASIK procedure. Visual outcomes after Contoura in normal eyes have been excellent, with uncorrected acuity of 20/20 or better in 93% of eyes and 20/12.5 or better in 34% of eyes.4 These results were achieved when the manifest refraction was used for surgical planning, and in eyes where the difference between the topographic astigmatism and manifest refractive cylinder were nominal.
As surgeons gained experience, they began treating eyes with larger differences between topographic astigmatism and refractive cylinder. This raised the question of what to base the actual treatment on. Past efforts have included retaining use of the manifest cylinder,5 use of the topographic astigmatism and axis,6 and some combinations of the above.7 Results have generally been good, but some refractive surprises have been experienced and there is often a requirement for subjective surgeon input. Perhaps the greatest limiting factor is that none of these approaches attempts to better delineate the source of the measured astigmatism differences between topography and the manifest refraction. Two eyes may have similar simulated keratometry from a topographer but very different looking surface power maps, for instance.
A relatively recent and novel approach is provided by the Phorcides Analytical Engine (Phorcides LLC, North Oaks, MN). It was developed based on a combination of geographic imaging software (GIS, often used to evaluate the topography of Earth’s terrain, but adapted for the eye in this application) and optics. The GIS allows more precise determination and characterization of irregularities on the corneal surface. This then allows the expected optical effect (on both sphere and cylinder) of “smoothing” these irregularities to be determined. Modifications to the treatment profile can then be made based on eliminating any number of identified irregularities. The analysis is specific to each eye, and automated, reducing inter-individual variability and reducing the potential for transcription error. The current requirement, in addition to those for standard Contoura treatment, is a data file from a Scheimpflug corneal imaging device, so that the anterior and posterior astigmatism can be appropriately characterized. Recent research has indicated that results obtained when Contoura surgery is planned with the Phorcides Analytical Engine are superior to those achieved when planned with the manifest refraction, based on theoretical8 and actual9 clinical outcomes.
The purpose of the current study was to determine if there were any differences in the refractive and visual acuity outcomes from topography-guided myopic LASIK treatment when planned with the Phorcides Analytical Engine vs planning based on the manifest refraction.
Methods
This study comprised a two-arm retrospective chart review of clinical outcomes after topography-guided LASIK at one site. The study was approved by an institutional review board (Salus IRB, Austin, TX, USA) and a waiver of informed consent was granted; all extracted data from charts were de-identified. The study was conducted in compliance with the tenets of the Declaration of Helsinki, International Harmonization (ICH) guidelines and Good Clinical Practice (GCP). As there was no clinical intervention, registration of the study with any clinical trial registry was not required. Data are not available for sharing.
Using an alpha of 0.05 and a beta (power) of 0.8, a two-sided test of two proportions indicated that 90 eyes in each group would be required to reliably confirm a presumed difference of 20% (40% vs 60%) in the percentage of eyes with 20/16 or better uncorrected visual acuity after surgery. The intent was to collect data from a minimum of 100 eyes treated using new surgical planning software (Phorcides Analytical Engine, PAE) and comparing those eyes to a similar group of eyes that were treated based on the manifest refraction (Manifest). In the Manifest group, the clinic’s specific nomogram adjustments were applied to calculate the programmed treatment. For the PAE group, proprietary software adjusts the manifest sphere and cylinder based on the topography to create the treatment plan. All other surgery parameters were the same. Flaps for all subjects in both groups were made with the Wavelight® FS200 Laser (Alcon, Fort Worth, TX, USA) using a flap thickness of 110 microns. Since this was a retrospective study, dropout was not expected.
Eligible subjects were those that received on-label topography-guided treatment for myopia or myopia with astigmatism using the Contoura treatment profile of the Wavelight excimer laser system (Alcon, Fort Worth, TX, USA). Subjects were excluded if they had clinically significant ocular pathology other than residual refractive error, a history of previous refractive surgery, calculated residual stromal bed thickness less than 250 microns, or suboptimal surgical outcomes that were not related to the method of treatment (eg, flap displacement).
