Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 15
Clinical Factors Associated with Serum Magnesium Concentration in Patients Undergoing Peritoneal Dialysis: A Single-Center Observational Study
Authors Kaneko S , Ookawara S, Morishita Y
Received 5 January 2022
Accepted for publication 19 May 2022
Published 24 May 2022 Volume 2022:15 Pages 185—195
DOI https://doi.org/10.2147/IJNRD.S357130
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 6
Editor who approved publication: Professor Pravin Singhal
Shohei Kaneko, Susumu Ookawara, Yoshiyuki Morishita
Department of Nephrology, Jichi Medical University Saitama Medical Center, Saitama, Japan
Correspondence: Yoshiyuki Morishita, Department of Nephrology, Jichi Medical University Saitama Medical Center, Saitama, Japan, Tel +81-48-647-2111, Fax +81-48-647-6831, Email [email protected]
Purpose: Magnesium (Mg) is an essential element that is associated with various physiological functions, such as maintenance of blood pressure, muscle contraction, and nerve function. In patients undergoing hemodialysis, hypomagnesemia is associated with cardiovascular and all-cause mortality. However, in patients undergoing peritoneal dialysis (PD), clinical factors associated with Mg have not been fully investigated.
Patients and Methods: Clinical factors including anthropometric data, comorbidities, laboratory data, medications, and dialysis methods were collected from the medical records of patients undergoing PD. The associations of these factors with the serum Mg concentration were investigated by univariate and multivariate analyses.
Results: Sixty patients undergoing PD were investigated. The univariate analysis showed that the serum Mg concentration was significantly associated with treatment by hybrid PD (daily PD + once-weekly hemodialysis) (β = 0.264, P = 0.04), administration of phosphate binders (β = 0.294, P = 0.02), the serum C-reactive protein concentration (β = − 0.318, P = 0.01), the serum potassium (K) concentration (β = 0.451, P < 0.01), and the serum intact parathormone concentration (β = − 0.333, P = 0.01). The multivariate analysis using these factors showed an independent association between the serum Mg and K concentrations (β = 0.333, P = 0.01).
Conclusion: The serum Mg concentration was independently associated with the serum K concentration in patients undergoing PD.
Keywords: peritoneal dialysis, magnesium, phosphate binder, potassium, intact parathormone
Introduction
Magnesium (Mg) is an essential element in the human body that acts as a coenzyme in more than 300 types of enzymatic reactions.1–3 Physiologically, Mg plays a critical role in maintenance of blood pressure, muscle contraction, nerve function, and bone structure.1–3 The human body contains 21 to 28 g of Mg. Approximately half of the total amount of Mg is contained in bones and teeth, and the remainder is present in muscles or non-muscular soft tissue such as nerves and brain.2 Blood is considered to contain <1% of the total Mg in the human body.2 Mg is supplied by daily intake of food such as meat, fish, nuts, and green vegetables.2,4 The kidneys maintain an appropriate amount of Mg in the body by excretion of excess Mg in urine.2
Hypomagnesemia is a frequent problem in the clinical setting.5,6 Hypomagnesemia is caused by malabsorption, gastrointestinal disorders, alcohol abuse, renal tubular disorders (Gitelman syndrome), and some medications (proton pump inhibitors, diuretics, amphotericin B, aminoglycosides, tetracyclines, and chemotherapy drugs).5–7 Additionally, hypomagnesemia is associated with various pathological conditions including seizures, arrhythmias, hypertension, vascular calcification, cardiovascular disease, insulin resistance, asthma, attention deficit hyperactivity disorder, Alzheimer’s disease, migraine, hypokalemia, hypocalcemia, and osteoporosis.5,6,8 In particular, arrhythmias resulting from hypomagnesemia can lead to fatal ventricular arrhythmias.9,10 Foglia et al10 investigated patients with Gitelman syndrome (hypomagnesemia and hypokalemia) and found that these electrolyte abnormalities prolong the duration of myocardial action potentials, leading to QT prolongation on the electrocardiogram. Moreover, such abnormalities of ventricular repolarization seem to originate from these abnormal electrolyte concentration gradients in the myocardial cell membrane.10 Among patients who require intensive care, 60% may develop hypomagnesemia.11 Therefore, appropriate monitoring of the serum Mg concentration is necessary to avoid the development of hypomagnesemia in the clinical setting.
