Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Clinical Feature, Lifestyle Behavior and Non-Communicable Diseases Comorbidities Among Psoriasis Patients in Shanghai: Gender Disparity Analysis Based on a Cross-Sectional Study
Authors Zheng Q, Kuai L, Jiang W, Qiang Y, Wei L, Chen S, Li B, Wang R
Received 22 October 2022
Accepted for publication 7 December 2022
Published 15 December 2022 Volume 2022:15 Pages 2751—2762
DOI https://doi.org/10.2147/CCID.S393697
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Qi Zheng,1,* Le Kuai,2,* Wencheng Jiang,1,* Yan Qiang,3 Lei Wei,2 Siting Chen,2 Bin Li,1 Ruiping Wang3,4
1Department of Traditional Chinese Medicine, Shanghai Skin Diseases Hospital, Tongji University, Shanghai, People’s Republic of China; 2Department of Dermatology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 3Clinical Research and Innovation Transformation Center, Shanghai Skin Diseases Hospital, Tongji University, Shanghai, People’s Republic of China; 4School of Public Health, Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bin Li; Ruiping Wang, Email [email protected]; [email protected]
Background: Gender difference is prevalent in clinical feature, disease severity for noncommunicable diseases (NCD), but studies on gender disparity in clinical feature, disease severity and NCD comorbidity among psoriasis patients are limited. This cross-sectional study explores gender differences in clinical feature, lifestyle behavior and NCD comorbidity among psoriasis patients.
Methods: Psoriasis patients were recruited through cluster survey method in two hospitals, and questionnaire interviews were applied to collect the demographic feature, lifestyle habits, clinical feature and NCD among patients.
Results: A total of 2102 psoriasis patients included 1332 males (63.4%), 70% were over 35 years old and approximately 50% of them were overweight or obesity. The median value for psoriasis initiation age and disease duration was 33 years old (34 for male and 32 for female) and 9 years (10 for male and 7 for female), respectively. The psoriasis recurrence was mainly in winter (73.4%) and autumn (34.2%) both for patients. The prevalence of tobacco smoking and alcohol drinking was 31.2% and 12.6%. Male patients had higher prevalence of tobacco smoking (odds ratio (OR) = 13.26, 95% confidence interval (CI): 9.54– 18.44) and alcohol drinking (OR = 14.44, 95% CI: 7.90– 26.40). The prevalence of diabetes, hypertension, hyperlipidemia, and metabolic syndrome were 13.2%, 28.5%, 23.4% and 21.5%, respectively. Male patients had higher prevalence of diabetes (OR = 1.53, 95% CI: 1.16– 2.02), hypertension (OR = 1.87, 95% CI: 1.52– 2.30), hyperlipidemia (OR = 2.34, 95% CI: 1.85– 2.95) and metabolic syndrome (OR = 2.06, 95% CI: 1.63– 2.62) than female patients. The proportions for 4 types of NCDs diagnosed after psoriasis onset were over 58%, which were also higher in males than females.
Conclusion: Female patients had shorter disease duration and with less NCD, and male patients had more body weight issue, with fewer sleep time and higher prevalence of tobacco smoking and alcohol drinking and NCDs. We recommend that dermatologist should notice the gender disparity in psoriasis patients, which is helpful for the disease diagnosis and treatment.
Keywords: psoriasis, gender disparity, tobacco smoking, alcohol drinking, overweight, metabolic diseases, comorbidity
Introduction
Psoriasis is a chronic and inflammatory disease that affects the skin, nails and joints and can be accompanied by various comorbidities.1 The overall prevalence of psoriasis ranges from 0.09% to 5.10%, and approximately 125 million people suffer from psoriasis worldwide.2 Psoriasis is more common in Caucasian, followed by yellow race and black race.3 Psoriasis has several sub-types based on its clinical feature, of which psoriasis vulgaris is the most common sub-type.4 The clinical manifestation of psoriasis is mainly erythema and scaly, and psoriasis can occur all over the body with scalp and extensor limbs being more common, and psoriasis is usually aggravated in winter. Due to the high prevalence, long disease course, nature of recurrence and heavy economic burden, psoriasis impacts patients physically and psychologically and is an important public health issue in the field of dermatology.
