Back to Journals » Clinical Ophthalmology » Volume 16
Clinical Features of Glaucoma Associated with Cytomegalovirus Corneal Endotheliitis
Authors Mori K, Ye Y, Yokogawa H , Nishino T, Kobayashi A, Mori N, Takemoto Y, Sugiyama K
Received 24 May 2022
Accepted for publication 27 July 2022
Published 19 August 2022 Volume 2022:16 Pages 2705—2711
DOI https://doi.org/10.2147/OPTH.S376039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Kazuya Mori,* Yunyan Ye,* Hideaki Yokogawa, Tsubasa Nishino, Akira Kobayashi, Natsuko Mori, Yuko Takemoto, Kazuhisa Sugiyama
Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Kanazawa, Japan
*These authors contributed equally to this work
Correspondence: Hideaki Yokogawa, Department of Ophthalmology, Kanazawa University Graduate School of Medical Science, Takara-machi 13-1, Kanazawa, Ishikawa-ken, 920-8641, Japan, Tel +81-76-265-2403, Fax +81-76-222-9660, Email [email protected]
Purpose: The purpose of this study was to highlight the manifestations of glaucoma associated with cytomegalovirus (CMV) corneal endotheliitis.
Methods: We reviewed the 34 patients that met the diagnostic criteria for CMV endotheliitis in our hospital, with special attention to the glaucoma status, including onset of glaucoma, glaucoma in the fellow eye, visual field defects, intraocular pressure, and final outcomes.
Results: Thirty-four eyes of 34 patients (mean age, 69.1 ± 13.1 years; 31 males [91.2%]) with CMV corneal endotheliitis were enrolled. Thirty-two eyes (94.1%) had a history of a glaucoma diagnosis, which had been treated for 10.0 ± 10.1 years. Glaucoma in the fellow eye was noted in 16 cases (47.1%) and a history of Posner-Schlossman syndrome was noted in 13 cases (38.2%). Visual fields measured using a Humphrey field analyzer were normal-to-early stage (MD>-6dB) in 16 eyes (47.1%) and middle-to-late stage (MD≤-6dB) in 18 eyes (52.9%). The intraocular pressure decreased from 22.4 ± 10.6 mmHg at the initial visit to 14.9 ± 7.9 mmHg after medical treatment, including 0.5% topical ganciclovir (GCV) with and without a systemic anti-CMV agent, corticosteroid eye drops, and an anti-glaucoma agent (p< 0.01). During the follow-up period of 4.8 ± 3.0 years (range, 0.2– 10 years), 16 eyes (47.1%) required glaucoma surgery, including filtering surgery (7 eyes) and trabeculotomy only (9 eyes).
Conclusion: Our case series showed that most of the patients with CMV corneal endotheliitis had glaucoma. Although medical therapy, including 0.5% topical GCV, had efficacy in lowering the intraocular pressure, one-half of the cases required glaucoma surgery. Therefore, ophthalmologists should strive to make an earlier diagnosis of CMV corneal endotheliitis by utilizing PCR testing of aqueous humor samples to prevent sight-threatening glaucomatous damage.
Keywords: cytomegalovirus, corneal endotheliitis, secondary glaucoma, antiviral treatment
Introduction
Cytomegalovirus (CMV), a member of the human herpesvirus family, often causes recurrent ocular inflammation of the anterior segment in immunocompetent patients, including corneal endotheliitis, iris atrophy, and anterior uveitis.1–6 CMV corneal endotheliitis was initially recognized by 2 groups in 2006–2007.2,3 Thereafter, CMV corneal endotheliitis has become an established clinical entity through series of reports from worldwide, mainly from Asian countries.1–9
CMV corneal endotheliitis characterized by corneal edema, linear or circular keratic precipitates (KPs), and mild anterior chamber reaction. It sometimes accompanies intraocular pressure (IOP) elevation.4–9 Polymerase chain reaction (PCR) of aqueous humor samples is helpful in confirming the diagnosis. Previous work has demonstrated the efficacy of treatment with topical ganciclovir (GCV) and systemic anti-CMV agent.4–9 In some cases, endothelial decompensation occurs, that may require endothelial keratoplasty.4,5 Also, persist IOP elevation require glaucoma surgery.4,5,11
Based on previous reports4,5,11 and our clinical experience involving patients with CMV corneal endotheliitis, we are of the opinion that glaucoma secondary to CMV corneal endotheliitis and a decrease in endothelial cell density may lead to irreversible visual impairment. The purpose of this study was to highlight manifestations of glaucoma associated with CMV corneal endotheliitis. We paid special attention to glaucoma status, including the onset of glaucoma, glaucoma in the fellow eye, visual field defects, intraocular pressure, and final outcomes.