A greater understanding of Mg abnormalities in patients with chronic kidney disease (CKD) is required.4,12 Clinicians have long recognized the need to be aware of the development of hypermagnesemia due to impaired urinary excretion of Mg in patients with CKD.13 In particular, Mg oxide as a laxative increases the risk of hypermagnesemia in patients with CKD, regardless of its low bioavailability.14 Hypermagnesemia causes various conditions including muscle weakness, hypotension, bradycardia, disturbance of consciousness, hypocalcemia, hyperkalemia, absence of the deep tendon reflex, and respiratory distress.15–17 However, even in patients with CKD, Mg is not an element that should simply be ignored; its concentration should be controlled within an appropriate range.4 In the clinical setting, we also see hypomagnesemia especially in patients with stage 5 CKD or end-stage kidney disease who pay particular attention to their diet.4 In patients with stage 5 CKD or end-stage kidney disease, intake of raw dark green vegetables and seafoods is restricted because these foods contain high concentrations of potassium (K) and phosphorus.4 However, these foods are also high in Mg.4 Therefore, this restriction of Mg-containing foods is considered to contribute to the development of hypomagnesemia.4 In patients undergoing hemodialysis (HD), hypomagnesemia is reportedly associated with cardiovascular mortality, all-cause mortality, bone fracture, erythropoietin resistance, vascular stiffness, vascular calcification, atherosclerosis, and poor muscle performance.18–21 Additionally, recent studies have unexpectedly suggested that relatively high serum Mg concentrations may be beneficial for reducing cardiovascular risk, preventing vascular calcification, and treating hypertension in patients with CKD.4,12,22 Even in patients undergoing peritoneal dialysis (PD), the importance of Mg is gradually becoming apparent.23–26 However, compared with HD, many factors are still unclear. In particular, only a few studies have targeted Asian patients undergoing PD.23–26 Clarifying the significance of Mg in patients undergoing PD, as has been done in patients undergoing HD, would contribute to a more favorable prognosis and improved quality of life. Therefore, we conducted this study to investigate the significance of Mg in patients undergoing PD. Specifically, we retrospectively investigated the association between the serum Mg concentration and clinical factors in patients undergoing PD.
Materials and Methods
Study Design
In this single-center, retrospective observational study, we analyzed the clinical factors associated with the serum Mg concentration in patients undergoing PD. This study was approved by the Saitama Medical Center, Jichi Medical University Ethics Committee (DAI-RIN 15–34) and was conducted in accordance with the Declaration of Helsinki. In accordance with the national legislation and the institutional requirements, an opt-out procedure replaced the need for provision of written consent.
Study Population and Setting
This study was conducted at Saitama Medical Center, Jichi Medical University from April 2018 to March 2019. The inclusion criteria were (i) patients undergoing PD, (ii) patients whose serum Mg concentration was measured for clinical purposes, and (iii) age of >18 years. The exclusion criteria were (i) patients with acute kidney injury, (ii) patients who underwent renal transplantation, and (iii) patients who refused to participate in the study. All patients undergoing PD were treated in accordance with the 2009 Japanese Society for Dialysis Therapy Guideline for PD and 2015 Japanese Society for Dialysis Therapy Guideline for Renal Anemia in Chronic Kidney Disease.27,28 The HD dialysate Mg concentration was 1.0 mEq/L (1.2 mg/dL). Phosphate binders that were approved in Japan at the time of the study were calcium carbonate, sevelamer hydrochloride, lanthanum carbonate, bixalomer, ferric citrate, and sucroferric oxyhydroxide. No patients were prescribed an Mg-containing phosphate binder because such phosphate binders were not approved by public health insurance in Japan at the time of the study.
