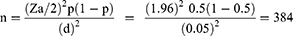Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 14
Clinical Non-Adherence and Its Associated Factors Among HIV-Positive Pediatric Patients Attending HIV Care in South Gondar Zone Public Health Facilities, Northwest Ethiopia, 2021
Authors Tiruneh CM , Emiru TD , Tibebu NS , Abate MW , Nigat AB , Bantie B , Belete A, Walle BG, Legas G , Getu BD
Received 10 December 2021
Accepted for publication 12 January 2022
Published 29 January 2022 Volume 2022:14 Pages 23—32
DOI https://doi.org/10.2147/HIV.S352386
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Chalie Marew Tiruneh,1 Tigabu Desie Emiru,1 Nigusie Selomon Tibebu,1 Moges Wubneh Abate,2 Adane Birhanu Nigat,2 Berihun Bantie,2 Amsalu Belete,3 Belete Gelaw Walle,4 Getasew Legas,3 Bisrat Dessie Getu5
1Department of Pediatrics and Child Health Nursing, College of Health Sciences, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Adult Health Nursing, Debre Tabor University, Debre Tabor, Ethiopia; 3Department of Psychiatry, College of Health Sciences, School of Medicine, Debre Tabor University, Debre Tabor, Ethiopia; 4Department of Pediatrics and Child Health Nursing, College of Health Science and Medicine, Wolaita Sodo University, Wolaita Sodo, Ethiopia; 5Department of Nursing, Debre Tabor Health Sciences College, Debre Tabor, Ethiopia
Correspondence: Chalie Marew Tiruneh, Email [email protected]; Tigabu Desie Emiru, Email [email protected]
Background: Poor clinical adherence is the main factor that hinders ART adherence level in children and its ultimate effect on viral load suppression and decreasing morbidity and mortality of children. Although data from different settings are necessary to tackle such types of problems, the pieces of evidence are limited in the case of clinical adherence level. Therefore, this study was intended to assess clinical non-adherence and its associated factors among HIV-infected pediatrics on highly active antiretroviral therapy.
Methods: A multi-center cross-sectional study was conducted from July 1 to August 30, 2021, among HIV-infected children receiving ART in the South Gondar Zone. Data were collected through face-to-face interviews, and reviewing patients’ documents using a structured checklist. Data were entered into Epi-data version 4.6 and exported to the Statistical Package for Social Science version 23 for analysis. Binary logistic regression was used to assess the association between the factors and the outcome variable. The significance of variables was declared when a p-value was less than 0.05.
Results: From 422 participants, 383 have involved in the study making the response rate of 90.7%. Almost half of the study participants 190 (49.6%) were girls. Two hundred ninety-one (76%) of caretakers were biological mothers, and 203 (53%) did not have adherence supporters. About 179 (46.7%) of caretakers did not disclose the status of the child about the illness. The overall prevalence of non-adherence among children on ART was 31.9% (95% CI: 27.2– 36.6). Rural residency, diagnostic status non-disclosure, no adherence supporter, having no biological caretaker and co-morbid illness were significantly associated with clinical non-adherence of HIV positive children.
Conclusion: Clinical non-adherence among children among HIV-positive children attending care in south Gondar zone health facilities is unacceptably high. Attention shall be given to HIV-positive pediatrics who reside in rural areas, whose status was not disclosed, had no adherence supporter, had a non-biological caretaker, and had comorbidity to have good clinical adherence on ART service.
Keywords: adherence, pediatrics, antiretroviral, south Gondar, Ethiopia
Background
By the end of 2019, about 36.9 million individuals were living with HIV/AIDS worldwide; of which 1.8 were children (age 0–14 years). At the end of 2017, about 21.7 million people living with HIV were accessing antiretroviral treatment.1 A region of East and Southern African countries constitutes around 52% of the global burden and in Ethiopia, nearly 56,514, pediatrics were living with it by the end of 2018. Of which, around 2994 had been newly infected cases.