Patients and Methods
Patients and Study Design
This retrospective observational study was approved by the Ethical Committee of Kanazawa University Graduate School of Medical Science and adhered to the tenets of the Declaration of Helsinki. We reviewed 34 patients who were diagnosed with CMV corneal endotheliitis from April 2010 to May 2020 in the Department of Ophthalmology, Kanazawa University Hospital. All patients met the diagnostic criteria established in 2015.4 In all cases had multiplex PCR analysis of aqueous humor samples detected CMV. The demographics and clinical manifestations of each patient were recorded at the time of the initial visit, including sex, age, medical history, distance best-corrected visual acuity (BCVA) measured with Landolt ring, intraocular pressure (IOP) measured using a Goldmann applanation tonometer, endothelial cell density (ECD) determined with a specular microscope (Konan Medical Inc., Hyogo, Japan), slit-lamp findings and photograph, gonioscopic findings and photograph, fundus findings and photograph, and visual field parameters (24–2 SITA-fast) based on the Humphrey field analyzer (Carl Zeiss Meditec, Dublin, CA, USA). At each follow-up visit, IOP, anterior inflammatory reactions, and treatment effects were recorded. If IOP is higher than 21mmHg, it is defined as glaucoma, and as secondary glaucoma, the optic disc and the visual field may be normal. Immediate after the diagnosis of CMV corneal endotheliitis, all patients received anti-CMV treatment. The effect of anti-CMV treatment was evaluated 1 month after initiation of anti-CMV treatment. When patients had insufficient reduction of IOP, and had progressive visual field defects despite anti-CMV treatment, filtering surgery (trabeculectomy or glaucoma tube), or trabeculotomy was performed. In general, filtering surgery was indicated in eyes with advanced stage visual field defects. The mean follow-up period was 4.8 ± 3.0-year (range, 0.2–10 years). We analyzed IOP outcomes in eyes with or without glaucoma surgery.
Diagnostic Criteria
CMV corneal endotheliitis was diagnosed based on the diagnostic criteria promulgated by the Japan Corneal Endotheliitis Study Group in 2015.4 According to the diagnostic criteria, there must be clinical evidence of corneal endotheliitis with KPs. Specifically, viral examination by multiplex PCR of the aqueous humor should be positive for CMV DNA, but negative for herpes simplex virus (HSV) DNA and varicella-zoster virus (VZV) DNA. The multiplex PCR was performed by a Japanese company specializing in laboratory testing services (SRL, Tokyo, Japan). The primers and the PCR conditions have been described elsewhere.10 CMV corneal endotheliitis was classified into typical and atypical based on the clinical manifestations. Typical CMV corneal endotheliitis is characterized by coin-shaped lesions or linear KPs similar to the rejection line, while atypical CMV corneal endotheliitis has localized corneal edema and KPs associated with two of the following signs: recurrent/chronic anterior uveitis, ocular hypertension/secondary glaucoma, and corneal endothelial cell loss.
Statistical Analyses
Statistical analysis was performed using SPSS (version 23; SPSS, Inc., Chicago, IL, USA). Quantitative variables are presented as the mean and standard deviation (mean ± SD). Before and after anti-CMV drug treatment comparisons of IOP were performed using Wilcoxon signed rank test. In addition, typical and atypical CMV corneal endotheliitis comparisons were performed using Mann–Whitney U-test. P-values < 0.05 were considered statistically significant.