The pathogenesis of psoriasis is still unclear. Previous studies indicate that many factors play a role in the pathogenesis of psoriasis.5 External risk factors for psoriasis include mechanical stress, air pollution, ultraviolet radiation, drugs, vaccination, infection, tobacco and alcohol exposure. Internal risk factors for psoriasis include genetic, immunity, obesity, diabetes, dyslipidemia, hypertension and mental stress.6 In recent years, growing evidence indicates that psoriasis can be combined with some systemic diseases, known as psoriasis comorbidity.7,8 Understanding the correlation between psoriasis and comorbidity is helpful for the early identification and treatment of comorbidities, and the treatment of comorbidities is meaningful to improve the psoriasis condition. The pathogenesis of comorbidity is complex. The disease course, severity and recurrence frequency of psoriasis, family history and other factors may all affect the occurrence of comorbidity.7,8
In medical disciplines, gender is an important demographic feature for human beings,9 which may affect the health status in terms of incidence, prevalence, disease severity, adverse effects, and response to therapy.10 Studies have shown that gender is associated with the differences in clinical characteristics, disease severity, psychological distress and life quality for many non-communicable diseases (NCD).11 However, there is limited evidence on the pathogenesis, treatment preference and comorbidity management for psoriasis in different genders. Therefore, understanding the gender disparity in disease onset, treatment preference, NCD comorbidities and satisfaction among psoriasis patients is critical to improve their treatment effect and life quality.
In this study, we aim to learn the clinical feature, lifestyle behavior and NCD comorbidity among psoriasis patients and to explore the gender differences to provide reference for clinical decision-making and comorbidity prevention and treatment for psoriasis.
Methods
Study Design
During January to December 2021, we conducted a cross-sectional study in Shanghai Skin Diseases Hospital and Yueyang Hospital. As reported by Wei L12 in 2021, the prevalence of hypertension and Type 2 diabetes mellitus (T2DM) were 31.85% and 20.41% in patients with psoriasis in Shanghai. In this study, we applied sample size formula 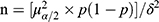 , and set prevalence of NCD equal 20% (p = 20%), α = 0.05, δ = 10% of p, with 20% of non-response rate, the sample size calculation indicated that at least 1845 psoriasis patients should be enrolled. In this study, we used cluster sampling method to recruit psoriasis patients. Ethics Review Committee of Yueyang Hospital, Shanghai University of Traditional Chinese Medicine reviewed and approved this study (2021–028), and all psoriasis patients signed the informed consent forms before questionnaire interview. In this study, 2102 psoriasis patients were included in the final analysis.
, and set prevalence of NCD equal 20% (p = 20%), α = 0.05, δ = 10% of p, with 20% of non-response rate, the sample size calculation indicated that at least 1845 psoriasis patients should be enrolled. In this study, we used cluster sampling method to recruit psoriasis patients. Ethics Review Committee of Yueyang Hospital, Shanghai University of Traditional Chinese Medicine reviewed and approved this study (2021–028), and all psoriasis patients signed the informed consent forms before questionnaire interview. In this study, 2102 psoriasis patients were included in the final analysis.
Psoriasis Patient Enrollment
In this study, psoriasis was confirmed according to guideline of the Chinese clinical dermatology.13 1) pathogenic site: with skin damage manifested as localized or systemic; 2) clinical symptoms: mainly red inflammatory papules, maculopapular rash, patches of varying sizes, covered with multiple layers of silvery-white scales. After scraping the scale, there is membranous appearance and punctate bleeding. In this study, both of male and female psoriasis patients aged >18 years were included and psoriasis patients with neurological or psychiatric abnormalities were excluded.
Data Collection
In this study, data were collected through a self-designed questionnaire during January and December 2021. We encourage patients with psoriasis to complete a face-to-face questionnaire survey. The main content of the questionnaire includes1 demographic feature: gender, age, education, personal monthly income, body mass index (BMI), etc.;2 lifestyle habits: tobacco smoking, alcohol drinking, tea drinking, physical exercise, sleep condition, etc.;3 medical history of NCD: diabetes, hypertension, hyperlipidemia, psoriasis subtype, disease duration, psoriasis recurrence and family history.4 Lab examination: blood biochemical indicators. Physical examination of psoriasis patients including height, weight, and waist circumference was extracted directly from their medical records. This study presented a homogenization of the management in the blood biochemical indicators between two hospitals.
Lifestyle Habits Definition
In this study, tobacco smoking was defined as those who smoked at least one cigarette a day for at least 6 months. Alcohol drinking was defined as a person who drinks alcohol at least 3 times a week for six months or more in a lifetime, and we define tea drinking as those who drinking tea (green, black, etc.) at least 3 times a week for over 6 months. Daily sleep time was the combined hours of daytime nap and nighttime sleep in the most recent month before the investigation. Low intensity (LI) physical exercise was defined as exercise with METs (Metabolic Equivalent) less than 3.0, and patients with daily LI exercise ≥30 minutes were then classified into groups of “Never”, “(1–2) times/week”, “(3–5) times/week” and “(6–7) times/week”.
