Back to Journals » Clinical Ophthalmology » Volume 13
Clinical Courses Of Corneal Endothelial Dysfunction Due To Gomphocarpus physocarpus Milky Latex-Induced Injury: A Case Series
Authors Ono T , Kinoshita K, Iwasaki T , Mori Y, Nejima R, Nakamura Y, Amano S, Aihara M , Miyata K
Received 5 September 2019
Accepted for publication 1 November 2019
Published 22 November 2019 Volume 2019:13 Pages 2293—2299
DOI https://doi.org/10.2147/OPTH.S230009
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser

Takashi Ono,1,2 Katsuhito Kinoshita,1 Takuya Iwasaki,1 Yosai Mori,1 Ryohei Nejima,1 Yasuko Nakamura,3 Shiro Amano,4 Makoto Aihara,2 Kazunori Miyata1
1Miyata Eye Hospital, Miyakonojo, Miyazaki, Japan; 2Department of Ophthalmology, The University of Tokyo, Tokyo, Japan; 3Kagoshima Miyata Eye Clinic, Kagoshima, Japan; 4Inouye Eye Hospital, Tokyo, Japan
Correspondence: Takashi Ono
Miyata Eye Hospital, 6-3, Kuraharacho, Miyakonojo, Miyazaki 885-0051, Japan
Tel +81-986-22-1441
Fax +81-986-24-2174
Email [email protected]
Purpose: To investigate the clinical courses of patients with corneal endothelial dysfunction due to Gomphocarpus physocarpus milky latex-induced injury.
Patients and methods: In this retrospective case series, we included consecutive patients who visited Miyata Eye Hospital or Kagoshima Miyata Eye Clinic between October 2010 and August 2017 and had corneal edema caused by G. physocarpus milky latex-induced injury. Patient information and data on central corneal thickness (CCT), corneal endothelial cell density (ECD), best-corrected visual acuity (BCVA), and treatment were retrospectively reviewed.
Results: Five eyes of four patients were included. The mean age was 79.0 ± 7.1 years. All patients complaining of symptoms visited the hospital 1 or 2 days after the injury. All patients had corneal edema; two of the five eyes showed hyperemia, whereas none showed corneal epithelial defect or blepharitis. The mean CCT was 699.8 ± 95.9 μm at the first visit and decreased to 563.2 ± 74.0 μm 1 week after the injury with treatment with topical steroids and antibiotics. The mean ECD and BCVA were 2695.8 ± 191.3 cells/mm2 and 0.22 ± 0.19 at the first visit and 2826.0 ± 132.9 cells/mm2 and 0.10 ± 0.09 one week after the injury, respectively.
Conclusion: G. physocarpus caused transient dysfunction of the corneal endothelium and thereby, corneal edema. Accurate diagnosis with history taking is important to ascertain the types of plants the patient has touched and to exclude other possible diagnoses.
Keywords: Asclepias physocarpus, corneal endothelial dysfunction, corneal edema, plant toxin
Introduction
Gomphocarpus physocarpus, which is generally known as balloon plant or milkweed, belongs to the Asclepiadoideae subfamily and grows in many regions such as Australia, China, Hawaii, and other Pacific islands.1 Also called Asclepias physocarpa, it reaches a height of approximately 1–2 m and bears large ballooned fruits filled with numerous seeds (Figure 1). Characteristically, this plant releases white milky latex from stalks, which is why it is also known as “milkweed.” Reportedly, the milky latex of G. physocarpus and related species of Asclepiadoideae is cytotoxic, containing cardenolide that binds and inhibits Na-K-ATPase activity2,3 and induces corneal endothelial dysfunction with decrease in visual acuity and pain.4–8 Because the acute symptoms caused by these plants resemble those of uveitis, corneal endotheliitis, or keratitis, differential diagnosis is important. Additionally, because G. physocarpus is prevalent in a home garden as an ornamental plant or used as a cut flower, injuries may be fairly common. However, only a few case studies have reported the effects, course, and treatment of ocular toxicity due to G. physocarpus-related injury,4–8 partly because it is difficult to identify the cause as this would require a detailed history of all plants the patient has handled. Therefore, we consider that reporting the characteristics and prognosis of this type of injury would be helpful for clinicians.