Results
Patients
Thirty-four eyes of 34 patients were enrolled in the current study (Table 1). The mean age was 69.1 ± 13.1 years (range, 27 ~ 87 years). Most of the patients were elderly men (28 cases [82.4%] ≥ 60 years of age). There were 18 patients with systemic diseases, including 13 with diabetes mellitus, 7 with hypertension, and 3 with collagen diseases that were treated with systemic immunosuppressants. It should be noted that most of the patients (32 eyes [94.1%]) had glaucoma and received anti-glaucoma eyedrop therapy prescribed by prior ophthalmologists for an average of 10.0 ± 10.1 years (range, 7 months-to-34 years). Thirteen eyes (38.2%) had a history of Posner-Schlossman syndrome (PSS). Sixteen eyes (47.1%) had glaucoma in the fellow eye, but these fellow eyes did not apply PCR examination of aqueous humor sample. Two fellow eyes accompanied mild anterior chamber reaction, while 14 fellow eyes did not. Seven eyes (20.6%) had previously received antiviral treatment with acyclovir/valacyclovir and all 34 eyes had a history of topical corticosteroid use.
 |
Table 1 Demographic and Medical History of 34 Cases |
Clinical Data at the Initial Visit
Table 2 summarizes the clinical data of 34 eyes at the time of the initial visit. Based on the diagnostic criteria, 16 eyes (47.1%) were classified as typical CMV corneal endotheliitis with coin-shaped lesions (Figure 1) or linear KPs, and the remaining 18 eyes (52.9%) were classified as atypical CMV corneal endotheliitis. At the time of the initial visit, the mean BCVA ± SD was LogMAR 0.48 ± 0.60 (decimal, 0.33). The mean IOP was 22.4 ± 10.6 mmHg (range, 4–43 mmHg) and 19 eyes (55.9%) had an elevated IOP > 21 mmHg. Twenty-eight eyes were open angle, excluding 6 eyes that had previous glaucoma surgery or penetrating keratoplasty. The mean ECD was 1471 ± 683 cells/mm2 (unmeasurable in 11 eyes), and the ECD was < 2000 cells/mm2 in 28 eyes (82.4%). Four eyes (11.8%) had pseudo-exfoliation. Glaucomatous visual field defects, as measured by a Humphrey field analyzer, were detected in 27 cases (79.4%), including 9 eyes (26.5%) in the early stage (MD>-6dB), 7 eyes (20.6%) in the middle stage (−12dB<MD≤-6dB), and 11 eyes (32.4%) in the late stage (MD≤-12dB).
 |
Table 2 Clinical Data of 34 Cases at the Initial Visit |
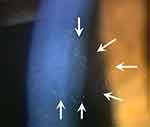 |
Figure 1 Slit-lamp photograph of typical CMV corneal endotheliitis. Close-up of coin-shaped lesion; keratic precipitates make up the circular arrangement (arrows). |
Clinical Course
After the diagnosis of CMV corneal endotheliitis was established, all patients were treated immediately with 0.5% topical GCV (6–8 applications/day). A topical corticosteroid and an anti-glaucoma agent were also applied. Concomitant systemic antivirals were administered (intravenous GCV [10 mg/kg daily×7d] or oral valganciclovir [VGCV; 1800 mg/day×21d]) to 22 patients (64.7%). The mean IOP decreased from 22.4 ± 10.6 mmHg at the time of the initial visit to 14.6 ± 7.9 mmHg 1 month after initiation of anti-CMV treatment (p<0.01; Table 3). There were no differences in the IOP before and after anti-CMV treatment between the typical and atypical CMV corneal endotheliitis groups (Table 3).
 |
Table 3 Intraocular Pressure Changes Before and 1 Month After Anti-CMV Drug Treatment |
During the 4.8 ± 3.0-year (range, 0.2–10 years) follow-up period, 18 eyes (52.9%) maintained a fair IOP without glaucoma surgery (final IOP, 11.0 ± 4.0 mmHg 5.1 ± 3.1 years after initiation of anti-CMV treatment; p<0.01), whereas 16 eyes (47.1%) required glaucoma surgery, including filtering surgery (trabeculectomy or glaucoma tube [7 eyes]) and trabeculotomy only (9 eyes). In all surgery cases, the final IOP was fair (10.1 ± 5.1 mmHg 5.3 ± 3.5 years after filtering surgery; p=0.01 and 11.8 ± 5.0 mmHg 4.2 ± 2.9 years after trabeculotomy; p<0.01). In contrast, 7 eyes required endothelial keratoplasty during follow-up. Among these 7 eyes, 2 had undergone previous glaucoma surgery and 5 had no previous glaucoma surgery. Inflammation did not recur after surgery under continuation of 0.5% topical GCV. We did not experience any adverse events with anti-CMV medications, such as drug toxicity with 0.5% topical GCV or neutropenia with systemic anti-CMV agents.
