Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Clinical, Imaging, Histological and Surgical Aspects Regarding Giant Paraovarian Cysts: A Systematic Review
Authors Stefanopol IA , Baroiu L , Neagu AI, Danila DM, Nechifor A , Miulescu M , Balan G, Vasile CI , Niculet E , Tatu AL
Received 8 February 2022
Accepted for publication 10 April 2022
Published 29 April 2022 Volume 2022:18 Pages 513—522
DOI https://doi.org/10.2147/TCRM.S361476
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Ioana Anca Stefanopol,1,2,* Liliana Baroiu,3,4 Anca-Iulia Neagu,1,5 Dumitru Marius Danila,2,6 Alexandru Nechifor,3,* Magdalena Miulescu,7,* Gabriela Balan,3,8,* Claudiu Ionut Vasile,3,9,* Elena Niculet,1,10 Alin Laurenţiu Tatu3,11– 13
1Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 2Department of Pediatric Surgery and Orthopedics, “Sf Ioan” Clinical Emergency Hospital for Children, Galați, Romania; 3Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 4Infectious Diseases Department, “Sf Cuv Parascheva” Clinical Hospital of Infectious Diseases, Galați, Romania; 5Department of Anatomopathology, “Sf Ioan” Clinical Emergency Hospital for Children, Galați, Romania; 6Clinical Surgical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 7Research Center in the Functional Cardiorespiratory and Neuromotor Exploration, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, Galați, Romania; 8Department of Gastroenterology, “Sf Ap Andrei” Emergency County Clinical Hospital, Galați, Romania; 9“Elena Doamna” Clinical Hospital of Psychiatry, Galaţi, Romania; 10Department of Pathology, “Sf Ap Andrei” Emergency County Clinical Hospital, Galați, Romania; 11Research Center in the Field of Medical and Pharmaceutical Sciences, ReFORM-UDJ, Galați, Romania; 12Dermatology Department, “Sf Cuv Parascheva” Clinical Hospital of Infectious Diseases, Galați, Romania; 13Multidisciplinary Integrated Center of Dermatological Interface Research MIC DIR, Dunarea de Jos” University, Galati, Romania
*These authors contributed equally to this work
Correspondence: Liliana Baroiu, Clinical Medical Department, Faculty of Medicine and Pharmacy, “Dunărea de Jos”University, 47 Domnească Street, Galați, 800010, Romania, Tel +40723201241, Email [email protected] Anca-Iulia Neagu, Department of Morphological and Functional Sciences, Faculty of Medicine and Pharmacy, “Dunărea de Jos” University, 47 Domnească Street, Galați, 800010, Romania, Tel +40754937354, Email [email protected]
Abstract: Paraovarian cysts (POCs) develop within the broad ligament of the uterus. POCs are considered to be giant when the threshold of 150 mm is exceeded. Clinical signs and symptoms occur as a consequence of the pressure effect on adjacent organs or due to complications. Abdominal ultrasonography, computed tomography or magnetic resonance imaging are useful imaging tools, but most often the exact origin of such voluminous cysts is revealed only by surgical exploration. The review aims to appraise and update the diagnostic, the histological aspects and the treatment of the giant POCs in rare cases. We carried out a systematic search in Medline-PubMed, Google Scholar and ResearchGate electronic databases. Twenty-seven papers fulfilling the selection criteria were included in the review. The data extracted included information about first author, year of publication, country, patient age, size and side of the POCs, symptoms, tumoral markers, imaging methods, preoperative diagnosis, surgical management and histopathological findings. Although not very numerous, all the studies highlighted the low incidence of giant POCs, the impossibility of establishing the origin of the cystic mass by clinical and imaging methods even with advanced technical tools and the low risk of torsion (11.1%). Despite the recognized benign nature of POCs, we found an unexpected high percent (25.9%) of borderline giant POCs. Surgical excision is the only treatment option. Ovarian-sparing surgery was performed in 85.1% of the cases, and minimally invasive techniques were applied in only 42.9% of the patients, which demonstrates the need of a high-level laparoscopic expertise. Knowledge of this pathology, its recognition as a possible etiology of an abdominopelvic cyst, and a higher awareness of the possibility of a borderline histology in giant POCs are required for the proper management of these particular cases.