In this retrospective case series, we investigated the clinical courses of patients with corneal endothelial dysfunction due to G. physocarpus milky latex-induced injury.
Methods
This was a retrospective observational case series. The institutional review board of Miyata Eye Hospital approved this study and the study protocol adhered to the tenets of the Declaration of Helsinki. The data of all patients were confidentially handled. Informed consent was obtained from each individual by the opt-out procedure providing a summary of the study on our hospital website (Case 1–3) or written document (Case 4). Consecutive patients who were referred to Miyata Eye Hospital or Kagoshima Miyata Eye Clinic and diagnosed with corneal edema caused by G. physocarpus milky latex-induced injury between October 2010 and August 2017 were included. Medical records were retrospectively reviewed for patient background, best-corrected visual acuity (BCVA), central corneal thickness (CCT), corneal endothelial cell density (ECD), percentage of hexagonal shape cells (HEX), coefficient of variation (CV), intraocular pressure (IOP), and treatment during the observation period. BCVA was measured with a 5-m Snellen chart and converted to the logarithm of the minimum angle of resolution (logMAR) for analyses. CCT was measured by pachymeter (AL-3000, Tomey, Nagoya, Japan) and anterior segment optical coherence tomography (SS-1000, Tomey). ECD, HEX, and CV were measured by non-contact specular microscopy (FA-3509, Konan, Nishinomiya, Japan). IOP was measured by non-contact tonometer (NT-4000, Nidek, Gamagori, Japan). All the data in this study are reported as mean ± standard deviation, unless otherwise specified.
Results
Five eyes of four patients were included in this study. Both eyes were injured in one patient and one of the eyes was injured in the other three patients. Patients' basic characteristics, symptoms, and clinical findings are shown in Table 1. Three of the patients were female and one was male. The mean age was 79.0 ± 7.1 years. The mean BCVA at the first visit was 0.22 ± 0.19. The mean CCT was 699.8 ± 95.9 μm. Two of the five eyes showed hyperemia, but none showed corneal epithelial defect or blepharitis. All patients complaining of symptoms visited the hospital 1 or 2 days after the milky latex of G. physocarpus entered the eyes. All patients were treated with topical steroid and antibiotic eye drops without washing of the ocular surface or the anterior chamber. The mean BCVA was 0.22 ± 0.19 at the first visit and 0.10 ± 0.09 1 week after the injury.
 |
Table 1 Patients’ Basic Characteristics And Clinical Findings On The First Visit |
The changes in CCT are shown in Figure 2. The mean CCT was reduced to 563.2 ± 74.0 μm 1 week after the injury. The CCT of all patients gradually decreased from the day of the first visit and it recovered within 2 weeks.
The changes in ECD are shown in Figure 3. At the first visit, ECD could be evaluated in all patients. The mean ECD was 2695.8 ± 191.3 cells/mm2 at the first visit and 2826.0 ± 132.9 cells/mm2 1 week after the injury. In contrast to corneal edema, the ECD of all patients remained higher than 2000 cells/mm2 from the early period after the injury throughout the observation period. At the first visit, the CV was 36.9 ± 16.1% and the HEX was 43.2 ± 10.5%. IOP rise higher than 20 mmHg was not observed during the follow-up period. The clinical course of each patient is summarized in the following section.