Discussion
We showed that most of the patients with CMV corneal endotheliitis (32/34 [94.1%]) had secondary glaucoma. Koizumi et al4 reported that the prevalence of glaucoma was 65.1% in 109 eyes with CMV corneal endotheliitis in a Japanese population. The reason for the higher prevalence of secondary glaucoma in patients with CMV corneal endotheliitis in our hospital compared to the Koizumi et al4 report is unclear. We speculate that the patients in the Koizumi et al4 study might have had more early cases of CMV corneal endotheliitis in the pre-perimetric stage. Nevertheless, detection of secondary glaucoma with CMV corneal endotheliitis and the proper management is of paramount importance to prevent glaucoma progression.11
The case series reported herein from our hospital had a very long history of glaucoma (mean, 10.0 ± 10.1 years; range, 7 months-to-34 years) before a definite diagnosis of CMV endotheliitis was established. As a result, greater than one-half of our cases had middle- to late-stage glaucoma at the time CMV DNA was detected. Thirteen eyes (38.2%) were previously diagnosed with PSS. Notably, the clinical significance of CMV corneal endotheliitis was not appreciated at that time and the differential diagnosis between CMV corneal endotheliitis and PSS was unclear. Typically, PSS patients have open angles, but experience recurrent unilateral attacks of mild iritis with an elevated IOP. Chee et al12 reported that 52% of aqueous humor samples from the affected eyes of PSS patients were positive for CMV based on PCR analysis. In addition, Murata et al13 showed that CMV-DNA was detected in 61.9% of Japanese patients with PSS. Furthermore, the ECD was lower in the affected eyes of CMV-positive PSS eyes than the contralateral unaffected eyes.14 Taken together, these findings suggest that CMV-positive PSS could be clinically regarded as glaucoma secondary to CMV corneal endotheliitis. A prompt diagnosis and appropriate treatment is essential to improve prognosis, not only in patients with CMV corneal endotheliitis, but also in patients with PSS.
In this study, 16 patients (47.1%) had glaucoma in the fellow eye. Although PCR examination of aqueous humor sample was not performed, two fellow eyes with mild anterior chamber reaction may be glaucoma secondary to CMV infection (bilateral CMV infection), while 14 fellow eyes without anterior chamber reaction may be primary glaucoma.
Recently, Yawata et al15 analyzed variants of polymorphic genes in 122 patients with CMV anterior uveitis, and concluded that the underlying immunopathogenesis likely involves CMV-responding natural killer cells co-expressing markers that developed on a genetic background. Recent studies have shown that human trabecular meshwork cells effectively support CMV replication, as well as corneal endothelial cells.16,17 CMV infection enhances the production of TGF-β, a cardinal cytokine that increases the resistance of trabecular meshwork outflow pathways, in human TM cells. Treatment with an antiviral agent decreased viral DNA replication, but an antiviral agent did not inhibit TGF-β production. The increase in TGF-β was ameliorated by treatment with corticosteroids.17 In the current study, the combined use of 0.5% topical GCV (6–8 applications/day) with or without a systemic anti-CMV agent, corticosteroid eyedrops, and an anti-glaucoma agent were effective in reducing the IOP. Fair IOP control was maintained without glaucoma surgery during the 5.1 ± 3.1 years follow-up period for 18 eyes (52.9%). Maintenance therapy with 0.5% topical GCV could be used due to the inhibition of CMV activity, facilitating IOP control, and preserving the corneal endothelium.18,19 In addition, topical GCV induces less frequent adverse events than systemic GCV, such as neutrocytopenia.5 The clinical effect of 0.15% GCV ophthalmic gel, which is approved for HSV keratitis, has been suggested in some case series even though 0.15% GCV ophthalmic gel has not been approved and marketed in Japan.20,21
In the current study, one-half of the eyes (47.1%) were classified as typical CMV corneal endotheliitis, in which coin-shaped lesions or linear KPs were present. We have previously confirmed that coin-shaped lesions may be CMV-infected swollen/necrotic endothelial cells which correspond to the owl’s eye cells observed under confocal microscopy.6,8,9 As shown in the current study, there were no differences in the IOP course between patients with typical and atypical CMV corneal endotheliitis.