Antiretroviral therapy (ART) has been shown to delay progression to AIDS, resulting in a greater and more sustained virological and immunological response and improving survival.2 Though Access to ART is increased currently, guidelines recommend that HIV-positive individuals need to have at least 90–95% of adherence towards ART to achieve ART-related goals and targets. However, sustaining adherence to antiretroviral therapy (ART) necessitates intensive and continuous monitoring, and this is a particular challenge for countries in sub-Saharan Africa.2,3
Different scholars agree that, if a patient requires the greatest benefits from HAART in terms of suppression of HIV, then that patient needs to comply with a near-perfect adherence to the clinical requirements of the HAART regimen. Inadequate clinical adherence leads to inadequate ART adherence to antiretroviral therapy which further leads to the emergence of the resistant virus with eventual exhaustion of treatment options and it leads to treatment failure.4
Individuals who have poor clinical adherence may have inadequate medication to take. This inadequacy may be due to missing clinical care follow-up or may not take proper medication due to missing periodic laboratory monitoring used to assess treatment success and to consider regimen change or they may have practices that interfere with the effect of medication. Avoiding practices are practices that are a hindrance for the success of ART treatment, like avoiding hesitating to get support if you need it; avoiding interrupting medication; avoiding despair; avoiding taking other medication without consultation; avoiding food high in sodium, sugar, and saturated fats; avoiding consuming; avoiding excessive alcohol).5
Various social and clinical obstacles confront clinical adherence, where inadequate suppression of viral replication by ART results due to poor adherence to therapy, low potency of the antiretroviral regimens, viral resistance to antiretroviral medications, and pharmacokinetic interactions.6 Transmission of the antiretroviral resistant viruses from person to person further compounds the problem as a clinical and public health challenge.7
Some caregivers may place too much responsibility for managing medications on older children and adolescents before they are developmentally able to undertake such tasks. Adherence can also be jeopardized by social and health issues within a family (eg, substance use, poor physical or mental health, unstable housing, poverty, violence, involvement with the criminal justice system, limited social support).8,9
Although there are different studies on HIV medication adherence,10–14 little is known about the prevalence and factors associated with clinical adherence in HIV-positive children on HAART in the study area. This study aimed to assess clinical non-adherence and its associated factors among HIV-infected pediatrics on highly active antiretroviral therapy in South Gondar zone public health facilities. The findings generated from this study will be used to improve the quality of care for HIV-infected pediatrics, and it could be important for planning interventions and effective strategies for maximizing long-term adherence to highly active antiretroviral therapy. The data collected will be used by organizations involved in this area to deal with the problems associated with clinical non-adherence.
Methods
Study Setting, Design, and Period
A multi-center cross-sectional study was conducted from July 1 to August 30, 2021, among Pediatrics Attending HIV care in the South Gondar Zone public health facilities, Northwest Ethiopia, 2021. The study was carried out in six selected health facilities (Debre Tabor compressive specialized hospital, Mekane Eyesus primary hospital, Nefas Mewucha primary hospital, Addis Zemen primary hospital, Woreta health center, and Wogeda hospital). All these and other health facilities in the South Gondar Zone offer chronic care and follow-up for more than 775 HIV-infected pediatrics.
Study Participants
The target population of this study was all confirmed HIV-infected pediatrics aged under 15 years who had chronic HIV care and follow-up in South Gondar Zone public health facilities. All HIV-infected pediatrics who had chronic HIV care and follow-up at the selected health facilities were included in the study population. Nevertheless, HIV-infected pediatrics with incomplete baseline information and those who did not have a caretaker to give consent, as well as caretakers diagnosed with mental problems and seriously ill, were excluded from the study.
Sample Size and Sampling Technique
Due to the absence of studies on the same problem across the region, the single population proportion formula was used to determine the minimum required sample size with the following statistical assumptions: 50% proportion (p) of clinical non-adherence was taken,5% margin of error; 5% non-response rate; and 95% confidence intervals (CI).
Where n = the required sample size, Zα/2= standard normal variation, p = prevalence (0.5) and d = margin of sampling error (0.05). By considering a 10% non-response rate, the final sample size of our study was 422.
To conduct this study, six public health facilities that provide chronic HIV care and follow-up services were included randomly in the South Gondar Zone. Firstly, a sampling frame was prepared using the patient’s medical registration number, and then, the total sample sizes were assigned proportionally to each health facility. Finally, study participants were taken from each selected health facility using a simple random sampling technique.