Collection of Clinical Information
The following clinical information was collected from the patients’ digital medical records: age, sex, body mass index, comorbidities (diabetes mellitus, hepatic diseases, and cardiac diseases), history of parathyroidectomy, PD period, hybrid HD (daily PD + once-weekly HD), medications (phosphate binders, calcium-sensing receptor activators, Mg oxide, diuretics, proton pump inhibitors, and vitamin D), PD methods, PD dialysate Mg concentration, dialysis efficacy (weekly Kt/V urea, peritoneal Kt/V urea, and renal Kt/V urea), 4-h dialysate/plasma creatinine ratio, and blood test results. PD methods were categorized as continuous ambulatory PD or automated PD. The former comprise methods in which patients manually change the dialysate, whereas in the latter, the dialysate is changed automatically by an automated cycler.29–32 Peritoneal weekly Kt/V urea was used as an indicator of the efficacy of PD, and renal weekly Kt/V was used as an indicator of residual renal function.33 Their sum was defined as total weekly Kt/V urea.33 The 4-h dialysate/plasma creatinine ratio is an indicator of the permeability of peritoneal membranes.34 Hybrid PD is a treatment modality that combines daily PD with once-weekly HD and is approved by the Japanese public medical insurance.35,36 This treatment modality is applied to patients whose fluid overload and urea toxin accumulation cannot be adequately treated by PD alone.35,36 The serum calcium concentration was corrected based on Payne’s formula: corrected calcium = serum calcium concentration + (4 − serum albumin concentration).37 Blood tests were performed for clinical purposes by the Department of Clinical Laboratory, Saitama Medical Center, Jichi Medical University.
Measurement of Serum Mg Concentration
The serum Mg concentration was measured for clinical purposes using the enzymatic method as previously described.38 The serum Mg concentration in this study represented the total serum Mg concentration; the Mg ion concentration was not measured.
Definition of Abnormal Serum Mg Concentration
Based on previous reports, a normal serum Mg concentration was defined as 1.70 to 2.43 mg/dL.2 Extreme hypomagnesemia was defined as a serum Mg concentration of <0.75 mg/dL and extreme hypermagnesemia as >13.50 mg/dL.39,40
Statistical Analysis
Quantitative variables were tested by the Shapiro–Wilk test to examine the normality of their distribution. Quantitative variables with a normal distribution are presented as mean ± standard deviation, and quantitative variables without a normal distribution are presented as median [interquartile range]. Categorical variables are presented as frequency and percentage. Univariate and multivariate analyses were performed to determine the clinical factors associated with the serum Mg concentration. The explanatory variables for the multivariate analysis of this study were set as factors that showed significant differences in the univariate analysis. Statistical analyses were performed using JMP 14 (SAS Institute Inc., Cary, NC, USA). A P value of <0.05 was considered statistically significant. In the univariate analysis, which was the main analysis in this study, the statistical power was 0.839 (n = 60).
Results
Patients’ Clinical Characteristics
Table 1 shows the clinical characteristics of the 60 patients who met the inclusion criteria for this study. Their median age was 62.5 [51.5–72.5] years, and 44 (73%) were male. Their median body mass index was 23.2 [20.8–25.3] kg/m2. The median PD period was 24 [12–45] months. The median total Kt/V urea, peritoneal Kt/V urea, and renal Kt/V urea were 1.6 [1.4–1.8], 1.1 [0.8–1.4], and 0.4 [0.2–0.8], respectively. The median serum Mg concentration was 2.1 [1.8–2.5] mg/dL. In this cohort, 7 of 60 (12%) patients were diagnosed with hypomagnesemia, and 16 of 60 (27%) patients were diagnosed with hypermagnesemia. No patients had extreme hypomagnesemia or extreme hypermagnesemia in our study cohort. Additionally, no patients had severe gastrointestinal disease or Gitelman syndrome. No patients were receiving amphotericin B, aminoglycosides, tetracyclines, or chemotherapy drugs.