Keywords: paraovarian cyst, paratubal cyst, giant, serous cystadenoma, torsion, management
Introduction
Both paratubal cysts and paraovarian cysts (POCs) are terms which define the same condition: cysts located within the mesosalpinx or the broad ligament.1,2 They can develop from the mesothelium but also from paramesonephric tissues or mesonephric remnants.3,4
POCs represent about 10% of all adnexal masses.5,6 They are the prerogative of reproductive ages being more common in the third or fourth decade of life.7 Only 4% occur in adolescence,8,9 while in postmenopausal women the prevalence is 6.25%.1
The estimated mean size of POCs is 75.1 mm (10–80 mm),10–12 and 95% of them have the diameter smaller than 20 mm.9 There is not an explicit definition of giant POCs. Some authors consider that the size must exceed the threshold of 150 mm,13,14 while others consider that 200 mm is a more appropriate dimension.1
POCs are ordinarily asymptomatic. In cases of giant POCs clinical signs and symptoms occur as a consequence of the pressure effect on adjacent organs or due to complications, such as torsion, rupture or hemorrhage.15,16 Because POCs develop into the broad ligament and have no pedicule, torsion is rare, with 2.1–16% incidence.9 When torsion occurs, it usually involves the fallopian tube, the infundibulopelvic ligament and the ipsilateral ovary.8 Although 97% of POCs are benign,8 borderline or malignant epithelial tumors have also been identified.17,18 Abdominal ultrasonography (USG), computed tomography (CT) and magnetic resonance imaging (MRI) are useful imaging tools, but there is a high risk of misdiagnose with ovarian cystic masses because they may not show a clear separation between the cyst and the ipsilateral adnexa.16,19 Most often, the diagnosis becomes evident only during surgical exploration.13,15 In order to preserve fertility, cystectomy with ovarian preservation remains the most appropriate treatment especially in young women or pediatric patients.3,20
The present review aims to systematically appraise and update the diagnostic and histological aspects of giant POCs, as well as the surgical treatment methods applicable in such rare POCs.
Materials and Methods
The research and extraction of eligible studies for the present systematic review were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines (PRISMA).21
Search Strategy
In order to identify all relevant papers in the medical literature, we conducted a comprehensive search in Medline-PubMed, Google Scholar and ResearchGate electronic databases. We focused on the following keywords in different combinations: paraovarian cyst, paratubal cyst, giant, serous cystadenoma, torsion, management. Taking into consideration the rarity of this pathology and the aim of the present review, we did not set a time frame for the publication dates, or a limit on the article type. We also screened the reference list of the selected articles searching for additional eligible publications.
Selection Criteria
Two reviewers worked independently screening all the titles and abstracts. Disagreements were settled through consensus. The inclusion criteria were (1) full-text articles; (2) papers written only in English; (3) patients with POCs larger than 150 mm, regardless of age. Reasons for exclusion were (1) articles which provided insufficient data; (2) articles on solid paraovarian tumors (benign or malignant neoplasms); articles on veterinary pathology.
Data Extraction
Two independent researchers extracted the data after creating a standardized extraction table including information about the first author, year of publication, country, patient age, size and side of the POCs, symptoms, tumoral markers, imaging methods, preoperative diagnosis, surgical management and histopathological findings. Discrepancies were resolved through discussion.
Results
A total of 401 records were identified. The flow diagram of the study selection process is shown in Figure 1. After removing the duplicates and after reviewers #1 and #2 selected papers based on their titles and abstracts, 94 records remained. Both researchers agreed to remove the articles, which did not meet some of the selection criteria. The remaining 53 reports assessed for eligibility were processed by reviewers #3 and #4. Finally, they decided to extract data for the present review from only 27 papers fulfilling the inclusion and exclusion criteria (Table 1).
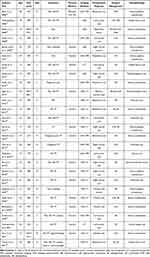 |
Table 1 The Reported Cases of Giant POCs (Larger Than 150 Mm) |
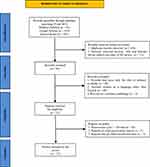 |
Figure 1 PRISMA flow diagram of the selection process. Notes: PRISMA figure adapted from: Page MJ, McKenzie JE, Bossuyt PM et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons CC BY 4.0 license21. |
We identified 21 case reports, 2 of them associated with a short literature review,1,12 1 short report,22 1 letter to the editor,23 and 4 reviews,11,16,24,25 but none of them included exclusively giant POCs or more than 10 studies.