Definition and Index Calculation
Metabolic syndrome in this study was defined as those individuals with ≥3 types of abnormalities including hypertension, diabetes (T2DM), increased waist circumference, and dyslipidemia. In accordance with the guidelines for T2DM prevention and treatment of in China (2020 edition), T2DM was defined as patient with Fasting Blood Glucose (FBG) ≥6.1 mmol/L or 2-h postprandial blood glucose ≥7.8 mmol/L in over 12 hours fasting. Hypertension was defined as patients with blood pressure ≥130/85 mmHg. Dyslipidemia was confirmed if triglycerides ≥1.7 mmol/L, reduced HDL was substantiated as HDL cholesterol <1.04 mmol/L, and the increased waist circumference was defined as waist circumference ≥90 cm in male and ≥85 cm in female. Disease duration of psoriasis patients was calculated as the time interval between the initial psoriasis diagnosis and the investigation which including the intermediate recovery times. The familial aggregation for psoriasis was classified as father, mother, siblings, and offspring. In this study, age was stratified into <35, 35–44, 45–55, and >55 years in accordance with quartile (P25, P50 and P75), marital status was categorized as unmarried, married and divorced/widows. Education level of patients was classified as junior high and lower, senior high, college and above. Occupation was classified as manual worker, mental worker and unemployment. Individual monthly income was categorized as <3000, 3000–5000, 5001–10,000, and >10,000 RMB (1000 Chinese Renminbi about US$160). BMI (kg/m2) of psoriasis patients was categorized as underweight (<18.5), normal weight (18.5–23.9), overweight (24.0–27.9) and obesity (≥28.0).
Statistical Analysis
In this study, data analysis was performed by applying SAS 9.4 (SAS Institute Inc, Cary, NC, USA). Quantitative data with normal distribution were expressed as mean and standard deviation (SD), and the Student’s t-test was used to test the differences between groups. Quantitative data with skewed distribution were expressed as median and interquartile range (IQR), and non-parametric rank-sum test was applied to examine the difference between groups. Qualitative data were expressed as frequency counts and percentage (%), and chi-square test was used to determine statistical difference between groups. Logistic regression was used to calculate odds ratio (OR) and 95% confidence interval (CI) to explore the gender disparity among psoriasis patients with different lifestyle habits and to investigate the association between gender and NCD comorbidity with the adjustment of potential confounding factors identified by the directed acyclic graphs (DAGs). In this study, a p-value of less than 0.05 (two-tailed) was considered as statistically significant.
Results
In this study, 2102 patients with psoriasis were enrolled, with 1332 (63.4%) patients were male. Approximately 73% of patients were over 35 years old, 82.2% of them were married, and 50% of them had an education of college and above. The proportion of mental worker and manual workers were 39.3% and 34.0%, respectively. About 62.5% of psoriasis patients were local residents, more than 59% of them had individual income over 5000 RMB per month, and over 50% of patients were overweight and obesity. Table 1 indicates that male patients had higher proportion of age over 45 years and higher proportion of married status than female patients, and the differences were statistically significant. Moreover, female patients had higher education level, higher proportion of unemployment, lower individual monthly income, and lower level of obesity and overweight, the differences were all statistically significant (P < 0.05) (Table 1).
 |
Table 1 The Demographic Feature Among Psoriasis Patients in Shanghai, China |
Gender Disparity in Lifestyle Habits Among Psoriasis Patients
Among 2102 patients with psoriasis, the prevalence of tobacco smoking, alcohol drinking and tea drinking was 31.2%, 12.6% and 23.9%, respectively. Table 2 indicates that male psoriasis patients had higher prevalence of tobacco smoking than female patients, the crude OR was 13.30 (95% CI: 9.69–18.26) and the adjusted OR was 13.26 (95% CI: 9.54–18.44). In comparison with female psoriasis patients with tobacco smoking, male patients had lower smoking initiation age, longer smoking duration and more daily cigarette smoke consumption, and differences were all statistically significant (P < 0.05) (Figure 1). For alcohol drinking, male patients were nearly 15 times more likely to be alcohol drinkers than females, even with the adjustment of potential confounders (OR = 14.44, [95% CI: 7.90–26.40]). Moreover, male patients had longer years of alcohol drinking duration (28 years for male and 8.5 years for female), and the difference was statistically significant (P<0.05) (Table 2, Figure 1).
