Back to Journals » Hepatic Medicine: Evidence and Research » Volume 14
Clinical, Laboratory and Bacterial Profile of Spontaneous Bacterial Peritonitis in Vietnamese Patients with Liver Cirrhosis
Authors Nguyen LC, Lo TTB, La HD, Doan HTN , Le NT
Received 26 April 2022
Accepted for publication 21 July 2022
Published 30 July 2022 Volume 2022:14 Pages 101—109
DOI https://doi.org/10.2147/HMER.S369966
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Gerry Lake-Bakaar
Long Cong Nguyen,1,2 Thuy Thi-Bich Lo,3 Huong Dieu La,1 Ha Thi-Ngoc Doan,1 Ngoan Tran Le4,5
1Gastroenterology and Hepatology Center, Bach Mai Hospital, Hanoi, Vietnam; 2University of Medicine and Pharmacy, Vietnam National University, Hanoi, Vietnam; 3Hung Vuong hospital, Ho Chi Minh City, Phu Tho, Vietnam; 4Institute of Research and Development, Duy Tan University, Da Nang, Vietnam; 5Department of Public Health, School of Medicine, International University of Health and Welfare, Tochigi, Japan
Correspondence: Long Cong Nguyen, Institution: Gastroenterology and Hepatology Center, Bach Mai hospital, Hanoi, Vietnam, Email [email protected]
Aim: To determine several clinical and laboratory features as well as the bacterial profile of spontaneous bacterial peritonitis (SBP) in 58 Vietnamese patients admitted to a single center due to liver cirrhosis.
Methods: We retrospectively analyzed bacteriological, clinical and laboratory characteristics of patients with SBP admitted to the Gastroenterology and Hepatology Center from July 2019 to July 2020.
Results: Out of a total 58 SBP patients, 41 (70.9%) had culture-negative neutrocytic ascites. The majority of patients experienced abdominal pain (93,1%) and large ascites (65,5%). Gram-negative bacteria formed the main pathogens (14/17). Escherichia coli (9/17) was the predominant cause followed by Burkholderia cepacia (2/17). Antibiotic sensitivity rate of E. coli for third generation cephalosporin was low but high for aminoglycoside and carbapenem antibiotics. The resistance of E. coli was significant against fluoroquinolones (100%). All 3 cases of gram-positive bacteria were sensitive to vancomycin.
Conclusion: Our study reported the bacteriological and clinical characteristics of patients with SBP and compared these findings between two groups: positive ascitic fluid culture and negative fluid culture. Ascitic fluid culture can guide for the right antibiotic choice since resistance to commonly prescribed antibiotics is common in SBP patients.
Keywords: liver cirrhosis, peritonitis, ascitic fluid, antibiotic sensitivity
Introduction
Spontaneous bacterial peritonitis (SBP) is a frequent and severe complication in patients with illnesses such as liver disease and ascites. Incidence of SBP varies between 25% to 46% per annum in patients with decompensated cirrhosis.1 A significant increase in the mortality associated with SBP has been observed from 30% to 63% since its first diagnosis after one month and one year respectively.2
Clinical presentation of SBP is highly variable and non-specific.3 A significant proportion of patients with SBP are even completely asymptomatic4,5 and hence diagnostic paracentesis to establish the diagnosis is recommended.6 Common symptoms reporting to have some associations with SBP include fever, diarrhea, abdominal pain or tenderness, vomiting, etc.7 A classic case of SBP is diagnosed on the basis of a positive ascitic fluid culture and a neutrophil count greater than 250/µL. Two variants of SBP are culture negative neutrocytic ascites (CNNA) and bacterascites (BA). CNNA has a negative culture with a high neutrophil count (≥250/µL) while in bacterascites, ascites fluid culture is positive but neutrophil count is <250/µL. The interaction between changes in intestinal microbiota, altered intestinal permeability, bacterial translocation, and systemic immune dysfunction represent the fundamental factors for the development of spontaneous bacterial peritonitis. These series of events facilitate bacterial translocation from intestinal lumen to mesenteric lymph nodes, and subsequently to portal and systemic circulation, from where eventually ascitic fluid will be colonized and, under proper conditions, infection will develop. There are several known risk factors for spontaneous bacterial peritonitis in patients with cirrhosis and ascites, including upper gastrointestinal bleeding, low ascitic protein concentration when combined with any of the following characteristics: Child-Pugh score ≥9, serum bilirubin level ≥3 mg/dL, impaired renal function (creatinine ≥1.2 mg/dL or blood urea nitrogen level ≥25 mg/dL), or hyponatremia (≤130 mEq/L).