Case Presentations
Case 1
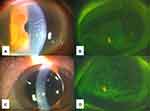
An 86-year-old woman visited our hospital for ocular pain. She had touched G. physocarpus while harvesting vegetables 2 days earlier and experienced ocular pain 1 day earlier. At first visit, her BCVA was 0.30 on the right eye and 0.097 on the left eye. Bilateral corneal edema and folds of Descemet’s membrane were observed without hyperemia, corneal defect, or ocular blepharitis (Figure 4A–D). Superficial punctate keratitis (SPK) was observed bilaterally (Figure 4B and D). CCT was 820 and 652 μm, ECD was 2535 and 2500 cells/mm2, the CV was 47.6% and 14%, and the HEX was 50% and 38.8% in the right and left eyes, respectively. IOP was 17 mmHg bilaterally. Few cells and slight flare inside the anterior chamber were observed but no sign of inflammation was found in the vitreous. Topical instillation of betamethasone six times a day and of gatifloxacin twice a day was initiated. CCT decreased to 754 and 640 μm on day 2 and to 700 and 630 μm on day 3 in the right and left eyes, respectively. One week later, CCT decreased to 641 and 640 μm and BCVA was 0.15 and 0.097 in the right and left eyes, respectively. The Descemet’s membrane folds improved, and the frequency of topical betamethasone instillation was changed to four times a day. Ruptured retinal microaneurysm was observed in the left eye with broad subretinal hemorrhage, but the patient’s macula was intact with no further decrease of visual acuity. Two months later, CCT was 593 and 563 μm, BCVA was 0.097 and 0.046, and ECD was 2356 and 2383 cells/mm2 in the right and left eyes, respectively, with no signs of inflammation in the anterior chamber. The CV improved to 25.5% and 26.7% and the HEX improved to 66% and 68% in the right and left eyes, respectively. Therefore, topical steroid instillation was discontinued.
Case 2
A 71-year-old woman visited our hospital complaining of decreased vision and pain in her left eye. She claimed that G. physocarpus milky latex had entered her left eye the previous day. Corneal edema was observed with hyperemia and folds of Descemet’s membrane. There was no corneal defect or SPK, but keratic precipitates were observed. Her left-eye BCVA was 0.15 and IOP was 10 mmHg. ECD was 3013 cells/mm2, the CV was 35%, the HEX was 58%, and CCT was 567 μm. Topical levofloxacin, betamethasone, and fradiomycin instillation four times a day were initiated. On day 4, her BCVA improved to 0.046 and CCT was 478 μm. The folds of Descemet’s membrane disappeared, and therefore all topical instillations were decreased to twice a day. One month later, corneal transparency was clear and BCVA improved to zero. The CCT was 467 μm, ECD was 2726 cells/mm2, CV was 30%, and HEX was 43%. Slight pigmented keratic precipitates were observed inside the cornea, and topical betamethasone and antibiotic instillation was discontinued at that time.
Case 3
A 74-year-old man visited our hospital complaining of ocular pain and blurred vision in his right eye. He claimed that G. physocarpus milky latex had entered his right eye the previous day. Slit-lamp examination showed decreased transparency in his right eye because of corneal edema and folds of Descemet’s membrane observed at his first visit. BCVA was 0.52 and IOP was 13 mmHg. ECD was 2661 cells/mm2, the CV was 56%, the HEX was 33%, and CCT was 713 μm. Cells were observed inside the anterior chamber, but there was no flare, hyperemia, blepharitis, or corneal epithelial defect. Topical levofloxacin and betamethasone instillation four times a day were initiated. BCVA improved to 0.30 and CCT decreased to 554 μm with slight keratic precipitates on the next day. One week later, BCVA was 0.22 and CCT was 517 μm with no folds of Descemet’s membrane, corneal edema, or cells in the anterior chamber. Hence, topical betamethasone and levofloxacin instillation were decreased to twice a day and discontinued 2 weeks after the injury. BCVA improved to 0.097, ECD improved to 3006 cells/mm2, the CV was 33%, and the HEX was 35% 3 months after the injury.
Case 4
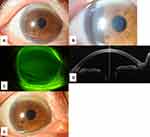
An 83-year-old woman was referred to our hospital complaining of ocular pain in her right eye. She had cut G. physocarpus in her garden the day before her visit. Her right cornea was edematous (Figure 5A and B). BCVA was 0.045 and IOP was 10 mmHg. There was no corneal defect, blepharitis, or inflammation inside the anterior chamber (Figure 5C). ECD was 2710 cells/mm2, the CV was 32%, the HEX was 36%, and CCT was 747 μm (Figure 5D). Topical betamethasone and levofloxacin instillation four times a day were initiated, and the pain disappeared the next day with the BCVA improving to zero. CCT also improved to 641 μm the next day and to 540 μm 1 week after the injury, and we, therefore, decreased the application of both topical instillations to twice a day. Two weeks after the injury, the folds of Descemet’s membrane and hyperemia had disappeared (Figure 5E). ECD was 2688 cells/mm2, the CV was 32%, and the HEX improved to 44%. Because BCVA recovered to −0.079 and CCT was 506 μm, topical instillation was discontinued.