 |
Table 2 The Lifestyle and Health Related Behaviors Among Psoriasis Patients in Shanghai, China (n = 2102) |
 |
Figure 1 Gender disparity in tobacco smoking and alcohol drinking among patients with psoriasis. Note: ▲The difference between male and female patients was statistically significant (p < 0.05). |
The prevalence of tea drinking among male patients was 4.37 times higher than that in female patients (95% CI: 3.36–5.69), even with the adjustment of potential confounders (OR = 4.24, 95% CI: 3.19–5.63). For daily physical exercise >30 minutes, 24.7% of patients chose never or occasional, 34.8% of them chose 6–7 times per week, but there was no statistical difference between male and female patients. The proportion of daily sleep time <8 hours was 81.8%, which was slightly higher in male patients (83.3%) than female patients (79.4%), but without statistical significance (Table 2).
Clinical Feature and NCD Comorbidity Among Psoriasis Patients
In the study, psoriasis vulgaris was the predominant subtype of psoriasis (95.5%). The median value for psoriasis initiation age and disease duration was 33 years old (IQR: 22.1–46.0) and 9 years (IQR: 4–18), respectively, and male patients had older age of disease initiation and longer disease duration than female patients. The recurrence of psoriasis was mainly in winter (73.4%) and autumn (34.2%). The familial aggregation of psoriasis was 8.9% in fathers, 6.0% in mothers, 9.4% in siblings, and 5.1% in offspring. Female patients reported higher familial aggregation of psoriasis than male patients both in fathers, mothers, siblings and offspring, but the difference between male and female patients was only statistically significant in offspring. The prevalence of diabetes, hypertension, hyperlipidemia, and metabolic syndrome were 13.2%, 28.5%, 23.4% and 21.5%, respectively (Table 3).
 |
Table 3 The Type, Feature of Psoriasis and NCD Comorbidity Among Psoriasis Patients in Shanghai, China |
Gender Disparity in NCD Comorbidity Among Psoriasis Patients
In this study, we incorporated diabetes, hypertension, hyperlipidemia and metabolic syndrome into the NCD comorbidity analysis. Table 4 indicates that the prevalence of diabetes, hypertension, hyperlipidemia and metabolic syndrome were associated with the gender, age, BMI, tobacco smoking and alcohol drinking among psoriasis patients. The prevalence of diabetes among male patients (14.9%) was higher than female patients (10.3%), and the OR was 1.53 (95% CI: 1.16–2.02). Logistic regression indicated that male patients had 1.87 times higher prevalence of hypertension than female patients (95% CI: 1.52–2.30), even with the adjustment of potential confounders (OR = 1.30, 95% CI: 1.00–1.68). Meanwhile, the prevalence of hyperlipidemia was 28.5% in male patients and 14.6% in female patients (OR = 2.34, 95% CI: 1.85–2.95), male patients had higher hyperlipidemia prevalence than female patients with control of potential confounding factors (OR = 1.63, 95% CI: 1.24–2.14). Male patients had 2.06 times higher prevalence of metabolic syndrome (95% CI: 1.63–2.62), even with the adjustment of confounders (OR = 1.60, 95% CI: 1.22–2.10) (Table 4).
 |
Table 4 The Gender Disparity in NCD Comorbidity Among Psoriasis Patients with the Adjustment of Potential Confounders in Shanghai, China |
Times for NCD Comorbidity Diagnosis Among Psoriasis Patients
In comparison with the psoriasis onset time, we classified psoriasis patients into Group A for those whose NCD diagnosis time was behind the onset of psoriasis and Group B for those whose NCD diagnosis time was ahead of the onset of psoriasis. In Part A of Figure 2, the proportion of patients with NCD diagnosed after psoriasis onset (Group A) was 58.84%, 60.54% and 66.60% for diabetes, hypertension and hyperlipidemia, respectively. The proportion of patients in Group A for diabetes, hypertension, and hyperlipidemia were all higher than that in Group B, both for male and female psoriasis patients. Moreover, male patients had a higher proportion of NCD diagnosed after psoriasis onset (Group A) than female patients for diabetes, hypertension and hyperlipidemia, the differences were all statistically significant (Figure 2A and B).