All the cases were reported by physicians working in University Hospitals from many countries in the world: Turkey,11,20,26,27 India,2,10,12,15,28 Serbia,29 Austria,22 Spain,30 Egypt,31 Italy,5 United States of America,16 South Korea,25,32 Japan,23 Romania,19 Lebanon,13 Portugal,33 Jordan,14 Nepal,34 Bosnia and Herzegovina,24,35 Indonesia,1 and Oman.3
Etiology
In females, both the uterus and the fallopian tubes develop from the paramesonephric (Müllerian) ducts.36 The mesonephric (Wolffian) ducts usually regress, but they may persist as embryonic remnants (epoophoron, paroophoron, Gartner’s ducts).37,38 Depending on their embryological origin, POCs have different location, histological features and evolution. Most often they arise from the peritoneal mesothelium or the paramesonephrotic structures,16 are located along the fallopian tube and are lined by a secretory epithelium responsible for cyst formation.11 In 2% of the cases, POCs are of mesonephric origin,6,9 located near the fimbria, with a non-secretory epithelium.16,39 Moreover, paramesonephric cysts are under hormonal influence, explaining their growth trend and prevalence in postmenarcheal ages,40 as well as their rapid growth in pregnant women.39 When a POC is located at the fimbriated end of the fallopian tube, pedunculated and smaller than 2 cm, it is considered to be a cystic hydatid of Morgagni.11,32,41
Clinical Aspects
We enlisted 27 cases of POCs larger than 150 mm in our review (Table 1). Thirty-seven percent (n = 10) were postmenarcheal pediatric patients (aged under 18 years), and 63% (n = 17) were adult women, with 3 cases of concomitant pregnancy.12,23,27 The only case of postmenopausal patient with a 260 mm POC was reported by Varras et al which was cited by Shah et al and Habek et al.28,42 Regarding the side location, we found 51.8% (n = 14) right-sided cysts, 37% (n = 10) left-sided and 11.1% (n = 3) bilateral.
The clinical signs and symptoms of POCs lack specificity. Most small cysts are asymptomatic, usually discovered during abdomino-pelvic imaging investigations or surgery for other pathologies.5,9,13,14 As the cysts grow, unspecific symptoms may occur, such as recurrent abdomino-pelvic pain, increase in abdominal volume or feeling of weight in the lower abdomen.11 As we found in the literature, the compression of neighboring organs may be reflected by cardiovascular or pulmonary complications,42 dysuria, pollakiuria or hydronephrosis,5,13,15,16,35 constipation,6,9,14,24,35 menstrual irregularities,3,15,25 dyspareunia,5,14 and even uterine prolapse.35 In all cases of giant POCs, the cyst presence may be confirmed by an increased abdominal volume and abdominal palpation. Vaginal bimanual examination (in sexually active patients) is helpful in diagnosing small or medium cysts.5 Like many other conditions (ie, mesenteric cyst, urachal cyst), POCs may also become symptomatic when associated with complications such as intracystic hemorrhage, perforation or torsion.43,44 In such cases, acute pain, hemoperitoneum or even hemorrhagic shock may be present.24 The most frequent and feared complication is ovarian torsion, with a higher probability of occurrence in POCs larger than 50 mm.13,16 Isolated fallopian tube torsion may be encountered, as well.45–47 The patients’ symptoms are acute abdominal pain, vomiting or nausea. Complicated POCs have clinical features similar to acute appendicitis, complicated ovarian cyst, ectopic pregnancy or pelvic inflammatory disease.11,33,48 Although the incidence of POCs in children is lower than in adults, the torsion rate seems to be higher due to a longer and looser infundibulopelvic ligament.40,49 The present literature review found 11.1% (n = 3) cases of torsioned giant POCs,29,30,33 only one case being in a pediatric patient.29 Another potential complication is malignancy, with a reported incidence of 2.9%, mostly in adult patients.39
Imaging Diagnostic and Tumor Markers