Historically, gram-negative bacteria were the main causative agents of spontaneous bacterial peritonitis, with Escherichia coli and Klebsiella spp. being the most frequently isolated organisms.8–10 Third generation cephalosporins are the first line antibiotics to treat spontaneous bacterial peritonitis; however, the initial treatment with cefotaxime, one of the most commonly used cephalosporins, failed more frequently than expected.11 However, major changes in the bacteriology of infections in patients with cirrhosis have occurred over the last few decades with an increasing prevalence of gram-positive, quinolone-resistant, and multidrug-resistant bacteria. A rising prevalence of gram-positive bacteria was reported over the past years in North America, South America, and Europe representing at present 48–62% of the isolated organisms. The most frequent gram positive isolates are Streptococcus spp., Enterococcus spp., and Staphylococcus spp.12,13 These changes are supposed to be due to indiscriminate use of antibiotics, increasing number of invasive procedures and hospitalization. In recent years, the emergence and spread of multi-drug resistant bacteria such as extended-spectrum beta-lactamases (ESBL)-producing enterobacteriaceae (34% in all cases) followed by carbapenemase producing enterobacteriaceae (27% in all cases) are becoming a great concern.14 Regarding SBP, an increasing prevalence of multi-drug resistant bacteria cases were also reported.12,15 It suggests a need for the constant assessment of common bacterial pathogen and their antibiogram to guide empirical treatment of SBP patients.
This study was conducted with the aim to find out some symptoms and laboratory findings with SBP. Further, we also aimed to identify the bacterial pathogens and their sensitivity pattern in order to choose the optimal antibiotic choice for patients.
Methods
Setting and Study Design
It was a descriptive cross-sectional study conducted from July 2019 to July 2020 at the Gastroenterology and Hepatology Center, in Bach Mai hospital, Vietnam. The hospital’s database holds records of clinical histories, disease manifestations, physical and laboratory findings. Bacteriology laboratory files were reviewed to identify all cirrhotic cases with SBP. Patients with secondary peritonitis were excluded from the study.
Definitions
The diagnosis of cirrhosis was based on clinical, biochemical, ultrasound and radiological findings. A diagnostic paracentesis was performed on all patients with peritonitis and suggested symptoms such as fever, chills, abdominal pain or abdominal tenderness. SBP was diagnosed when ascitic fluid polymorphonuclear leucocytes (PMN) count ≥250 cells/μL. Unclear triggers of hepatorenal syndrome and hepatic encephalopathy were also considered symptoms of peritonitis.
Treatment Algorithms
Antibiotic choices: we followed the guidance of AASLD 2009.16
IV antibiotics were started empirically (before obtaining culture results) in all patients with an ascites PMN count >250/mm3. Third-generation cephalosporins were the first choices where MDROs are not prevalent. For patients with nosocomial infection or recent hospitalization and critically ill patients admitted in the intensive care unit we tended to use carbapenems (Table 1).