Discussion
This study confirmed winter as the major recurrence season and psoriasis vulgaris as the main clinical subtype. The occurrence of psoriasis was strongly associated with older age, tobacco smoking, alcohol consumption, and being overweight or obesity. This study obtained outcomes consistent with already available data.14,15 In this study, gender disparity analysis indicated that male patients had higher prevalence of tobacco smoking, alcohol drinking and tea drinking, had younger age of smoking and alcohol drinking initiation, with longer duration of tobacco smoking and alcohol drinking than female patients. Moreover, male patients had elder age of disease initiation and longer disease duration than female patients, and the prevalence of diabetes, hypertension, hyperlipidemia, and metabolic syndrome in male patients were higher than female patients.
Data in this study showed that psoriasis patients were predominantly males, which were consistent with previous research.16 Male psoriasis patients also had longer disease duration than female patients, and this might be due to the different lifestyle habits such as higher prevalence of tobacco smoking, alcohol drinking and lower proportion of physical exercise in male patients. However, female were more likely to be affected by itching, resulting in female patients being less satisfied with their treatment than male.17 This study indicated that the disease onset age among female patients was younger than male patients, which was also in line with previous literature.18 The first report on the association between psoriasis and obesity was a Swedish study which included 159,200 residents and was followed up for over 10 years, and this study demonstrated that being overweight or obesity was closely related with psoriasis onset.19 In this study, we identified that the prevalence of overweight and obesity in male patients was higher than in female patients, and this might attribute to the fact that female patients had higher proportion of body weight anxiety and were prone to engage in weight control measures. Stutts et al20 reported that males were more likely than females to underestimate their BMI and fewer males tried to lose weight than females.
Tobacco smoking and alcohol consumption were identified as important risk factors for psoriasis. In this study, the prevalence of tobacco smoking and alcohol drinking was 31.2% and 12.6%, which was in line with previous studies.12 Meanwhile, the prevalence of tobacco smoking and alcohol drinking was higher in male patients than female patients. Gazel et al21 reported that patients who smoked over 20 cigarettes per day had twice higher severity of psoriasis risk and had more severe skin damage than those who smoked less than 10 cigarettes a day. Nicotine can stimulate the production of IL-12 by dendritic cells and increase the expression of CD40 and CD86, thereby stimulating T cell activation,22 and dioxins produced by tobacco burning can bind to the aromatic hydrocarbon receptors and regulate IL-17 and IL-22 transcription,23 all of which relate closely with the onset and progress of psoriasis. Brenaut et al24 found that alcohol drinking was a risk factor for psoriasis in almost 80% studies through database analysis. A prospective study25 in the United States indicated that drinking alcohol 2 to 3 times a week increased the risk of psoriasis by 1.72 times. In this study, gender disparity in the prevalence of tobacco smoking and alcohol drinking might be due to the fact that the tobacco smoking and alcohol drinking are predominantly prevalent in males than females in the general population in China.26
It is well documented that physical exercise is associated with the preventive as well as therapeutic health-related benefits for a range of chronic diseases. Previously, we learned through a meta analysis that psoriasis patients with fewer physical exercise had more severe psoriasis lesions, and more physical exercise could reduce the recurrence of psoriasis.27 In this study, male psoriasis patients were slightly more physically active than female patients, but gender disparity in the proportion of low intensity physical exercise over 30 minutes was not statistically significant. In this study, we could not explore the association between physical exercise and psoriasis prevalence by gender, because all recruited subjects were psoriasis patients and the prevalence of psoriasis could not be calculated, so the incorporation of community population should be considered in future study.
This study demonstrated that psoriasis recurrence was mainly in winter and autumn, which was consistent with previous studies,28,29 and this was related to differences in ultraviolet radiation and vitamin D absorption in different seasons. Ultraviolet radiation can reverse the common cytokine profile by up-regulating expression of T helper (Th) 2 cytokines and down-regulating the Th1/Th17 proinflammatory pathway in psoriasis.30 Moreover, vitamin D can regulate immunity, inhibit proliferation, promote apoptosis, promote keratinocyte differentiation, and participate in maintaining the integrity of the skin barrier in psoriasis patients.31 Ultraviolet radiation is strongest in summer and weakest in winter, and Vitamin D produced by the body will decrease with the decline of ultraviolet radiation. In winter, the cold air and higher probability of upper respiratory tract infection also contributed to the recurrence of psoriasis.32
Previous studies indicate that the prevalence of psoriasis is higher among immediate family members of psoriasis patients than the general population, demonstrating that genetic factors play an important role in the pathogenesis of psoriasis.33 Studies have shown that the proportion of psoriasis patients with family history is about 30% in foreign countries and 10–20% in China.34 In this study, psoriasis patients reported that 5.1% to 9.4% of their family members were victims of psoriasis, which was in line with previous studies.34 In this study, among the 559 patients with familial aggregation of psoriasis, female patients reported higher prevalence of psoriasis among family members than male patients, especially among their offspring, suggesting that mothers with psoriasis might have a greater impact on their offspring, but reasons for this phenomenon are unclear.