Data Collection Tool and Procedure
The checklist was prepared from the current National Consolidated Guidelines for comprehensive HIV prevention, care, and treatment in Ethiopia’s Federal Ministry of Health ART clinic intake and follow-up forms. The checklist focused on the socio-demographic characteristics of study participants, the socio-demographic characteristics of caretakers, and different clinically related factors. A one-day training was given to data collectors and supervisors about the aims of the study, the contents of the tool, and data collection procedures, and a pretest was done on 5% of the sample size at Ebinat hospital. The data was collected by trained health professionals through face-to-face interviews and document reviews. Children who had clinical non-adherence problems during the data collection time were linked to case managers. The assigned supervisors and principal investigators carried out close monitoring and supervision during the data collection process.
Operational Definitions
Clinical non-adherence: is defined as HIV-positive children who do not adhere to regularly attending clinical care follow-ups and periodic laboratory monitoring with avoiding practices that interfere with the effect of medication.2
Data Management and Statistical Analysis
The consistency and completeness of the collected data were examined during data management and analysis. Data were first entered into EpiData version 4.6 before being exported to SPSS version 25 for analysis. Binary logistic regression analysis was used to determine the association between the dependent and independent variables. Bi-variable logistic regression was performed to assess the association of each independent variable with the outcome variable, and variables with a P ≤ 0.25 in the bi-variable analysis were included in the final model of multi-variable analysis to control all possible confounders. The goodness-of-fit was tested by the Hosmer-Lemeshow statistic and the Omnibus tests. Frequencies and cross-tabulations were used to check for missed values of variables and to describe the study population’s relevant variables. Furthermore, percentages, proportions, and summary statistics were used to summarize the study population characteristics. The odds ratio with 95% CI was used to determine the direction and strength of the statistical association. Using multivariable analysis in binary logistic regression, the adjusted odds ratio and 95% CI were estimated to identify factors associated with clinical adherence. In this study, a P-value of 0.05 was considered statistically significant.
Result
Socio-Demographic Characteristics of Study Participants
Out of 422 participants, 383 have been involved in the study making the response rate of 90.7%. Almost half of the study participants 180 (47%) were girls and 157 (41%) were from rural areas. Study participants aged less than 60 months were 77 (20.1%), while 147 (37.3%) of them were between 121 and 180 months. The majority 291 (76%) of caregivers were biological mothers, and 174 (45.4%) of the caregivers were unable to read and write. Two hundred three children (53%) did not have treatment supporters (Table 1).
 |
Table 1 Socio Demographic Characteristics of HIV Positive Pediatrics Attending Care in South Gondar Zone Public Health Facilities |
Clinically Related Factors
One hundred twenty-six (32.9%) of study participants had WHO clinical stage 4 defining illness. Almost one-third of participants (36%) had different co-morbid illnesses. About half (51.7%) of study participants had less than sixty months of duration on antiretroviral treatment. One hundred seventy-nine (46.7%) of caretakers did not disclose the status of the child about the illness. Eighty-six (22.5%) of the respondents did not have a continuous drug supply (Table 2).
 |
Table 2 Clinical Related Factors of HIV Positive Pediatrics Attending Care in South Gondar Zone Public Health Facilities |
Prevalence of Clinical Non-Adherence
The overall prevalence of non-adherence among children on ART was 31.9% (95% CI: 27.2–36.6) (Figure 1).
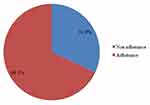 |
Figure 1 Prevalence of clinical none adherence among HIV positive pediatrics attending care in South Gondar Zone public health facilities. |
Factors Associated with Clinical Non-Adherence
This study assessed the relationship between the different variables and clinical non-adherence. In bi-variable analysis, residency, age, marital status of caretakers, having a co-morbid illness, diagnostic status disclosure, having an oral lesion, having adherence support, caretaker relationship with the child, and having diarrhea in the months were eligible for an adjustment in multi-variable analysis. Only diagnostic status disclosure, residency, having adherence support, caretaker relationship with the child, and co-morbid illness remained statistically significant associations with clinical non-adherence in the multi-variable analysis.