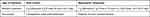 |
Table 1 Antibiotic Choice for Specific Infection |
Microbiological methods
A total of 20 mL ascitic fluid was collected from each patient for the ascitic fluid detailed report and culture. Blood samples of the patients were also collected to perform WBC, Hb, PLT, INR, CRP, AST, ALT, creatinine, AFP, serum bilirubin, serum total protein and serum albumin tess on. 3 mL of ascitic fluid was inoculated in blood culture bottles using the aseptic technique, WBC and PMN were counted by a Cell-dyn 3700 system. 10 mL of ascitic fluid was cultured with BD BACTEC™ FX blood culture system. Isolated pathogens were tested for antimicrobial susceptibility using BD Phoenix automated identification and susceptibility testing system. Blood culture bottles were incubated for 5−7 days at 37 °C and monitored daily for any signs of positive culture. Bottles showing any signs of positivity were sub-cultured on blood agar and chocolate agar.
Statistical Analysis
The data feeding and analysis was done on computer package SPSS (Statistical Package for Social Sciences), version 20.0. Quantitative variables were given as the mean ± standard deviation or the median (interquartile range). Qualitative variables were given as frequencies and percentages. Student’s t-test or Mann–Whitney U-test was used to compare continuous variables, and the Chi-square (χ2) test or Fisher’s exact test was used to compare categorical variables. A p-value ≤0.05 is considered statistically significant.
Results
During 1 year, a total of 58 patients with SBP were enrolled including 53 males and 5 females with sex ratio was 10.6/1. The mean age of participants was 55.36 ± 12.32 years. Out of the total of 58 patients, 17 (29.3%) patients had positive ascitic fluid culture results. Viral markers were available for 21 (36.2%) patients, out of these 19 (32.8%) patients were HBV positive, 2 (3.4%) were HCV positive. The majority of patients were addicted to alcohol (42/58, 72.4%). No statistically significant differences associated with HBV, HCV or alcohol rate were observed between the two groups: positive fluid culture and negative fluid culture.
Regarding the clinical presentation of patients we only found the significant associations of fever and hepatic encephalopathy between the two groups (p = 0.012, p = 0.001). Serum laboratory data (alanine transaminase/ALT, aspartate aminotransferase/AST, creatinine, albumin, total bilirubin, WBC, PMN and prothrombin time) are also shown in Table 2. Similarly, there were only significant differences in the international normalized ratio (INR) between the positive and negative ascitic fluid culture groups (p = 0.034).
 |
Table 2 Association of Different Symptoms, Clinical and Laboratory Features in Positive and Negative Ascitic Fluid Culture Groups |
In our study, we recorded 100% of patients with positive ascitic fluid culture had opaque fluid color whereas 63.4% patients of negative groups had this sign (p = 0.003). The ascitic WBC and PMN count of the positive fluid culture were significantly higher than that of the negative group (p = 0.003, p = 0.006 respectively). There are some risk factors for developing an episode of SBP: patients with an ascitic fluid total protein less than 1.5 g/dL and with impaired renal function (creatinine ≥1.2 mg/dl, BUN ≥25 mg/dl or serum Na ≤130 mmol/l) or liver failure (Child score ≥9 and bilirubin ≥3 mg/dl). However in our study we could not find a difference between the two groups when it comes to the mean of ascitic fluid protein (Table 3).
 |
Table 3 Ascitic Fluid Laboratory Findings in Positive and Negative Fluid Culture Groups |
The share of pathogens among patients is indicated in Figure 1. E. coli was the predominant pathogen that was isolated in 9 (52.94%) cases. Sensitivity pattern of E. coli pathogens is depicted in Figure 2 which shows that sensitivity rates to commonly prescribed antibiotics like cephalosporin (cefotaxime, ceftazidime, ceftriaxone, cefuroxime, cefepime, cefoperazone) were quite low. 100% of patients with E. coli infection were resistant to quinolone antibiotics (levofloxacin and ciprofloxacin). However most of the isolates were sensitive to carbapenem group (imipenem, meropenem, ertapenem). Three patients with positive-gram infection were sensitive to vancomycin. There was one patient infected with Staphylococcusaureus, which was sensitive to cephalosporins, carbapenems and piperacillin/tazobactam.