In recent years, growing evidences demonstrate that metabolic diseases are the most common comorbidities of psoriasis, including obesity, dyslipidemia, diabetes, etc.35 An increasing number of studies have confirmed that the prevalence of metabolic diseases is higher in patients with psoriasis than that in normal population,35 and patients with severe psoriasis have greater risk for metabolic syndrome than those with mild psoriasis.36 In this study, the prevalence of psoriasis comorbidities ranged from 13.2% (diabetes) to 28.5% (hypertension), which were in line with previous studies.35 Moreover, male psoriasis patients had higher prevalence of diabetes, hypertension, hyperlipidemia and metabolic syndrome than female patients, which was also inconsistent with previous studies.37 Findings in this study suggest that dermatologist should consider the gender disparity in NCD comorbidities among psoriasis patients and pay more attention to the metabolic diseases for its early detection and treatment among psoriasis patients.
In particular, this study examined the temporal relationship between the onset of psoriasis and the onset of metabolic diseases including hypertension, diabetes, and dyslipidemia. Findings in this study indicated that the proportion of metabolic diseases diagnosed after the onset of psoriasis was higher than that diagnosed before the onset of psoriasis, suggesting that the onset of psoriasis was a potential risk factor for metabolic diseases. A study implemented in Taiwan showed that patients with psoriasis were more likely to develop metabolic disorders and severe vascular disease.38 Another study indicated that the initiation of psoriasis would induce serious systemic inflammation, which in turn lead to insulin resistance and result in various metabolic abnormalities such as diabetes and cardiovascular diseases,39 so we recommend dermatologist to pay attention to the diagnosis and treatment of metabolic disorders among psoriasis patients, which is helpful for their psoriasis recovery.
There are several limitations in this study. First, we recruited psoriasis patients only in two hospitals in Shanghai, and patients were over 18 years old, so results of the study had limited representation of the total number of psoriasis patients. Second, information of tobacco consumption among tobacco smokers, information of alcohol drinking and tea drinking were collected through face-to-face interview, which might lead to recall bias. Third, indicators for psoriasis severity analysis were not included in this study, so we missed the chance to evaluate the gender disparity in disease severity of psoriasis, and the incorporation of indicators for psoriasis severity and life quality including Psoriasis Area and Severity Index (PASI), Physician’s Global Assessment (PGA), Dermatology Life Quality Index (DLQI), Professional Quality of Life Scale (PRQoL) and Visual Analogue Scale (VAS) should be considered in future study. Fourth, the nature of cross-sectional study limited the calculation of incidence for metabolic diseases among psoriasis patients. All of these aforementioned limitations in this study would restrict the interpretations of clinical findings in some degree.
Conclusion
Female psoriasis patients tended to be younger, had higher education, had shorter disease duration and with less NCD problem, whereas male psoriasis patients had more body weight issue, with fewer sleep time and higher prevalence of tobacco smoking and alcohol drinking and NCDs. We recommend that dermatologist should notice the gender disparity in psoriasis patients, which is helpful for the disease diagnosis and treatment.
Abbreviations
NCD, Noncommunicable diseases; OR, odds ratio; CI, confidence interval; LI, low intensity; METs, metabolic equivalent; FBG, fasting blood glucose; SD, standardized deviation; IQR, interquartile range; DAGs, directed acyclic graphs; PASI, psoriasis area and severity index; PGA, physician’s global assessment; DLQI, dermatology life quality index; PRQoL, professional quality of life scale; VAS, visual analogue scale.
Data Sharing Statement
Data in this study can be made available upon request to the corresponding author.
Ethics Approval and Consent to Participate
The ethics approval of this study was approved by the Ethics Review Committee of Yueyang Hospital, Shanghai University of Traditional Chinese Medicine (Approval number: 2021-028). Informed consent was provided by each participant before the questionnaire interview. The study complies with the Declaration of Helsinki.
Acknowledgment
We would like to thank the doctors and nursing staff of Yueyang Hospital (affiliated with Shanghai University of Traditional Chinese Medicine), and Shanghai Skin Diseases Hospital (affiliated with Tongji University) for fieldwork assistance.