The imaging investigation of choice for abdomen or pelvis is USG (transabdominal, transvaginal or transrectal).8,11,14 On USG, uncomplicated cysts are thin-walled, usually unilocular with clear content.1,9 Corroborating the symptoms with the USG images, the differential diagnosis of uncomplicated POCs should include ovarian cyst, mesenteric cyst, abdominal lymphangiomas, pancreatic pseudocyst, echinococcal cyst, cystic intestinal duplication, or cystic mesothelioma.6,16,50 Evocative for POC is the “split sign”, meaning the separation of the cyst from the ovary by pushing the transducer.11,33,40,51 In the study conducted by Gupta et al, USG had a 87.5% accuracy in diagnosing POCs, but the mean diameter of the lesions was 7.5 cm.52 Whenever a cyst torsion is suspected, Doppler studies are mandatory, although the presence of vascular flow may not exclude a twisted ovary.8,11 Findings like papillary projections, septum or intramural nodules should raise the suspicion of borderline or malignant tumor.32,53,54 Whenever an adnexal mass presents imaging signs of malignancy, it is mandatory to test the values of the serum tumor markers: CA 125, carcinoembryonic antigen (CEA), lactate dehydrogenase (LDH), alpha-fetoprotein (AFP), beta-human chorionic gonadotropin (β HCG).11,30 These markers could be useful in differentiating between benign and malignant cysts, although their values are elevated in only 54% of the adnexal malignant neoplasms and in 6.5% of the benign lesions.13,55 There are insufficient studies on the predictive value of CA 125 levels for malignant POCs.1
CT or MRI seem to be more accurate in showing a delineation between the unilocular cystic lesion and a normal ovary.3,24,48 The MRI is preferred, because it avoids radiation, which is essential especially in pediatric patients.5,56 Despite the technical imaging progress, the correct diagnosis is usually established by abdominopelvic surgical exploration.11,16,57 Barloon et al cited by Tjokroprawiro found that only 1 out of 15 (6.66%) patients received a correct diagnosis before surgery.1 The present literature review revealed that none of the giant POCs were correctly diagnosed, and in 62.9% (n = 17) of the cases, the preoperative diagnosis was ovarian cyst. Other preoperative diagnoses were abdominal cyst or tumor (n = 6), mesenteric cyst (n = 2), and ovarian torsion (n = 2). As the findings above show, it seems unlikely to establish the origin within the broad ligament of a giant abdomino-pelvic cyst by clinical and imaging methods. Moreover, the diagnosis of POC has not been considered in the differential diagnosis in any of the cases found in the literature.
Treatment Options
POCs larger than 30 mm should be excised, due to their constant growth and torsion risk.20,24,40 Smaller cysts may be aspirated, but there is a high risk of recurrence.8 It is obvious that the appropriate way to treat giant POCs is the surgical excision, but there is no consensus on whether it should be by laparoscopic or open techniques.1,58 Generally, the limiting factors for laparoscopy are the giant size of the mass, the signs of malignancy or an insufficient expertise of the surgeon.28,33 Whether open or minimally invasive techniques are preferred, the preoperative administration of dexamethasone has a prophylactic effect on postoperative nausea and vomiting.59 The anesthesiologists prefer the inhalational induction with sevoflurane, because of its rapid action, pleasant odor and absence of airways irritation,60 but they should be aware of the intraoperative allergic reactions caused by the neuromuscular blocking agents.61 The literature review showed that open surgery has been preferred by 57.1% (n = 16) of the authors, with 8 cases of midline incision,1,10,11,20,29,32,33,35 2 of Pfannenstiel incision,16,24 and 1 of paramedian incision.15 In the last decade, there have been reported 22 giant POCs, but only 9 (40.9%) were treated laparoscopically, and in all these cases, an open-entry technique was used transumbilical,5,25 in the left umbilical fold,22 supraumbilical,3,13,29 subumbilical,14 or in Palmer’s point.34 Aspirating the cyst content was mandatory for an accurate diagnosis and for an easier cyst dissection. The cyst drainage was performed with the Veress needle,22,34 or with a high pressure suction cannula introduced transumbilically. In order to avoid the spillage of the fluid in the peritoneal cavity, surgeons may use a closed system (wound protector, a purse-string suture around the trocar).13,14 We also found one case of laparoscopic excision through a minilaparotomy without pneumoperitoneum, in a pregnant woman,23 and one case of laparo-endoscopic single-site surgery for a borderline POC.25 The main purpose of the treatment should be sparing the ovarian tissue necessary to preserve fertility as well as to a proper sexual development of the pediatric patients. Cystectomy is the standard treatment,1,3,11 but removal of a giant POC sometimes requires associated tubal excision or even oophorectomy. The present review found only 70.3% (n = 19) cystectomies. There were 4 cases of associated salpingectomy,14,20,25,35 2 salpingo-oophorectomies,15,26 and 2 adnexectomies.29,33