 |
Table 1 Clinical Characteristics of the Patients (n = 60) |
Clinical Factors Associated with Serum Mg Concentration
Table 2 shows the associations between the serum Mg concentration and other clinical factors. In the univariate analysis, the serum Mg concentration was significantly associated with treatment by hybrid PD (β = 0.264, P = 0.04), administration of phosphate binders (β = 0.294, P = 0.02), the serum C-reactive protein concentration (β = −0.318, P = 0.01), the serum K concentration (β = 0.451, P < 0.01) and the serum intact parathormone concentration (β = −0.333, P = 0.01). The multivariate analysis using these factors as explanatory variables showed that the serum K concentration (β = 0.333, P = 0.01) was significantly and independently associated with the serum Mg concentration. Figure 1 shows a scatter plot depicting the significant correlation between the serum Mg concentration and serum K concentration. The PD dialysate Mg concentration did not affect the serum Mg concentration.
 |
Table 2 Clinical Factors Associated with Serum Mg Concentration (n = 60) |
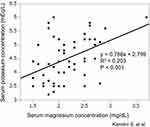 |
Figure 1 Significant correlation between serum magnesium concentration and serum potassium concentration in patients undergoing peritoneal dialysis. |
Discussion
In this study, the multivariate analysis showed that the serum K concentration was independently associated with the serum Mg concentration. Although this study was small and observational, this finding is important because few studies have investigated the importance of Mg in patients undergoing PD.23–26 Clinically, the serum Mg concentration may not be routinely measured in all patients. However, appropriate monitoring of the serum Mg concentration is important because the serum K concentration is associated with the serum Mg concentration in patients undergoing PD.
Many reports have highlighted the positive relationship between Mg and K in the general population.41,42 Hypokalemia and hypomagnesemia can occur simultaneously from common causes: adverse effects of diuretics, chronic diarrhea, drugs that induce nephrotoxic tubular lesions (aminoglycosides and cisplatin), alcohol abuse, and dietary restrictions due to CKD.41,42 Additionally, Gitelman syndrome is the most common primary renal tubular disorder known to be associated with hypomagnesemia and hypokalemia.43 Mg can influence urinary excretion of K through inhibition of the renal outer medullary K (ROMK) channel, which is located in the renal tubules.41 In the ROMK channel, intracellular and extracellular K ion migration is controlled by the K ion concentration gradient and membrane potential.41 Under physiological conditions, K ions basically move outward through the ROMK channel.41 This function of the ROMK channel is strongly modulated by the intracellular Mg concentration. Intracellular Mg binds to the ROMK channel and inhibits the outward movement of K.41 Conversely, hypomagnesemia promotes K excretion.41 Although this relationship may be affected by renal function, to the best of our knowledge no studies have been performed to investigate the relationship. How renal function affects the relationship between ROMK channels and Mg would be an interesting topic for future research. Hyperkalemia and hypermagnesemia may occur simultaneously in patients with CKD.15–17 However, Surprisingly, there are few reports on how hypermagnesemia affects hyperkalemia in contrast to the large number of reports on the relationship between hypokalemia and hypomagnesemia.44 Hudali and Takkar44 reported unexplained hyperkalemia in patients after intravenous Mg supplementation for the treatment of preeclampsia. They hypothesized that Mg supplementation suppresses aldosterone production by translocating calcium intracellularly or that K excretion is suppressed through excessive suppression of the ROMK channel by an increased serum Mg concentration.44
Patients undergoing PD are clearly different from the general population in terms of K metabolism because of the effects of renal dysfunction, the PD dialysate, their restricted diet, and their abnormal acid–base balance.27 However, our study revealed that as in the general population, Mg and K are correlated even in patients undergoing PD. Appropriate control of the serum K concentration is important in patients undergoing PD because persistent hypokalemia is a risk factor for all-cause mortality and development of PD-related peritonitis.45 We believe that clinicians should measure the serum Mg concentration when patients undergoing PD develop unexplained serum K abnormalities. Furthermore, correction of the serum Mg concentration may contribute to better control of the serum K concentration. One way to accomplish this would be to optimize the PD dialysate Mg concentration. Some studies have shown a correlation between the PD dialysate Mg concentration and serum Mg concentration, although this correlation was denied in our study.46 This is an interesting topic for future intervention studies.