Author Contributions
All authors listed in this paper made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from Intelligence Funds of Shanghai Skin Disease Hospital (2021KYQD01), Shanghai Shenkang Hospital Development Center Management Research Program (2020SKMR-32), Shanghai Talent Development Fund (2021073), China Fund for Medical Equipment (IIT2022-01), Shanghai Shenkang second round of clinical three-year action plan project (SHDC2022CRS053) and Shanghai Sailing Program (21YF1441500). The funder had no role in study design, data collection and analysis, decision for publication, or preparation of the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Griffiths CEM, van der Walt JM, Ashcroft DM., et al. The global state of psoriasis disease epidemiology: a workshop report. Br J Dermatol. 2017;177(1):e4–e7. doi:10.1111/bjd.15610
2. Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31(2):205–212. doi:10.1111/jdv.13854
3. Kaufman BP, Alexis AF. Psoriasis in Skin of Color: insights into the Epidemiology, Clinical Presentation, Genetics, Quality-of-Life Impact, and Treatment of Psoriasis in Non-White Racial/Ethnic Groups. Am J Clin Dermatol. 2018;19(3):405–423. doi:10.1007/s40257-017-0332-7
4. Griffiths CE, Barker JN. Pathogenesis and clinical features of psoriasis. Lancet. 2007;370(9583):263–271. doi:10.1016/S0140-6736(07)61128-3
5. Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: a Review. JAMA. 2020;323(19):1945–1960. doi:10.1001/jama.2020.4006
6. Lee EB, Wu KK, Lee MP, Bhutani T, Wu JJ. Psoriasis risk factors and triggers. Cutis. 2018;102(5s):18–20.
7. Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi:10.1016/j.jaad.2016.07.064
8. Amin M, Lee EB, Tsai TF, Wu JJ. Psoriasis and Co-morbidity. Acta dermato-venereologica. Acta dermato-venereologica. 2020;100(3):adv00033. doi:10.2340/00015555-3387
9. Lippi D, Bianucci R, Donell S. Gender medicine: its historical roots. Postgrad Med J. 2020;96(1138):480–486. doi:10.1136/postgradmedj-2019-137452
10. Grego S, Pasotti E, Moccetti T, Maggioni AP. “Sex and gender medicine”: il principio della medicina di genere [Sex and gender medicine: the foundation of gender medicine]. G Ital Cardiol (Rome). 2020;21(8):602–606. Italian. doi:10.1714/3405.33894
11. Napolitano M, Mastroeni S, Fania L, et al. Sex- and gender-associated clinical and psychosocial characteristics of patients with psoriasis. Clin Exp Dermatol. 2020;45(6):705–711. doi:10.1111/ced.14218
12. Wei L, Chen S, Qiang Y, et al. Tobacco smoking was positively associated with metabolic syndrome among patients with psoriasis in Shanghai: a cross-sectional study. Tob Induc Dis. 2022;20:05. doi:10.18332/tid/144228
13. Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nature Rev Dis Primers. 2016;2:16082. doi:10.1038/nrdp.2016.82
14. Nowowiejska J, Baran A, Grabowska P, Lewoc M, Kaminski TW, Flisiak I. Assessment of life quality, stress and physical activity among patients with psoriasis. Dermatol Ther (Heidelb). 2022;12(2):395–406. doi:10.1007/s13555-021-00662-1
15. Purzycka-Bohdan D, Kisielnicka A, Zabłotna M, et al. Chronic plaque psoriasis in Poland: disease severity, prevalence of comorbidities, and quality of life. J Clin Med. 2022;11(5):1–16. doi:10.3390/jcm11051254
16. Chen L, Huang X, Xiao Y, Su J, Shen M, Chen X. Prevalence and risk factors of atopic dermatitis, psoriasis, acne, and urticaria in China. J Central South Univ Med Sci. 2020;45(4):449–455. doi:10.11817/j.issn.1672-7347.2020.190115
17. Murer C, Sgier D, Mettler SK, et al. Gender differences in psoriasis: a Swiss online psoriasis survey. Arch Dermatol Res. 2021;313(2):89–94. doi:10.1007/s00403-020-02066-1
18. Iskandar IYK, Parisi R, Griffiths CEM, Ashcroft DM. Systematic review examining changes over time and variation in the incidence and prevalence of psoriasis by age and gender. Br J Dermatol. 2021;184(2):243–258. doi:10.1111/bjd.19169
19. Lindegård B. Diseases associated with psoriasis in a general population of 159,200 middle-aged, urban, native Swedes. Dermatologica. 1986;172(6):298–304. doi:10.1159/000249365
20. Stutts LA, Blomquist KK. A longitudinal study of weight and shape concerns and disordered eating groups by gender and their relationship to self-control. Eat Weight Disord. 2021;26(1):227–237. doi:10.1007/s40519-020-00844-4
21. Gazel U, Ayan G, Solmaz D, Akar S, Aydin SZ. The impact of smoking on prevalence of psoriasis and psoriatic arthritis. Rheumatology. 2020;59(10):2695–2710. doi:10.1093/rheumatology/keaa179