Histopathological Aspects
Histopathologically, POCs are mostly benign: simple cysts (74.6%) and benign neoplastic lesions (25.4%) – cystadenomas and cystadenofibromas.11,24,52 Malignant or borderline paraovarian epithelial tumors have also been reported.20,28 Borderline tumors (of low malignant potential) are defined by malignant features without stromal invasion.25,32 Reviewing the literature we found the following histopathological types of giant POCs: 33.3% (n = 9) serous cystadenomas,3,12,14,20,22,24,27,29,34 18.5% (n = 5) simple serous cysts,1,15,19,31,35 22.2% (n = 6) paratubal/paraovarian cysts,13,16,23,28 7.4% (n = 2) paramesonephric cyst,2,5 and 25.9% (n = 7) borderline serous papillary cystadenomas (only one in a 17-year-old patient).10,11,25,26,30,32,33
We found an unexpected high percentage (25%) of borderline giant POCs. Serum marker testing was indicated in 17 cases, but Ca 125 levels were elevated only in the case reported by Bayar et al.26 Of the 7 cases included in the present review, papillary projections inside the cyst were found on USG,26,32 on CT,11,25 or intraoperatively.10,30,33 Some authors recommend supplementary intraoperative measures for cyst’s drainage in these situations, as well as the confirmation of cyst malignancy by frozen sections.1,44
Conclusions
Although not very numerous, all the studies highlighted the low incidence of giant POCs, the impossibility of establishing the origin of the cystic mass by clinical and imaging methods even with advanced technical tools and the low risk of torsion. Despite the recognized benign nature of the giant POCs, the present review found 25% of the lesions to be borderline tumors. Surgical excision is the only treatment option. Ovarian-sparing surgery was performed in 85.1% of cases, and minimally invasive techniques were applied in only 42.9% of the patients, which demonstrates the need for a high-level laparoscopic expertise.
Knowledge of this pathology, its recognition as a possible etiology of an abdominopelvic cyst, and a higher awareness of the possibility of a borderline histology in giant POCs are required for the proper management of these particular cases.
Abbreviations
POC, paraovarian cyst; POCs, paraovarian cysts; USG, ultrasonography; CT, computed tomography; MRI, magnetic resonance imaging.
Data Sharing Statement
The datasets used and/or analysed during the current study are available from Dr Liliana Baroiu, the corresponding author on reasonable request.
Ethics Approval and Informed Consent
Not applicable.
Aknowledgments
The linguistic review of the present article was made by Antoanela Marta Mardar, member of the Research Center “Interface Research of the Original and Translated Text. Cognitive and Communicative Dimensions of the Message”, Faculty of Letters, “Dunărea de Jos” University of Galati, Romania.
The present work was academically supported by “Dunarea de Jos” University of Galati, through the research center Multidisciplinary Integrated Center of Dermatological Interface Research (MIC-DIR).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The APC for the present study was supported by the ‘Dunarea de Jos’ University of Galati through an internal grant (grant no. RF3668/01.10.2021).
Disclosure
The authors declare that they have no financial competing interests.
References
1. Tjokroprawiro BA. Huge paratubal cyst: a case report and a literature review. Clin Med Insights Case Rep. 2021;14:11795476211037549. doi:10.1177/11795476211037549
2. Sagili H, Krishnan M, Dasari P. Huge bilateral paramesonephric cysts in a 25 year old nulliparous woman. J Clin Diagn Res. 2013;7(11):2589–2590. doi:10.7860/JCDR/2013/6563.3597
3. Kiran S, Jabri SS, Razek YA, Devi MN. Non-tender huge abdominal mass in an adolescent: bilateral paraovarian cysts. Sultan Qaboos Univ Med J. 2021;21(2):e308–e311. doi:10.18295/squmj.2021.21.02.022
4. Bohîlțea RE, Cîrstoiu MM, Turcan N, Ionescu CA. Ultrasound diagnostic of mesonephric paraovarian cyst - case report. J Med Life. 2016;9(3):280–283.
5. Leanza V, Coco L, Genovese F, et al. Laparoscopic removal of a giant paratubal cyst complicated by hydronephrosis. G Chir. 2013;34(11–12):323–325.