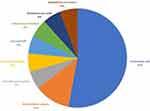 |
Figure 1 Distribution of pathogens among SBP patients. |
 |
Figure 2 Antibiotic sensitivity patterns of E. coli. |
In this study, all three patients with septicemia (two cases of Burkholderia cenocepacia and one case of Staphylococcusaureus) developed sepsis and severe sepsis, and one patient died.
Discussion
Spontaneous bacterial peritonitis (SBP) is a frequent complication in patients with chronic liver disease and ascites. In this study, we retrospectively investigated the clinical and bacteriological characteristics of SBP patients and compared these features between two groups: positive ascitic fluid culture and negative ascitic fluid culture. Regarding the epidemiology of patients, this study's results are in concordance with some local studies which show that cirrhosis occurs mostly in male patients with the dominant rate of alcoholic cirrhosis (72.4%). Hepatitis B is still a major issue in Vietnam with the rate of infection in our study being 32.8%. As for SBP variants, CNNA was the most common among patients. A similar pattern of distribution of different SBP variants has been reported by Evans.17 Some local studies shared slightly different results which showed a higher percentage of positive ascitic fluid culture. A higher rate of classic SBP has been reported by Oladimeji (67.7%) and Shizuma (35–65%).18,19
With reference to the clinical presentation of patients we also found that abdominal pain or tenderness, fever and diarrhea are the most common symptoms suggesting SBP with the predominant rate of abdominal pain (93.1% in all patients) which is consistent with local and international data. The proportion of patients with abdominal pain in our study is higher than that in the study by Cheong et al (50.8%).20 Abdominal pain is diffuse throughout the abdomen, sometimes it is a vague feeling of abdominal pain or distention, sometimes it is very intense, even some patients have abdominal guarding. We only recorded a statistically significantly higher rate of fever and hepatic encephalopathy in the group of positive ascitic fluid culture.
Regarding biochemical parameters, we noted that the mean value of serum albumin and platelet count in positive culture cases was lower than that in negative culture cases, whereas the mean value of serum total bilirubin and the mean INR value were higher in the positive culture group. This finding is in concordance with the study of Oliveira et al.21 However we only recorded significant differences in the mean INR value and PLT count between the two groups. This can be explained by the fact that 100% of patients who had positive ascitic culture results were classified into Child-Pugh C group. 100% of patients with positive culture had opaque ascitic fluid, it suggests that the color of ascitic fluid is a factor in predicting whether the bacteria can be isolated.
Ascitic fluid culture was positive in 29.3% of SBP cases. This result is quite different from some local studies and had a lower rate of culture positivity compared to some international studies. Oladimeji recorded the rate as 67.7%,19 whereas Shizuma found the rate varied from 35–65%.18 Literature suggests a culture positivity rate of 31–71%. The differences in frequency of isolation rate could be attributed due to different culture techniques and our change in selection criteria as only cirrhotic patients who had a higher count of PMN (≥250 cells/µL) were enrolled therefore some patients who had BA may have been missed.
Gram-negative bacilli were isolated from 82.3% of culture positive cases with Escherichia coli being the leading pathogen (64.3%). These results are consistent with the findings observed by other recent local and international studies. Research by author Cheong et al recorded 72.9% of SBP cases are gram-negative bacteria in which E. coli accounted for 43.2%.20 Another study by author Oliveira reported that gram-negative bacteria accounted for 58.2%.21 The main reason may be bacterial translocation from gut hence the commonly isolated pathogens are usually enteric gram-negative rods followed by Streptococcus species.22,23 However, in our study, the second most common bacteria was Burkholderiacepacia. In both the cases, the blood culture results coincided with the ascitic fluid culture results. The condition of the two patients was severe, with sepsis, pneumonia and SBP. In recent years, studies on ascites infections around the world have shown that there is an increase in the rate of infections caused by gram-positive bacteria. According to a report by Fiore et al, this prevalence has been reported in North America, South America, Europe, Asia, and Africa, on average, ranging from 48% to 62%. The most common strains of gram-positive bacteria are Streptococcus spp., Enterococcus spp., and Staphylococcus spp.24 We reported three cases of gram-positive including Enterococcusfaecium, Streptococcus crista and Staphylococcusaureus, which belong to the above strains.