Eligible clinical records from January to August of 2018 were extracted for inclusion in the Manifest group; the practice was using topography-guided treatments based solely on the manifest refraction at that time. Those for the PAE group were extracted from June to December 2019, after the software had been implemented in the practice.
The primary measure of interest was the uncorrected visual acuity (UDVA) achieved after surgery. Other measures compared were the residual refractive cylinder, the spherical equivalent refractive error, and the best-corrected visual acuity (CDVA). Postoperative CDVA and UDVA were also compared to the preoperative CDVA. Note that standard graphs comparing nomograms are not relevant to the analysis of results, as Phorcides applies proprietary algorithms (not nomogram-adjusted) to the manifest refraction to adjust the treatment sphere and cylinder. Comparative data are limited to the actual clinical results achieved.
Electronic data records were reviewed to identify eyes meeting the relevant inclusion and exclusion criteria outlined above; de-identified data from preoperative examination and all postoperative examinations from a period between 1 month and 6 months after surgery were collected from eligible records. For each eye, the postoperative exam with a follow-up period closest to 90 days was selected for analysis. Both eyes of any subject could be included. Age, sex, preoperative refractive error and CDVA were extracted from clinical files for each subject/eye, along with the surgical planning parameters and the actual treatment parameters. Postoperative data included the follow-up time, the postoperative CDVA, UDVA and the manifest refraction. Visual acuities were recorded in Snellen notation but converted to the equivalent log of the minimum angle of resolution (logMAR) notation for statistical analysis. All visual acuity data are monocular.
Statistical analyses were performed using Statistica 12 (TIBCO Software Inc., Palo Alto, CA, USA). An analysis of variance (ANOVA) was used for the comparison of parametric variables. A Chi-squared test was used for non-parametric comparisons. In both cases, p ≤ 0.05 was considered significant.
Results
A review of clinical records identified 115 eyes eligible for inclusion in the PAE group and 133 eyes eligible for inclusion in the Manifest group. Table 1 provides a summary of relevant demographics, including the preoperative refractive status and the postoperative follow-up time. The two groups were reasonably well-matched, with three statistically significant differences between them. The Manifest group had slightly higher preoperative refractive astigmatism, but the mean difference was less than 0.30 D and the treated astigmatism was not statistically significantly different. Follow-up time was also slightly longer in the Manifest group (22 days). However, around 90% of eyes in both groups fell within a 30- to 120-day postoperative follow-up period. No adverse events were identified, and no safety concerns were evident in the data set extracted. Finally, the preoperative best-corrected visual acuity was statistically significantly better in the PAE group, but the mean difference was the equivalent of only a half of a logMAR letter or 1/10 of a line of visual acuity.
 |
Table 1 Demographics, Preoperative Refractive Status and Follow-Up Time |
Table 2 contains the summary of visual acuity and refractive outcomes for the two groups. The mean logMAR UDVA and CDVA were both statistically significantly better in the PAE group, but the differences were minor. Figures 1 and 2 show the distribution of postoperative UDVA and CDVA by group, respectively, to the nearest line of Snellen acuity. Significantly more eyes in the PAE group had a CDVA of 20/15 or better (p = 0.05) and a UDVA of 20/15 or better (p = 0.05). Significantly more eyes in the Manifest group had a UDVA of 20/25 or worse (13/133 vs 1/115 in the PAE group, p = 0.002). Overall CDVA was excellent, with 98% and 99% of eyes in the Manifest and PAE groups, respectively, having 20/20 or better best-corrected acuity. All eyes had a CDVA of 20/25 or better and all but one eye had a UDVA of 20/25 or better (one eye in the Manifest group had a UDVA of 20/40).
 |
Table 2 Clinical Outcomes (n = 133 Manifest, 115 PAE) |
 |
Figure 1 Postoperative uncorrected visual acuity by group. |
 |
Figure 2 Postoperative best-corrected visual acuity by group. |
There was no statistically significant difference in the refractive sphere or spherical equivalent refractions between groups. There was a statistically significant difference in refractive cylinder, but the mean difference was only 0.06 D; both groups had mean values less than 0.10 D. Figure 3 shows the distribution of residual refractive cylinder. A chi-squared test showed that the number of eyes with no residual cylinder was higher in the PAE group than in the Manifest group (90% vs 77%, respectively, p = 0.004).