6. Felipe JH, Alcantar AR, Franco RF. Adolescent with paraovarian cyst. Surgical treatment. Cir Cir. 2017;85(6):535–538. doi:10.1016/j.circir.2016.08.002
7. De Sanctis V, Soliman AT, Elsedfy H, et al. An adolescent with an asymptomatic adnexal cyst: to worry or not to worry? Medical versus surgical management options. Acta Biomed. 2017;88(2):232–236. doi:10.23750/abm.v88i2.6050
8. Thakore SS, Chun MJ, Fitzpatrick K. Recurrent ovarian torsion due to paratubal cysts in an adolescent female. J Pediatr Adolesc Gynecol. 2012;25(4):85–87. doi:10.1016/j.jpag.2011.10.012
9. Dietrich JE, Heard MJ, Edwards C. Uteroovarian ligament torsion of the due to a paratubal cyst. J Pediatr Adolesc Gynecol. 2005;18(2):125–127. doi:10.1016/j.jpag.2005.01.009
10. Agrawal N, Gupta N, Chandra S, Gupta S, Fayyaz S. An unusual presentation of huge paraovarian cyst as papillary serous cyst adenofibroma: a rare case report. Int J Reprod Contracept Obstet Gynecol. 2018;7(9):3887–3889. doi:10.18203/2320-1770.ijrcog20183815
11. Kiseli M, Caglar GS, Cengiz SD, Karadag D, Yılmaz MB. Clinical diagnosis and complications of paratubal cysts: review of the literature and report of uncommon presentations. Arch Gynecol Obstetrics. 2012;285(6):1563–1569. doi:10.1007/s00404-012-2304-8
12. Katke RD, Pagare P, Raina J, Singh K. A rare case of successful pregnancy outcome with giant paraovarian cyst: a case report and review of literature. Int J Reprod Contracept Obstet Gynecol. 2013;2(4):730–732. doi:10.5455/2320-1770.ijrcog20131255
13. Skaff B, Zoorob D, El Assaad R, Abou-Baker M. Minimally invasive excision of a giant paratubal cyst: case report and management review. Case Rep Obstet Gynecol. 2019;2019:3458230. doi:10.1155/2019/3458230
14. Atileh LIA, Dahbour D, Hammo H, Abdullattif M. Laparoscopic removal of a 40-cm paratubal cyst in a morbidly obese patient. Gynecol Minim Invasive Ther. 2020;9(1):39–41. doi:10.4103/GMIT.GMIT_110_18
15. Mukhopadhyay S. Giant paraovarian cyst. J Obstet Gynecol India. 2006;56(4):352–353.
16. Asare EA, Greenberg S, Szabo S, Sato TT. Giant paratubal cyst in adolescence: case report, modified minimal access surgical technique, and literature review. J Pediatr Adolesc Gynecol. 2015;28(5):e143–e145. doi:10.1016/j.jpag.2014.11.002
17. Haykal T, Fleifel S, Jalla K, Safadi B. Borderline serous papillary tumor arising in a paraovarian cyst: a case report and an extensive review of the literature. Int J Clin Res. 2021;2(1):81–92. doi:10.38179/ijcr.v2i1.72
18. Mehawej J, El Helou N, Wang L, Mhawech-Fauceglia P. Paratubal serous borderline tumor in an 85 years old woman: a case report. Gynecol Oncol Rep. 2020;32:100559. doi:10.1016/j.gore.2020.100559
19. Mărginean CO, Mărginean C, Meliţ LE, Săsăran VŞ, Poruţiu M, Mărginean CD. An incidental diagnosis of a giant paraovarian cyst in a female teenager: a case report. Medicine. 2018;97(48):e13406. doi:10.1097/MD.0000000000013406
20. Erikci VS, Payza D, Hoşgör M. Giant paraovarian cyst: a case report. J Surg. 2015;5(3):214–216.
21. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
22. Saxena AK, Petnehazy T, Schalamon J, Höllwarth E. Giant paraovarian cyst in adolescent female: presentation and laparoscopic management. Eur J Pediatr. 2008;167:487–488. doi:10.1007/s00431-007-0519-7
23. Tsuji S, Kimura F, Hanada T, et al. Successful minimally invasive resection of a huge paratubal cyst in pregnancy. Gynecol Minim Invasive Ther. 2017;6(3):141–142. doi:10.1016/j.gmit.2016.08.007
24. Zvizdic Z, Bukvic M, Murtezic S, Skenderi F, Vranic S. Giant paratubal serous cystadenoma in an adolescent female: case report and literature review. J Pediatr Adolesc Gynecol. 2020;33(4):438–440. doi:10.1016/j.jpag.2020.03.010
25. Lee S, Ahn KH, Park HT, et al. Paratubal borderline malignancy: a case of a 17-Year-old adolescent female treated with laparo-endoscopic single-site surgery and a review of the literature. J Pediatr Adolesc Gynecol. 2016;29(1):74–76. doi:10.1016/j.jpag.2014.07.012