According to a recent study, 70% of isolated bacteria in SBP patients were resistant to quinolones.18 This is particularly worrisome, as norfloxacin remains the antibiotic of choice for the prophylaxis of SBP. Regarding multidrug-resistant bacteria, they are found mainly in nosocomial bacterial peritonitis. However, 4% to 16% of SBP caused by community-acquired bacteria also causes multi-antibiotic resistance.24 Third generation cephalosporins are the recommended drugs of choice for treating SBP empirically. But recent studies have shown that cephalosporins are effective only in 70% of community acquired and 56% of hospital acquired SBP. In our study, the susceptibility rates of E. coli bacteria to cephalosporin antibiotics (cefotaxime, ceftazidime, ceftriaxone, cefuroxime, and cefepime) are 22.2%, 33.3%, 22.2%, 22.2%, and 22.2% respectively which are much lower than that in one previous local study where the rates varied from 54.2% to 62.9%. Just 44.4% patients with E. coli isolated were sensitive to cefoperazone and sulbactam combination which raises a great concern of inefficient empirical prescription of these medications to treat SBP. In this study, the third generation cephalosporin resistance rate of E. coli cases is 66.7–77.8%. This rate is higher than that in a study by Shizuma (33–75%).18 This is because ESBL-producing gram-negative bacteria are resistant to these antibiotics. However, in this study, the sensitivity rates of E. coli to carbapenems (imipenem, meropenem, ertapenem) was 100%. A similar pattern was recorded in aminoglycoside antibiotics: amikacin (100%) and gentamicin (88.9%) which is higher than some previous studies. Higher results indicate that these may have been effective and considered the treatment of choice in some cases. A combination of piperacillin and beta lactamases inhibitor tazobactam, showed better results, for which antibiotic sensitivity in E. coli was 100% suggesting that this antibiotic can still be used for the treatment of SBP. Although isolated E. coli was highly resistant to quinolones, at the rate of 100% for both ciprofloxacin and levofloxacin, which is quite alarming because of the increasingly widespread use of this class of antibiotics in clinical practice, their prolonged use for the prevention of SBP in cirrhotic patients, and the increase of multidrug resistant (MDR) and extensively drug resistant (XDR) bacteria.20,25–27 Marciano et al found a quinolones resistance rate of 70%.28 Five other cases of gram-negative isolated were sensitive to different cephalosporins. One patient with a Klebsiella pneumoniae infection was mostly sensitive to all types of cephalosporins and quinolones as well. Gram-positive pathogens were increasingly recognized as important causative bacteria in patients with SBP, in our study all three cases of gram-positive infection were sensitive to vancomycin. The patient who had Staphylococcusaureus infection was sensitive to cephalosporins, carbapenems and piperacillin/tazobactam.
Multidrug resistant bacteria and gram-positivity have become a bigger problem nowadays. Meropenem combined with glycopeptides or daptomycin has been suggested as the primary approach for healthcare-associated spontaneous bacterial peritonitis or in severe infections in areas with high prevalence of multidrug-resistant organisms, and for nosocomial spontaneous bacterial peritonitis in general. Carbapenems alone or with daptomycin, vancomycin, or linezolid if high prevalence of multidrug-resistant bacteria, gram-positive bacteria or sepsis. The results of a recent prospective cohort study serve as a platform to propose a treatment algorithm according to the severity of infection. Briefly, the authors state that empirical treatment of infections should consider patient’s Quick Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation (APACHE II) scores, and in those with greater risk, a more aggressive empirical treatment should be indicated.28 In this study, all three patients with septicemia (two cases of Burkholderia cenocepacia and one case of Staphylococcusaureus) developed sepsis and severe sepsis, and one patient died. The role of antibiogram is crucial, early and appropriate use of antibiotics significantly improves outcomes in most serious cases, especially in the situation where multidrug resistant bacteria is increasing worldwide.