Our univariate analysis showed that the serum C-reactive protein concentration, the serum intact parathormone concentration, performance of hybrid PD, and administration of phosphate binders were associated with the serum Mg concentration, although an independent relationship was not shown in the multivariate analysis. These results have several implications. Research has shown that the serum Mg and C-reactive protein concentrations are inversely correlated.47–49 Additionally, Mg supplementation for hypomagnesemia decreases the serum C-reactive protein concentration.47–49 These findings suggest that hypomagnesemia may be related to chronic inflammation and various chronic diseases.47–49 In our study, the serum Mg and C-reactive protein concentrations were inversely correlated in patients undergoing PD, consistent with previous studies.
It is widely reported that parathormone has complex dependence on Mg.39,50 Mg has an affinity for calcium-sensing receptors in the parathyroid glands, and this could explain the inverse correlation between the serum Mg and intact parathormone concentrations in our study.39,50 However, severe and chronic hypomagnesemia (serum Mg concentration of <0.75 mg/dL) inhibits secretion of parathormone, which is usually stimulated by hypocalcemia.39 This may occur because of inhibition of cyclic AMP production in the parathyroid gland by hypomagnesemia.39 In such cases, Mg supplementation can result in rapid recovery of parathormone secretion.39 The reason for the observed linear inverse correlation between the serum Mg and intact parathormone concentrations in our study may be that none of the patients had severe and chronic hypomagnesemia.
Unexpected results were obtained regarding the relationship between Mg and hybrid PD. In this study, the HD dialysate Mg concentration was 1.0 mEq/dL (1.2 mg/dL) in all patients. This concentration was lower than the serum Mg concentration (2.1 mg/dL). Mg removal by hybrid PD was predicted to decrease the serum Mg concentration, but the univariate analysis unexpectedly showed high serum Mg concentrations in patients undergoing hybrid PD. Typically, hybrid PD is indicated for patients with impaired residual renal function and those who have difficulty with dietary restrictions.35,36 Therefore, background factors may have facilitated the accumulation of Mg.
To our knowledge, no reports have focused on the relationship between phosphate binders and the serum Mg concentration in patients undergoing PD. In patients undergoing HD, Japanese researchers have reported that oral administration of calcium carbonate is significantly correlated with high serum Mg concentrations.51 Although that study was observational and did not demonstrate a causal relationship, the authors explained that Mg as a drug additive may have an effect on the correlation.51 The relationship between phosphate binders and Mg is an interesting topic for future research.
This study had several important limitations. First, we did not investigate causality because this was a cross-sectional and observational study. Second, we could not investigate the effect of Mg on all-cause mortality or the utility of Mg supplementation. Third, whether the total amount of Mg in the body can be evaluated by only measuring the serum Mg concentration remains unclear. Fourth, we did not measure the serum Mg ion concentration because Japanese public insurance did not cover such measurement at the time of the study. Fifth, because of the small sample size of this study, the effects of bias and error cannot be ignored. Therefore, future large-scale interventional research is needed to clarify the importance of the serum Mg concentration and the effect of Mg supplementation in patients undergoing PD.
Conclusions
In this study, the serum Mg concentration was independently associated with the serum K concentration in patients undergoing PD. Mg may have diverse significance not only in patients undergoing HD but also in patients undergoing PD. Future large-scale interventional studies are needed to clarify the importance of Mg in patients undergoing PD.
Abbreviations
Mg, magnesium; K, potassium; CKD, chronic kidney disease; HD, hemodialysis; PD, peritoneal dialysis; ROMK channel, renal outer medullary potassium (K) channel.
Institutional Review Board Statement
This study was approved by the ethics committee of Saitama Medical Center, Jichi Medical University (DAI-RIN 15-34) and was conducted in accordance with the Declaration of Helsinki. Patients were notified of their option to opt out of the study.
Data Sharing Statement
The datasets in this study are available from the corresponding author on reasonable request.
Consent for Publication
All authors give consent for publication.
Acknowledgments
We thank the medical staff of the dialysis department of Saitama Medical Center, Jichi Medical University for assisting with this study. We also thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Author Contributions
All authors contributed to the study conception, design, and execution; data acquisition, analysis, and interpretation; and writing and editing of the manuscript. All authors have agreed with submission to the International Journal of Nephrology and Renovascular Disease and agreed on all versions of the article (before submission, during revision, and the final version accepted for publication). All authors have agreed to take responsibility and be held accountable for the contents of the article.