26. Bayar Ü, Özcan O, Basaran M, et al. Giant paraovarian cyst: a case report. Case Rep Clin Pract Rev. 2006;7:155–158.
27. Gorkem U, Efeturk T, Sahiner T, Bas Y, Dolapci M, Gungor T. A rare case of paratubal cystadenoma during pregnancy. J Surg Case Rep. 2016;2016(1):rjv169. doi:10.1093/jscr/rjv169
28. Shah NH, Kale KG, Paranjape SH, Shah VN. Successful laparoscopic management of a giant paraovarian cyst in an adolescent female. J Postgrad Gynecol Obstet. 2016;3(7):e308.
29. Kostov M, Mijović Z, Mihailović D. Giant paraovarian cyst in a child complicated with torsion. Vojnosanit Pregl. 2008;65(11):843–846. doi:10.2298/VSP0811843K
30. Borrás Suñer D, Cazorla Amorós E, Urgal Ayala A, Fortuño Salais S, Díaz-García C. Torsion of a giant para-ovarian cyst. Conservative laparoscopic treatment. Gynecol Surg. 2009;6(1):67–69. doi:10.1007/s10397-008-0392-z
31. Kandil M, Sayyed T, Zakaria M. Laparoscopic trocar management of a giant paraovarian cyst: a case report. F1000Res. 2013;2:29. doi:10.12688/f1000research.2-29.v2
32. Shin Y-J, Kim J-Y, Lee HJ, Park J-Y, Nam J-H. Paratubal serous borderline tumor. J Gynecol Oncol. 2011;22(4):295–298. doi:10.3802/jgo.2011.22.4.295
33. Alpendre F, Pedrosa I, Silva R, Batista S, Tapadinhas P. Giant paratubal cyst presenting as adnexal torsion: a case report. Case Rep Women’s Health. 2020;27:e00222. doi:10.1016/j.crwh.2020.e00222
34. Bhansakarya R, Subedi S. Laparoscopic management of large right paratubal cyst: a case report. J Nepal Med Assoc. 2020;58(227):501–504. doi:10.31729/jnma.4982
35. Čančar V, Ivanović R, Lalović N, Milinković B, Sladoje D. Paraovarian cyst as the cause of uterine prolapse. Arch Oncol. 2021;27(1):12–14. doi:10.2298/AOO190528001C
36. Stefanopol IA, Baroiu L, Constantin GB, et al. Diagnostic and management of undescended ovary - a preoperative dilemma: a case-based systematic review. Int J Women’s Health. 2022;14:15–27. doi:10.2147/IJWH.S345742
37. Schoenwolf GC, Bleyl SB, Brauer PR, Francis-West PH. Development of the urogenital system. In: Livingstone C, editor. Larsen’s Human Embryology.
38. Sadler TW. Urogenital system. In: Kluwer W, editor. Langman’s Medical Embryology.
39. Okada T, Yoshida H, Matsunaga T, et al. Paraovarian cyst with torsion in children. J Pediatr Surg. 2002;37(6):937–940. doi:10.1053/jpsu.2002.32922
40. Tzur T, Smorgick N, Sharon N, Pekar-Zlotin M, Maymon R, Melcer Y. Adnexal torsion with paraovarian cysts in pediatric and adolescent populations: a retrospective study. J Pediatr Surg. 2021;56(2):324–327. doi:10.1016/j.jpedsurg.2020.05.023
41. Wittich AC. Hydatid of Morgagni with torsion diagnosed during cesarean delivery. A case report. J Reprod Med. 2002;47(8):680–682.