Our study was conducted by prospective methods, therefore we could directly monitor all the events during the treatment process, collecting all relevant data according to the research record form. However, because of the small number of patients, our results may not be representative of the population. When it comes to selection criteria, we only selected cirrhotic patients with ascitic fluid PMN count ≥250 cells/mm3, therefore, the rate of positive ascites culture will be lower than other studies. We have not yet classified the patients as community-acquired or hospital-acquired, health-facility-related infections, so when we gave the antibiotic resistance rate of bacteria it was not as clear as studies in the world and also difficult to compare the results with studies in the world. Further studies with larger sample sizes should be planned to address these issues.
Conclusions
Patients with positive ascitic fluid culture all had opaque ascitic fluid and severe clinical conditions. Resistance against antibiotics like fluoroquinolones and cephalosporins was high which must be strictly controlled to reduce antibiotic resistance pathogens. Carbapenems and aminoglycosides may be considered as alternative treatment choices for SBP patients.
Abbreviations
SBP, spontaneous bacterial peritonitis; E. coli, Escherichia coli; CNNA, culture negative neutrocytic ascites; BA, bacterascites; ESBL, extended-spectrum beta-lactamases; PMN, polymorphonuclear leucocytes; WBC, white blood count; Hb, hemoglobin; PLT, platelet count; INR, international normalised ratio; CRP, c-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AFP, alpha-fetoprotein.
Ethics Approval and Consent to Participate
This study was approved by Institutional Review Board at Bach Mai Hospital and complied with the Declaration of Helsinki. Participants were informed sufficiently about the study and asked for their consent by documents. Written informed consent has been obtained from the study participants prior to study commencement.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Alaniz C, Regal RE. Spontaneous bacterial peritonitis: a review of treatment options. Pharmacol Ther. 2009;34(4):204.
2. Arvaniti V, D’Amico G, Fede G, et al. Infections in patients with cirrhosis increase mortality four-fold and should be used in determining prognosis. Gastroenterology. 2010;139(4):1246–1256. e5. doi:10.1053/j.gastro.2010.06.019
3. Ginés P, Quintero E, Arroyo V, et al. Compensated cirrhosis: natural history and prognostic factors. Hepatology. 1987;7(1):122–128. doi:10.1002/hep.1840070124
4. Bandy SM, Tuttle A. Spontaneous bacterial peritonitis. E-medicine from WebMD; 2008
5. Riggio O, Angeloni S. Ascitic fluid analysis for diagnosis and monitoring of spontaneous bacterial peritonitis. World J Gastroenter. 2009;15(31):3845. doi:10.3748/wjg.15.3845
6. Căruntu FA, Benea L. Spontaneous bacterial peritonitis: pathogenesis, diagnosis, treatment. J Gastrointestin Liver Dis. 2006;15(1):51–56.
7. Navarro VJ. Spontaneous bacterial peritonitis. Curr Treat Options Gastroenterol. 1999;2(6):457–462. doi:10.1007/s11938-999-0049-7
8. Piano S, Brocca A, Mareso S, et al. Infections complicating cirrhosis. Liver Int. 2018;38:126–133. doi:10.1111/liv.13645
9. Piotrowski D, Boroń-Kaczmarska A. Bacterial infections and hepatic encephalopathy in liver cirrhosis–prophylaxis and treatment. Adv Med Sci. 2017;62(2):345–356. doi:10.1016/j.advms.2016.11.009
10. Conn HO, Rodes J, Navasa M. Spontaneous Bacterial Peritonitis: The Disease, Pathogenesis and Treatment. CRC Press; 2000.