Funding
This research received no external funding.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Gröber U, Schmidt J, Kisters K. Magnesium in prevention and therapy. Nutrients. 2015;7(9):8199–8226. doi:10.3390/nu7095388
2. Pickering G, Mazur A, Trousselard M, et al. Magnesium status and stress: the vicious circle concept revisited. Nutrients. 2020;12:12. doi:10.3390/nu12123672
3. Volpe SL. Magnesium in disease prevention and overall health. Adv Nutr. 2013;4(3):378s–83s. doi:10.3945/an.112.003483
4. Sakaguchi Y. The emerging role of magnesium in CKD. Clin Exp Nephrol. 2022;26(5):379–384. doi:10.1007/s10157-022-02182-4
5. Hansen BA, Bruserud Ø. Hypomagnesemia in critically ill patients. J Intensive Care. 2018;6:21. doi:10.1186/s40560-018-0291-y
6. Pham PC, Pham PA, Pham SV, Pham PT, Pham PM, Pham PT. Hypomagnesemia: a clinical perspective. Int J Nephrol Renovasc Dis. 2014;7:219–230. doi:10.2147/IJNRD.S42054
7. Gröber U. Magnesium and drugs. Int J Mol Sci. 2019;20:9. doi:10.3390/ijms20092094
8. Blaszczyk U, Duda-Chodak A. Magnesium: its role in nutrition and carcinogenesis. Rocz Panstw Zakl Hig. 2013;64(3):165–171.
9. Mozos I. Laboratory markers of ventricular arrhythmia risk in renal failure. Biomed Res Int. 2014;2014:509204. doi:10.1155/2014/509204
10. Foglia PE, Bettinelli A, Tosetto C, et al. Cardiac work up in primary renal hypokalaemia-hypomagnesaemia (Gitelman syndrome). Nephrol Dial Transplant. 2004;19(6):1398–1402. doi:10.1093/ndt/gfh204
11. Wong ET, Rude RK, Singer FR, Shaw ST
12. Floege J. Magnesium in CKD: more than a calcification inhibitor? J Nephrol. 2015;28(3):269–277. doi:10.1007/s40620-014-0140-6
13. Wyskida K, Witkowicz J, Chudek J, Więcek A. Daily magnesium intake and hypermagnesemia in hemodialysis patients with chronic kidney disease. J Ren Nutr. 2012;22(1):19–26. doi:10.1053/j.jrn.2011.03.001
14. Mori H, Suzuki H, Hirai Y, et al. Clinical features of hypermagnesemia in patients with functional constipation taking daily magnesium oxide. J Clin Biochem Nutr. 2019;65(1):76–81. doi:10.3164/jcbn.18-117
15. van de Wal-Visscher ER, Kooman JP, van der Sande FM. Magnesium in chronic kidney disease: should we care? Blood Purif. 2018;45(1–3):173–178. doi:10.1159/000485212
16. Bokhari SR, Siriki R, Teran FJ, Batuman V. Fatal hypermagnesemia due to laxative use. Am J Med Sci. 2018;355(4):390–395. doi:10.1016/j.amjms.2017.08.013
17. Van Laecke S. Hypomagnesemia and hypermagnesemia. Acta Clin Belg. 2019;74(1):41–47. doi:10.1080/17843286.2018.1516173
18. Sakaguchi Y, Fujii N, Shoji T, Hayashi T, Rakugi H, Isaka Y. Hypomagnesemia is a significant predictor of cardiovascular and non-cardiovascular mortality in patients undergoing hemodialysis. Kidney Int. 2014;85(1):174–181. doi:10.1038/ki.2013.327
19. Yu L, Song J, Lu X, Zu Y, Li H, Wang S. Association between serum magnesium and erythropoietin responsiveness in hemodialysis patients: a cross-sectional study. Kidney Blood Press Res. 2019;44(3):354–361. doi:10.1159/000500921
20. Hori M, Yasuda K, Takahashi H, Yamazaki C, Morozumi K, Maruyama S. Impact of serum magnesium and bone mineral density on systemic fractures in chronic hemodialysis patients. PLoS One. 2021;16(5):e0251912. doi:10.1371/journal.pone.0251912
21. Lu C, Wang Y, Wang D, et al. Hypomagnesemia and short-term mortality in elderly maintenance hemodialysis patients. Kidney Dis. 2020;6(2):109–118. doi:10.1159/000504601