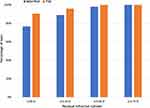 |
Figure 3 Residual refractive cylinder by group. |
Table 3 shows the difference between the preoperative CDVA and the postoperative UDVA and CDVA by group. There were no statistically significant differences between groups for the number of eyes gaining a line of UDVA or CDVA. In both cases and for both groups about 1 in 4 eyes had a VA increase of 1 or more lines. No eye in either group had a postoperative CDVA a line or worse than their preoperative CDVA and 98%/99% of eyes in the Manifest/PAE groups, respectively, had a postoperative UDVA within 1 line or better of their preoperative CDVA.
 |
Table 3 Difference Between Preoperative CDVA and Postoperative UDVA and CDVA |
As a final note, a review of the clinical records showed that 198 eyes (133 Manifest, 65 PAE) had a primary treatment more than 200 days before data extraction. Three of these eyes, all in the Manifest group, had an enhancement in that ~7-month period (2.2%), with no PAE eyes enhanced in the same time period. No post-enhancement acuity or refractive data were included in the analysis above.
Discussion
The data here demonstrate the value of the Phorcides Analytical Engine in the planning of topography-guided ablations. Results achieved utilizing the PAE demonstrate improved visual outcomes relative to those that have been reported for Contoura ablations planned with a variety of approaches, where 54% of eyes had UDVA of 20/15 (versus 60% for Phorcides in the current study) and 94% had UDVA of 20/20 (versus 99% for Phorcides).8 Interestingly, averaging the percentages for the two groups in the current study yields rates of 54% and 95%. Results are also remarkably similar to those reported in a recent study of retrospective data from four surgeons at four different sites (62% of eyes with UDVA of 20/16), which speaks to the relative stability of the outcomes achieved. This is likely a function of the objective nature of the surgical planning.
Visual acuity and residual refractive cylinder data using PAE are better than those reported for topography-guided ablations in some previous large studies using the same laser but using the manifest refraction to plan the ablation profile, while the Manifest results here appear similar or slightly better than those previously reported with the use of the manifest refraction as the treatment input in the same studies; note that inclusion and exclusion criteria may vary between studies.5,10 The PAE results are also superior to those reported in the trial conducted for FDA approval of the Contoura procedure,4 despite the fact that the PAE group eyes were not limited by consideration of the difference between the preoperative corneal and refractive astigmatism values. They also appear better than the results achieved using the topographic cylinder and axis,6 or some combination of the topographic and manifest cylinder axis.7 The approach of identifying specific irregularities on the cornea and compensating for them is the likely reason for this – it is not a function of trying to determine an optimal ratio between two fixed numbers, but of making eye-specific calculations.
As noted earlier, surgeons are unlikely to adopt a treatment algorithm that has the potential for more variability in exchange for slightly improved overall outcomes. The elimination or reduction of outliers is a critical consideration. No PAE eye in the current study had a residual refractive cylinder greater than 0.50D. Every PAE eye had a postoperative UDVA within 1 line or better of their preoperative CDVA, and there were no reported retreatments in the PAE group. This was achieved with a system that objectively determines the treatment refraction, reducing the potential for surgeon ‘hunches’ to result in suboptimal outcomes.
There are limitations to the current study. As a retrospective study, stringent inclusion and exclusion criteria could not be applied. Further, standardization of testing, while very likely in a single site study such as this, cannot be presumed. In a clinical environment, few clinicians would bother to test patients’ vision down to 20/10. Nor is it likely that a refraction would be conducted if a patient had 20/15 uncorrected visual acuity at distance. An important observation here is that these limitations would apply to both groups, so observed differences between the groups in this study are likely to be reliable. In addition, more detailed data such as corneal aberration measurements and subjective satisfaction were not available.