After anti-CMV treatment, 47.1% of the patients underwent glaucoma surgery in our case series, which is consistent with the 25.7–60% previously reported in the literature.11,22 Indeed, PSS patients with a higher ECD reduction ratio and CMV-positive patients with a disease duration > 5 years were more likely to undergo glaucoma surgery.14 In our surgery cases, the final IOP was fair after filtering surgery and/or trabeculotomy.
Limitations of this study were the retrospective design and relatively small sample size. In eyes with pseudo-exfoliation, we cannot exclude pseudo-exfoliation as a cause of glaucoma. In addition, use of anti-CMV medication was not uniform and the indications for glaucoma surgery were not strictly defined. Therefore, a large prospective long follow-up study is warranted.
In conclusion, our case series revealed that most of the patients with CMV corneal endotheliitis had glaucoma. Although medical therapy, including 0.5% topical GCV, was effective in reducing the IOP, one-half of the patients required glaucoma surgery. Therefore, ophthalmologists should strive to establish a timely diagnosis of CMV corneal endotheliitis by PCR testing of aqueous humor samples to prevent sight-threatening glaucomatous damage.
Patient Consent
This retrospective clinical study was approved by the Ethical Committee of Kanazawa University Graduate School of Medical Science (approval number 3412) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained with opt-out method. This report does not contain any personal identifying information.
Funding
There was no funding or grant support for this study.
Disclosure
Kazuya Mori and Yunyan Ye are co-first authors. The corresponding investigator (H.Y.) has full access to all data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis. All authors have no funding or conflicts of interest to disclose.
References
1. Carmichael A. Cytomegalovirus and the eye. Eye. 2012;26:237–240. doi:10.1038/eye.2011.327
2. Koizumi N, Yamasaki K, Kawasaki S, et al. Cytomegalovirus in aqueous humor from an eye with corneal endotheliitis. Am J Ophthalmol. 2006;141:564–565. doi:10.1016/j.ajo.2005.09.021
3. Chee SP, Bacsal K, Jap A, et al. Corneal endotheliitis associated with evidence of cytomegalovirus infection. Ophthalmology. 2007;114:798–803. doi:10.1016/j.ophtha.2006.07.057
4. Koizumi N, Inatomi T, Suzuki T, et al. Clinical features and management of cytomegalovirus corneal endotheliitis: analysis of 106 cases from the Japan corneal endotheliitis study. Br J Ophthalmol. 2015;99:54–58. doi:10.1136/bjophthalmol-2013-304625
5. La Distia Nora R, Putera I, Mayasari YD, et al. Clinical characteristics and treatment outcomes of cytomegalovirus anterior uveitis and endotheliitis: a systematic review and meta-analysis. Surv Ophthalmol. 2021;67(21):1014–1030.