22. Naldi L. Psoriasis and smoking: links and risks. Psoriasis. 2016;6:65–71.
23. Armstrong AW, Armstrong EJ, Fuller EN, Sockolov ME, Voyles SV. Smoking and pathogenesis of psoriasis: a review of oxidative, inflammatory and genetic mechanisms. Br J Dermatol. 2011;165(6):1162–1168. doi:10.1111/j.1365-2133.2011.10526.x
24. Brenaut E, Horreau C, Pouplard C, et al. Alcohol consumption and psoriasis: a systematic literature review. J Eur Acad Dermatol Venereol. 2013;27(Suppl 3):30–35. doi:10.1111/jdv.12164
25. Qureshi AA, Dominguez PL, Choi HK, Han J, Curhan G. Alcohol intake and risk of incident psoriasis in US women: a prospective study. Arch Dermatol. 2010;146(12):1364–1369. doi:10.1001/archdermatol.2010.204
26. Zheng GY, Wei SC, Shi TL, Li YX. Association between alcohol, smoking and HLA-DQA1*0201 genotype in psoriasis. Acta Biochim Biophys Sin (Shanghai). 2004;36(9):597–602. doi:10.1093/abbs/36.9.597
27. Zheng Q, Sun XY, Miao X, et al. Association between physical activity and risk of prevalent psoriasis: a MOOSE-compliant meta-analysis. Medicine. 2018;97(27):e11394. doi:10.1097/MD.0000000000011394
28. Ferguson FJ, Lada G, Hunter HJA, et al. Diurnal and seasonal variation in psoriasis symptoms. J Eur Acad Dermatol Venereol. 2021;35(1):e45–e7. doi:10.1111/jdv.16791
29. Wu Q, Xu Z, Dan YL, et al. Seasonality and global public interest in psoriasis: an infodemiology study. Postgrad Med J. 2020;96(1133):139–143. doi:10.1136/postgradmedj-2019-136766
30. Kim E, Lee G, Fischer G. Use of narrowband ultraviolet B (NBUVB) in paediatric psoriasis: a systematic literature review and meta-analysis. Australas J Dermatol. 2021;62(2):124–129. doi:10.1111/ajd.13471
31. Stanescu AMA, Simionescu AA, Diaconu CC. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients. 2021;13(1):163. doi:10.3390/nu13010163
32. Rademaker M, Agnew K, Anagnostou N, et al. Psoriasis and infection. A clinical practice narrative. Australasian j Dermatol. 2019;60(2):91–98. doi:10.1111/ajd.12895
33. Huang YH, Kuo CF, Huang LH, Hsieh MY. Familial Aggregation of Psoriasis and Co-Aggregation of Autoimmune Diseases in Affected Families. J Clin Med. 2019;8(1):115. doi:10.3390/jcm8010115
34. Dand N, Mahil SK, Capon F, Smith CH, Simpson MA, Barker JN. Psoriasis and Genetics. Acta dermato-venereologica. Acta dermato-venereologica. 2020;100(3):adv00030. doi:10.2340/00015555-3384
35. Gisondi P, Fostini AC, Fossà I, Girolomoni G, Targher G. Psoriasis and the metabolic syndrome. Clin Dermatol. 2018;36(1):21–28. doi:10.1016/j.clindermatol.2017.09.005
36. Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and metabolic syndrome: a systematic review and meta-analysis of observational studies. J Am Acad Dermatol. 2013;68(4):654–662. doi:10.1016/j.jaad.2012.08.015
37. Salihbegovic EM, Kurtalic S, Omerkic E. Comorbidity in Men with Psoriasis. Med Arch. 2021;75(1):31–34. doi:10.5455/medarh.2021.75.31-34
38. Su YS, Yu HS, Li WC, et al. Psoriasis as initiator or amplifier of the systemic inflammatory march: impact on development of severe vascular events and implications for treatment strategy. J Eur Acad Dermatol Venereol. 2013;27(7):876–883. doi:10.1111/j.1468-3083.2012.04599.x
39. Boehncke WH, Boehncke S, Tobin AM, Kirby B. The ‘psoriatic march’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 2011;20(4):303–307. doi:10.1111/j.1600-0625.2011.01261.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