42. Habek D, Dmitrovic B, Popovic Z, et al. Giant paraovarian myxoma. J Obstet Gynaecol Res. 2006;32(2):212–215. doi:10.1111/j.1447-0756.2006.00379.x
43. Stefanopol IA, Miulescu M, Baroiu L, Anghele AD, Danila DM, Tiron Z. An unusual case of Meckel diverticulitis misdiagnosed as an infected urachal cyst. Medicina. 2021;57(5):495. doi:10.3390/medicina57050495
44. Durairaj A, Gandhiraman K. Complications and management of paraovarian cyst: a retrospective analysis. J Obstet Gynaecol India. 2019;69(2):180–184. doi:10.1007/s13224-018-1152-2
45. Syed S, Amin A, Ullah M. Fallopian tube torsion secondary to paraovarian fimbrial cyst: a difficult to diagnose and a rare cause of acute abdomen in adolescent. Cureus. 2021;13(9):e17888. doi:10.7759/cureus.17888
46. Qian L, Wang X, Li D, Li S, Ding J. Isolated fallopian tube torsion with paraovarian cysts: a case report and literature review. BMC Women's Health. 2021;21(1):345. doi:10.1186/s12905-021-01483-2
47. Khaitov D, Gabbur N. Contralateral recurrence of fallopian tube torsion: a case report. Case Rep Women’s Health. 2021;30:e00307. doi:10.1016/j.crwh.2021.e00307
48. Low S-CA, Ong C-L, La S-L, Beh S-T. Paratubal cyst complicated by tubo-ovarian torsion: computed tomography features. Australas Radiol. 2005;49(2):136–139. doi:10.1111/j.1440-1673.2005.01405.x
49. Yilmaz Y, Ozen IO, Caliskan D, Dilmen U. Paraovarian cyst torsion in children: report of two cases. Pediatr Int. 2013;55(6):795–797. doi:10.1111/ped.12145
50. Paul PG, Annal A, Chowdary KA, Paul G. A retroperitoneal cyst masquerading as a para-ovarian cyst in a postmenopausal woman. Gynecol Minim Invasive Ther. 2021;10(3):195–196. doi:10.4103/GMIT.GMIT_11_21
51. Savelli L, Ghi T, De Iaco P, Ceccaroni M, Venturoli S, Cacciatore B. Paraovarian/paratubal cysts: comparison of transvaginal sonographic and pathological findings to establish diagnostic criteria. Ultrasound Obstet Gynecol. 2006;28(3):330–334. doi:10.1002/uog.2829
52. Gupta A, Gupta P, Manaktala U, Khurana N. Clinical, radiological, and histopathological analysis of paraovarian cysts. J Midlife Health. 2016;7(2):78–82. doi:10.4103/0976-7800.185337
53. Zhao F, Zhang H, Ren Y, Kong F. Transvaginal sonographic characteristics of paraovarian borderline tumor. Int J Clin Exp Med. 2015;8(2):2684–2688.
54. Terek MC, Sahin C, Yeniel AO, et al. Paratubal borderline tumor diagnosed in the adolescent period: a case report and review of the literature. J Pediatr Adolesc Gynecol. 2011;24(5):e115–e116. doi:10.1016/j.jpag.2011.05.007
55. Depoers C, Martin FA, Nyangoh Timoh K, et al. A Preoperative scoring system for adnexal mass in children and adolescents to preserve their future fertility. J Pediatr Adolesc Gynecol. 2019;32(1):57–63. doi:10.1016/j.jpag.2018.08.009
56. Kishimoto K, Ito K, Awaya H, Matsunaga N, Outwater EK, Siegelman ES. Paraovarian cyst: MR imaging features. Abdom Imaging. 2002;27(6):685–689. doi:10.1007/s00261-002-0014-6
57. Darwish AM, Amin AF, Mohammad SA. Laparoscopic management of paratubal and paraovarian cysts. JSLS. 2003;7(2):101–106.
58. Dotters-Katz SK, James AH, Jaffe TA. Paratubal/Paraovarian masses: a study of surgical and non-surgical outcomes. Med J Obstet Gynecol. 2014;2:1019–1023.
59. Ciobotaru OR, Lupu MN, Rebegea L, et al. Dexamethasone-Chemical structure and mechanisms of action in prophylaxis of postoperative side effects. REV CHIM. 2019;70(3):843–847. doi:10.37358/RC.19.3.7017
60. Lupu M, Miulescu M, Stefanopol IA, et al. Effect of 2,6-diisopropylphenol and 1,1,1,3,3,3-hexafluoro-2-(fluoromethoxy) propane as anesthetic. REV CHIM. 2019;70(5):1888–1892. doi:10.37358/RC.19.5.7239
61. Ciobotaru OR, Stoleriu G, Ciobotaru OC, et al. Postanesthetic skin erythema due to succinylcholine versus atracurium. Exp Ther Med. 2020;20(3):2368–2372.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