6. Kobayashi A, Yokogawa H, Higashide T, et al. Clinical significance of owl eye morphologic features by in vivo laser confocal microscopy in patients with cytomegalovirus corneal endotheliitis. Am J Ophthalmol. 2012;153:445–453. doi:10.1016/j.ajo.2011.07.026
7. Yokogawa H, Kobayashi A, Takemoto Y, et al. Development of cytomegalovirus corneal endotheliitis during long-term topical tacrolimus and steroid treatment for chronic ocular surface inflammatory diseases. Cornea. 2021;40:1491–1497. doi:10.1097/ICO.0000000000002674
8. Yokogawa H, Kobayashi A, Yamazaki N, et al. In vivo imaging of coin-shaped lesions in cytomegalovirus corneal endotheliitis by anterior segment optical coherence tomography. Cornea. 2014;33:1332–1335. doi:10.1097/ICO.0000000000000269
9. Yokogawa H, Kobayashi A, Sugiyama K. Mapping owl’s eye cells of patients with cytomegalovirus corneal endotheliitis using in vivo laser confocal microscopy. Jpn J Ophthalmol. 2013;57:80–84. doi:10.1007/s10384-012-0189-5
10. Ogata N, Koike N, Yoshikawa T, et al. Human herpesvirus 6-associated uveitis with optic neuritis diagnosed by multiplex PCR. Jpn J Ophthalmol. 2011;55:502–505. doi:10.1007/s10384-011-0069-4
11. Touhami S, Qu L, Angi M, et al. Cytomegalovirus anterior uveitis: clinical characteristics and long-term outcomes in a French series. Am J Ophthalmol. 2018;194:134–142. doi:10.1016/j.ajo.2018.07.021
12. Chee SP, Jap A. Presumed fuchs heterochromic iridocyclitis and Posner-Schlossman syndrome: comparison of cytomegalovirus-positive and negative eyes. Am J Ophthalmol. 2008;146:883–889. doi:10.1016/j.ajo.2008.09.001
13. Murata K, Ishida K, Ozawa K, et al. The characteristics of Posner-Schlossman syndrome: a comparison in the surgical outcome between cytomegalovirus-positive and cytomegalovirus-negative patients. Medicine. 2019;98:e18123. doi:10.1097/MD.0000000000018123
14. Su CC, Hu FR, Wang TH, et al. Clinical outcomes in cytomegalovirus-positive Posner-Schlossman syndrome patients treated with topical ganciclovir therapy. Am J Ophthalmol. 2014;158:1024–1031. doi:10.1016/j.ajo.2014.08.007
15. Yawata N, Shirane M, Woon K, et al. Molecular signatures of natural killer cells in CMV-associated anterior uveitis, a new type of CMV-induced disease in immunocompetent individuals. Int J Mol Sci. 2021;22:3623. doi:10.3390/ijms22073623
16. Shimizu D, Miyazaki D, Shimizu Y, et al. Infection of endotheliotropic human cytomegalovirus of trabecular meshwork cells. Jpn J Ophthalmol. 2018;62(6):667–676. doi:10.1007/s10384-018-0618-1
17. Choi JA, Kim JE, Noh SJ, et al. Enhanced cytomegalovirus infection in human trabecular meshwork cells and its implication in glaucoma pathogenesis. Sci Rep. 2017;7:43349. doi:10.1038/srep43349
18. Kitazawa K, Jongkhajornpong P, Inatomi T, et al. Topical ganciclovir treatment post-Descemet’s stripping automated endothelial keratoplasty for patients with bullous keratopathy induced by cytomegalovirus. Br J Ophthalmol. 2018;102:1293–1297. doi:10.1136/bjophthalmol-2017-311145
19. Hwang JH, Ha M, Park Y, et al. The effect of topical ganciclovir and corticosteroid on cytomegalovirus corneal endotheliitis in Korean patients. Ocul Immunol Inflamm. 2019;27:338–344. doi:10.1080/09273948.2018.1486436
20. Koizumi N, Miyazaki D, Inoue T, et al. The effect of topical application of 0.15% ganciclovir gel on cytomegalovirus corneal endotheliitis. Br J Ophthalmol. 2017;101:114–119. doi:10.1136/bjophthalmol-2015-308238
21. Waduthantri S, Zhou L, Chee SP. Intra-cameral level of ganciclovir gel, 0.15% following topical application for cytomegalovirus anterior segment infection: a pilot study. PLoS One. 2018;13:e0191850. doi:10.1371/journal.pone.0191850
22. Accorinti M, Gilardi M, Pirraglia MP, et al. Cytomegalovirus anterior uveitis: long-term follow-up of immunocompetent patients. Graefes Arch Clin Exp Ophthalmol. 2014;252:1817–1824. doi:10.1007/s00417-014-2782-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