11. Angeloni S, Leboffe C, Parente A, et al. Efficacy of current guidelines for the treatment of spontaneous bacterial peritonitis in the clinical practice. World J Gastroenter. 2008;14(17):2757. doi:10.3748/wjg.14.2757
12. Alexopoulou A, Papadopoulos N, Eliopoulos DG, et al. Increasing frequency of gram‐positive cocci and gram‐negative multidrug‐resistant bacteria in spontaneous bacterial peritonitis. Liver Int. 2013;33(7):975–981. doi:10.1111/liv.12152
13. Acevedo J. Multiresistant bacterial infections in liver cirrhosis: clinical impact and new empirical antibiotic treatment policies. World J Hepatol. 2015;7(7):916. doi:10.4254/wjh.v7.i7.916
14. Piano S, Singh V, Caraceni P, et al. Epidemiology, predictors and outcomes of multi drug resistant (MDR) bacterial infections in patients with cirrhosis across the world: final results of the’Global study’. Dig Liver Dis. 2018;50:2–3. doi:10.1016/j.dld.2018.01.007
15. Fiore M, Maraolo AE, Gentile I, et al. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: a systematic review. World J Gastroenter. 2017;23(25):4654. doi:10.3748/wjg.v23.i25.4654
16. Runyon BA. AASLD practice guidelines committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology. 2009;49(6):2087–2107.
17. Evans LT, Kim WR, Poterucha JJ, Kamath PS. Spontaneous bacterial peritonitis in asymptomatic outpatients with cirrhotic ascites. Hepatology. 2003;37(4):897–901. doi:10.1053/jhep.2003.50119
18. Shizuma T. Spontaneous bacterial and fungal peritonitis in patients with liver cirrhosis: a literature review. World J Hepatol. 2018;10(2):254. doi:10.4254/wjh.v10.i2.254
19. Oladimeji AA, Temi AP, Adekunle AE, Taiwo RH, Ayokunle DS Prevalence of spontaneous bacterial peritonitis in liver cirrhosis with ascites. Pan Afr Med J. 2013;15(1). doi: 10.11604/pamj.2013.15.128.2702
20. Peck KR, Kang C-I, Lee J, et al. Clinical significance and outcome of nosocomial acquisition of spontaneous bacterial peritonitis in patients with liver cirrhosis. Clin Infect Dis. 2009;48(9):1230–1236. doi:10.1086/597585
21. Oliveira AM, Branco JC, Barosa R, et al. Clinical and microbiological characteristics associated with mortality in spontaneous bacterial peritonitis: a multicenter cohort study. Eur J Gastroenterol Hepatol. 2016;28(10):1216–1222. doi:10.1097/MEG.0000000000000700
22. Ghosh G, Jesudian AB. Small intestinal bacterial overgrowth in patients with cirrhosis. J Clin Exp Hepatol. 2019;9(2):257–267. doi:10.1016/j.jceh.2018.08.006
23. Wang C, Li Q, Ren J. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Front Immunol. 2019;10:1873. doi:10.3389/fimmu.2019.01873
24. Fiore M, Maraolo AE, Gentile I, et al. Current concepts and future strategies in the antimicrobial therapy of emerging Gram-positive spontaneous bacterial peritonitis. World J Hepatol. 2017;9(30):1166. doi:10.4254/wjh.v9.i30.1166
25. Rostkowska KA, Szymanek-Pasternak A, Simon KA. Spontaneous bacterial peritonitis–therapeutic challenges in the era of increasing drug resistance of bacteria. Clin Exp Hepatol. 2018;4(4):224. doi:10.5114/ceh.2018.80123
26. Pitout JD, Laupland KB. Extended-spectrum β-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159–166. doi:10.1016/S1473-3099(08)70041-0
27. Singh N, Wagener M, Gayowski T. Changing epidemiology and predictors of mortality in patients with spontaneous bacterial peritonitis at a liver transplant unit. Clin Microbiol Infect. 2003;9(6):531–537. doi:10.1046/j.1469-0691.2003.00691.x
28. Marciano S, Díaz JM, Dirchwolf M, et al. Spontaneous bacterial peritonitis in patients with cirrhosis: incidence, outcomes, and treatment strategies. Hepat Med. 2019;11:13. doi:10.2147/HMER.S164250
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