When compared to HIV-positive pediatrics living in urban areas, the likelihood of clinical non-adherence was about two times higher in rural areas (AOR = 2.122; 95% CI = 1.299–3.468). In terms of co-morbid illness, clinical non-adherence was approximately one point seven times (AOR = 1.741; 95% CI = 1.066–2.842) more likely in HIV-positive pediatricians with co-morbid illness than in those without co-morbidity. HIV-positive pediatrics who were told of their diagnosis were twice as likely to have clinical non-adherence (AOR = 2.011; 95% CI = 1.265–3.195) as HIV-positive pediatrics who were not told of their diagnosis (AOR = 2.011; 95% CI = 1.265–3.195). When compared to those who received adherence support, the likelihood of current clinical non-adherence was two and a half times higher in HIV-positive pediatrics who did not receive adherence support (AOR = 2.424; 95% CI = 1.492–3.937). Furthermore, HIV-positive children with non-biological mothers were three times more likely (AOR = 3.409; 95% CI = 1.979–5.869) to have clinical non-adherence than those with biological mothers as caregivers (Table 3).
 |
Table 3 Bivariable and Multivariable Analysis of HIV Positive Children Attending ART Care at Public Health Institutions in South Gondar, Amhara, Ethiopia, 2021 |
Discussion
For various reasons, clinical non-adherence is commonly encountered in the treatment of children and adolescents with HIV. In the current study, the prevalence of clinical non-adherence among HIV-positive pediatrics was 31.9% (95% CI: 27.2–36.6).
This study revealed that HIV-positive pediatrics who lived in rural areas were more likely to be clinically non-adherent as compared to those who lived in urban areas. This is because rural residents have a low chance of being involved in social support. After all, they have difficulty accessing health facilities and adherence supporters as compared to urban residents. This can also be justified as rural residents may have a low level of understanding about the disease and treatment, which affects adherence level. Moreover, rural residents have transportation problems that need to be addressed in the health setting at the appointment time and for conducting requested laboratory tests.
In this study, children who had a comorbid illness were more likely to be clinically non-adherent compared to those who did not have a comorbid illness. This might be due to those who have comorbid illnesses may have difficulty adhering to a schedule, adhering to the advice given by their health-care provider, and conducting the ordered laboratory request. This can also be justified as those who have comorbid illnesses may have an additional schedule in the health facilities for co-morbid illnesses,15 which makes them fatigued from multiple schedules.
This study also revealed that the prevalence of clinical non-adherence was higher in those HIV-positive children who had non-biological caretakers. This might be due to non-biological caretakers of HIV-positive children could have low attention spans for the child, which leads the child to have clinical non-adherence. Children who have no biological mother may be exposed to depression that leads the child to have a decreased interest in applying what the health-care provider orders for him/her. This might also be due to challenges in the trusty relationship-building process between the child and caretaker, which further leads the child to have poor clinical adherence.16
This study revealed that children who did not know their diagnosis status were more likely to have clinical non-adherence. This is in line with the study conducted on drug adherence at Gondar,14 Wollo,11 and in a tertiary hospital in Ethiopia.13 This is due to keeping the child’s status secret and not telling his/her problem having a deleterious effect on the discussion of HIV and ART with infected children, which increases the chance of the child cooperating with his/her treatment. This also leads the child to not seek the support they need and a loss of trust with caretakers, which affects their clinical adherence.2
In this study, children who did not have an adherence supporter were more likely to be clinical non-adherent. This is in line with the scientific evidence that inconsistent ongoing support and care services provided by different relatives to children result in poor adherence to follow-up and medication schedules. This is also supported by the scientific evidence that shows that management of adherence in adolescents is particularly challenging because of the emotional and psychosocial issues that are unique to their developmental stage, which needs consistent and continuous support from adherence supporters.2,5
Conclusion
Clinical non-adherence among children among HIV-positive children attending care in south Gondar zone health facilities is unacceptably high. To ensure successful clinical adherence, pediatric HIV patients who are rural residents, have an undisclosed HIV status, do not have an adherence supporter, have a non-biological caretaker, and have a co-morbid illness should be given special attention.
Abbreviations
AIDS, Acquired Immune Deficiency Syndrome; AOR, Adjusted odds ratio; ART, Antiretroviral Therapy; CI, Confidence Interval; COR, Crud odd ratio; HIV, Human immunodeficiency Virus; OI, Opportunistic Infection; SPSS, Statistical Package for Social science; WHO, World Health Organization.