While not part of the results, the surgeons currently using Contoura at this practice commented that they had initially attempted to utilize topography-guided ablations as their preferred treatment, but the highly subjective nature of their early efforts to determine surgical planning drastically limited their use of the technology. For a short time, they also applied a more objective approach;6 while overall results were good, there were several refractive surprises that led them to return to wavefront-optimized ablation profiles. At that time, they felt the visual benefits expected from Contoura in most patients did not outweigh the rare risk of an unpredictable and unsatisfactory outcome. Since adopting the objective approach of the Phorcides Analytic Engine, which implements eye-specific topographic corrections, they have returned to Contoura as their preferred treatment for all eyes that qualify for such treatment.
In summary, topography-guided refractive surgery planned with the Phorcides Analytical Engine yielded improved postoperative uncorrected visual acuity and lower variability in residual astigmatism than treatments based solely off the manifest refraction. The objective nature of the planning appeared to reduce the likelihood of outliers.
Acknowledgments
Angelique Tarazona, BS, COMT from Mann Eye Institute and Laser Centers assisted with data extraction and data checking. This research was conducted with an investigator-initiated study grant from Alcon (IIT #57548373).
Disclosure
Richard Potvin is a consultant to Alcon and Carl Zeiss Meditec. Phillip Brunson is a consultant to Allergan Pharmaceuticals, Eyevance Pharmaceuticals and Novartis Pharmaceuticals. Paul Michael Mann and Paul M Mann II report grants from Alcon, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Pallikaris IG, Papatzanaki ME, Stathi EZ, Frenschock O, Georgiadis A. Laser in situ keratomileusis. Lasers Surg Med. 1990;10:463–468. doi:10.1002/lsm.1900100511
2. Liedel KK, Pettit GH. Alcon CustomCornea platform with LADARWave and LADARVision. Ophthalmol Clin North Am. 2004;17(2):161–vi. doi:10.1016/j.ohc.2004.02.006
3. Holland S, Lin DT, Tan JC. Topography-guided laser refractive surgery. Curr Opin Ophthalmol. 2013;24(4):302–309. doi:10.1097/ICU.0b013e3283622a59
4. Stulting RD, Fant BS, Group TCS, et al. Results of topography-guided laser in situ keratomileusis custom ablation treatment with a refractive excimer laser. J Cataract Refract Surg. 2016;42(1):11–18. doi:10.1016/j.jcrs.2015.08.016
5. Wallerstein A, Gauvin M, Cohen M. Topography-guided ablation targeting the anterior corneal astigmatism yields inferior outcomes vs targeting the manifest refractive astigmatism. J Refract Surg. 2019;35(12):815. doi:10.3928/1081597X-20191030-015
6. Kanellopoulos AJ. Topography-modified refraction (TMR): adjustment of treated cylinder amount and axis to the topography versus standard clinical refraction in myopic topography-guided LASIK. Clin Ophthalmol. 2016;10:2213–2221. doi:10.2147/OPTH.S122345
7. Motwani M. The use of WaveLight(R) Contoura to create a uniform cornea: the LYRA protocol. Part 3: the results of 50 treated eyes. Clin Ophthalmol. 2017;11:915–921. doi:10.2147/OPTH.S133841
8. Stulting RD, Durrie DS, Potvin RJ, et al. Topography-guided refractive astigmatism outcomes: predictions comparing three different programming methods. Clin Ophthalmol. 2020;14:1091–1100. doi:10.2147/OPTH.S244079
9. Lobanoff M, Stonecipher K, Tooma T, Wexler S, Potvin R. Clinical outcomes after topography-guided LASIK: comparing results based on a new topography analysis algorithm with those based on manifest refraction. J Cataract Refract Surg. 2020;46(6):814–819. doi:10.1097/j.jcrs.0000000000000176
10. Ozulken K, Yuksel E, Tekin K, et al. Comparison of wavefront-optimized ablation and topography-guided contoura ablation with LYRA protocol in LASIK. J Refract Surg. 2019;35(4):222–229. doi:10.3928/1081597X-20190304-02
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