22. de Francisco AL M, Rodríguez M. Magnesium - its role in CKD. Nefrologia. 2013;33(3):389–399. doi:10.3265/Nefrologia.pre2013.Feb.11840
23. Cho MS, Lee KS, Lee YK, et al. Relationship between the serum parathyroid hormone and magnesium levels in continuous ambulatory peritoneal dialysis (CAPD) patients using low-magnesium peritoneal dialysate. Korean J Intern Med. 2002;17(2):114–121. doi:10.3904/kjim.2002.17.2.114
24. Ye H, Zhang X, Guo Q, et al. Prevalence and factors associated with hypomagnesemia in Southern Chinese continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 2013;33(4):450–454. doi:10.3747/pdi.2012.00164
25. Takahashi S, Okada K, Yanai M. Magnesium and parathyroid hormone changes to magnesium-free dialysate in continuous ambulatory peritoneal dialysis patients. Perit Dial Int. 1994;14(1):75–78. doi:10.1177/089686089401400115
26. Tsai S, Zhao H, Wu B, Zuo L, Wang M. Serum magnesium abnormality and influencing factors of serum magnesium level in peritoneal dialysis patients: a single-center study in northern China. Blood Purif. 2018;45(1–3):110–117. doi:10.1159/000485315
27. Working Group Committee for Preparation of Guidelines for Peritoneal Dialysis JSfDT, Japanese Society for Dialysis Therapy. 2009 Japanese society for dialysis therapy guidelines for peritoneal dialysis. Ther Apher Dial. 2010;14(6):489–504. doi:10.1111/j.1744-9987.2010.00901.x
28. Yamamoto H, Nishi S, Tomo T, et al. 2015 Japanese society for dialysis therapy: guidelines for renal anemia in chronic kidney disease. Ren Replace Ther. 2017;3(1):36.
29. van Hoeck KJM, Rusthoven E, Vermeylen L, et al. Nutritional effects of increasing dialysis dose by adding an icodextrin daytime dwell to Nocturnal Intermittent Peritoneal Dialysis (NIPD) in children. Nephrol Dial Transplant. 2003;18(7):1383–1387. doi:10.1093/ndt/gfg120
30. Badve SV, Zimmerman DL, Knoll GA, Burns KD, McCormick BB. Peritoneal phosphate clearance is influenced by peritoneal dialysis modality, independent of peritoneal transport characteristics. Clin J Am Soc Nephrol. 2008;3(6):1711–1717. doi:10.2215/CJN.00190108
31. Diaz-Buxo JA, Walker PJ, Chandler JT, Burgess WP, Farmer CD. Experience with intermittent peritoneal dialysis and continuous cyclic peritoneal dialysis. Am J Kidney Dis. 1984;4(3):242–248. doi:10.1016/S0272-6386(84)80099-2
32. Venkataraman V, Nolph KD. Utilization of PD modalities: evolution. Semin Dial. 2002;15(6):380–384. doi:10.1046/j.1525-139X.2002.00095.x
33. Lo W-K, Bargman JM, Burkart J, et al. Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit Dial Int. 2006;26(5):520–522. doi:10.1177/089686080602600502
34. Teitelbaum I, Burkart J. Peritoneal dialysis. Am J Kidney Dis. 2003;42(5):1082–1096. doi:10.1016/j.ajkd.2003.08.036
35. Masakane I, Tsubakihara Y, Akiba T, Watanabe Y, Iseki K. The most recent trends of peritoneal dialysis in Japan. Perit Dial Int. 2008;28(Suppl 3):S27–S31. doi:10.1177/089686080802803s06
36. Kawanishi H, Marshall MR, Zhao J, et al. Mortality, hospitalization and transfer to haemodialysis and hybrid therapy, in Japanese peritoneal dialysis patients. Perit Dial Int. 2021;42(3):305–313. doi:10.1177/08968608211016127
37. Ohbal T, Shiraishi T, Kabaya T, Watanabe S. [Evaluation of Payne’s formula for the correction of calcium: comparison with improved calcium and albumin measurement methods]. Rinsho Byori. 2014;62(2):133–138. Japanese.