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the Corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
Ethical clearance and approval were obtained from Debre Tabor University, College of Health Sciences, research, and community service coordinator through an ethical letter with protocol number CHS2519/2021. The study was also done as per the declaration of Helsinki. Participants were informed about voluntarism and could withdraw at any time during the study if they chose not to respond. For those who volunteered to participate, verbal informed consent was obtained from the parent/legal guardian of the children involved in this study. The process of verbal informed consent was approved by Debre Tabor University. Furthermore, the confidentiality of data was kept at all levels of the study and was not used for any other purposes than the stated study objectives.
Acknowledgments
We would like to express our genuine appreciation to all the selected study site officers with whom we have communicated and recognized for conducting this study. Our special thanks also extend to the study participants, data collectors, supervisors, and staff.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declared that they have no conflicts of interest for this work.
References
1. UNAIDS. Global HIV & AIDS statistics−2020 fact sheet; 2020.
2. FMoH E. National Consolidated Guidelines for Comprehensive HIV Prevention, Care and Treatment. Geneva: World Health Organization; 2020.
3. Kharsany AB, Karim QA. HIV infection and AIDS in sub-Saharan Africa: current status, challenges and opportunities. Open AIDS J. 2016;10:34. doi:10.2174/1874613601610010034
4. Williams AB. Adherence to HIV regimens: 10 vital lessons. Am J Nurs. 2001;101(6):37–43. doi:10.1097/00000446-200106000-00019
5. Ameri M, Movahed E, Farokhzadian J. Effect of information, motivation, and behavioral skills model on adherence to medication, diet, and physical activity in HIV/ADIS patients: a health promotion strategy. J Educ Health Promot. 2020;9:317. doi:10.4103/jehp.jehp_188_20
6. Unaids, HIV/AIDS. JUNPo, Program WBGHA. The Global Economic Crisis and HIV Prevention and Treatment Programmes: Vulnerabilities and Impact. World Health Organization; 2009.
7. Wainberg MA, Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279(24):1977–1983. doi:10.1001/jama.279.24.1977
8. Naar-King S, Montepiedra G, Nichols S, et al. Allocation of family responsibility for illness management in pediatric HIV. J Pediatr Psychol. 2009;34(2):187–194. doi:10.1093/jpepsy/jsn065
9. Cluver LD, Hodes RJ, Toska E, et al. ‘HIV is like a tsotsi. ARVs are your guns’: associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. Aids. 2015;29:S57–S65. doi:10.1097/QAD.0000000000000695
10. Bezabhe WM, Chalmers L, Bereznicki LR, Peterson GM, Bimirew MA, Kassie DM. Barriers and facilitators of adherence to antiretroviral drug therapy and retention in care among adult HIV-positive patients: a qualitative study from Ethiopia. PLloS One. 2014;9(5):e97353. doi:10.1371/journal.pone.0097353
11. Arage G, Tessema GA, Kassa H. Adherence to antiretroviral therapy and its associated factors among children at South Wollo Zone Hospitals, Northeast Ethiopia: a cross-sectional study. BMC Public Health. 2014;14(1):1–7. doi:10.1186/1471-2458-14-365
12. Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. BMC Pediatr. 2008;8(1):1–9. doi:10.1186/1471-2431-8-53
13. Biressaw S, Abegaz WE, Abebe M, Taye WA, Belay M. Adherence to antiretroviral therapy and associated factors among HIV infected children in Ethiopia: unannounced home-based pill count versus caregivers’ report. BMC Pediatr. 2013;13(1):1–9. doi:10.1186/1471-2431-13-132
14. Dachew BA, Tesfahunegn TB, Birhanu AM. Adherence to highly active antiretroviral therapy and associated factors among children at the University of Gondar Hospital and Gondar Poly Clinic, Northwest Ethiopia: a cross-sectional institutional based study. BMC Public Health. 2014;14(1):1–6. doi:10.1186/1471-2458-14-875
15. Monroe AK, Chander G, Moore RD. Control of medical comorbidities in individuals with HIV. J Acquir Immune Defic Syndr. 2011;58(5):458. doi:10.1097/QAI.0b013e31823801c4
16. Ogden J, Esim S, Grown C. Expanding the care continuum for HIV/AIDS: bringing carers into focus. Health Policy Plan. 2006;21(5):333–342. doi:10.1093/heapol/czl025
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.