38. Tabata M, Totani M, Murachi T. A chemiluminometric method for NADPH and NADH using a two-enzyme bioreactor and its application to the determination of magnesium in serum. Biomed Chromatogr. 1990;4(3):123–127. doi:10.1002/bmc.1130040310
39. Rude RK, Oldham SB, Singer FR. Functional hypoparathyroidism and parathyroid hormone end-organ resistance in human magnesium deficiency. Clin Endocrinol. 1976;5(3):209–224. doi:10.1111/j.1365-2265.1976.tb01947.x
40. Ishida Y, Tabuchi A. Severe hypermagnesemia with normal renal function can improve with symptomatic treatment. Case Rep Emerg Med. 2020;2020:2918249. doi:10.1155/2020/2918249
41. Huang CL, Kuo E. Mechanism of hypokalemia in magnesium deficiency. J Am Soc Nephrol. 2007;18(10):2649–2652. doi:10.1681/ASN.2007070792
42. Fulchiero R, Seo-Mayer P. Bartter syndrome and Gitelman syndrome. Pediatr Clin North Am. 2019;66(1):121–134. doi:10.1016/j.pcl.2018.08.010
43. Gug C, Mihaescu A, Mozos I. Two mutations in the thiazide-sensitive NaCl co-transporter gene in a Romanian Gitelman syndrome patient: case report. Ther Clin Risk Manag. 2018;14:149–155. doi:10.2147/TCRM.S150483
44. Hudali T, Takkar C. Hypocalcemia and hyperkalemia during magnesium infusion therapy in a pre-eclamptic patient. Clin Case Rep. 2015;3(10):827–831. doi:10.1002/ccr3.356
45. Davies SJ, Zhao J, Morgenstern H, et al. Low serum potassium levels and clinical outcomes in peritoneal dialysis-international results from PDOPPS. Kidney Int Rep. 2021;6(2):313–324. doi:10.1016/j.ekir.2020.11.021
46. Katopodis KP, Koliousi EL, Andrikos EK, Pappas MV, Elisaf MS, Siamopoulos KC. Magnesium homeostasis in patients undergoing continuous ambulatory peritoneal dialysis: role of the dialysate magnesium concentration. Artif Organs. 2003;27(9):853–857. doi:10.1046/j.1525-1594.2003.07193.x
47. Simental-Mendia LE, Sahebkar A, Rodriguez-Moran M, Zambrano-Galvan G, Guerrero-Romero F. Effect of magnesium supplementation on plasma c-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Curr Pharm Des. 2017;23(31):4678–4686. doi:10.2174/1381612823666170525153605
48. Nielsen FH. Dietary magnesium and chronic disease. Adv Chronic Kidney Dis. 2018;25(3):230–235. doi:10.1053/j.ackd.2017.11.005
49. Hamedifard Z, Farrokhian A, Reiner Ž, et al. The effects of combined magnesium and zinc supplementation on metabolic status in patients with type 2 diabetes mellitus and coronary heart disease. Lipids Health Dis. 2020;19(1):112. doi:10.1186/s12944-020-01298-4
50. Chase LR, Slatopolsky E, Krinski T. Secretion and metabolic efficacy of parathyroid hormone in patients with severe hypomagnesemia. J Clin Endocrinol Metab. 1974;38(3):363–371. doi:10.1210/jcem-38-3-363
51. Nagano N, Ito K, Honda M. The magnesium included as a pharmaceutical excipient in phosphate binders might affect the serum magnesium levels of dialysis patients. J Jpn Soc Dial Ther. 2016;49(9):571–580. doi:10.4009/jsdt.49.571
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