Discussion
This is the first study to retrospectively examine multiple cases of corneal endothelial dysfunction owing to G. physocarpus milky latex-induced injury. The prognosis of all patients was good; BCVA improved and ECD did not decrease. Corneal transparency is maintained by Na-K-ATPase in the corneal endothelium and functiona disruption leads to corneal opacity and decrease of visual function. Based on the findings of this study, the ECD did not change but corneal edema was observed in the patients. Therefore, it is suggested that G. physocarpus caused transient dysfunction of the corneal endothelium, although it did not cause cytotoxicity. This transient effect might inhibit the function of Na-K-ATPase, which may have caused the corneal edema that led to increased CCT from 2 weeks to 2 months.
Longer than 1 day after the injury was required for the symptoms to appear in all patients in this study. Because corneal epithelial defect was minor or not observed in the patients, the plant toxin is assumed to have penetrated the cornea and entered the corneal endothelium.8 Hence, it is suggested that the toxin required longer than 1 day to penetrate the corneal endothelium and inhibit Na-K-ATPase activity, causing corneal edema as the result of decreased normal pumping function of corneal endothelial Na-K-ATPase. Because we could measure the ECD in all patients at the first visit, it is suggested that the corneal edema at that time was not too severe for evaluation.
Corneal toxicity caused by some plant species belonging to the Asclepiadoideae subfamily has been reported, such as by Asclepias curassavica,4,9 Asclepias physocarpa (G. physocarpus),5,6 and Asclepias tuberosa.7 Cytotoxicity caused by other types of plants producing milky latex, such as by Calotropis procera, was also reported.10 Skin irritation might occur because of the cytotoxicity of the G. physocarpus milky latex. However, no patient showed blepharitis in this study. The milky latex of G. physocarpus includes substances that are used as ingredients in Chinese medicine. Cardenolide, one of the substances, inhibits Na-K-ATPase activity and has been investigated; it has been clarified that cardenolide varies in structure and biological activities depending on the species and that the latex of plants more strongly inhibits Na-K-ATPase than do leaf extracts.2,3 Cardenolide might have contributed to the corneal dysfunction of the patients in this study through inhibition of Na-K-ATPase activity.
The mean ECD at the first visit and 1 week after the injury was 2695.8 ± 191.3 cells/mm2 and 2826.0 ± 132.9 cells/mm2, respectively, i.e. the values were not low. This result suggested that G. physocarpus milky latex-induced injury does not necessarily occur to people with low ECD. Therefore, it is necessary for all individuals, not only those with low ECD, to be careful when coming in contact with G. physocarpus in the garden or the road. In the current study, all patients were older than 70 years. G. physocarpus is seen at home garden as a popular ornamental plant in Japan. Therefore, it is expected that retired people who enjoyed gardening as hobby, would have more chance of exposure to milky latex.
Topical steroid instillation was effective for all patients in this study. Steroid instillation was reported to induce Na-K-ATPase activity in the corneal endothelium.11 An alternative treatment suggested for this injury is irrigation of the anterior chamber, which was not performed in this study. Irrigating and washing out the toxins and inflammatory cytokines from the anterior chamber might affect and reduce the symptoms, but inflammation of the anterior chamber was observed in only three of the five eyes, suggesting that the inflammation was not severe. Because corneal endotheliitis and uveitis, which can become exacerbated with irrigation, are included in the differential diagnosis, whether to perform irrigation of the anterior chamber should be carefully decided. Furthermore, in the current cases we used topical antibiotic administration as the first treatment, it may be unnecessary in cases without cells inside anterior chamber, epithelial defect, or hyperemia. Additionally, when corneal edema keeps and corneal thickness does not decrease, use of 5%NaCl instillation would be effective as treatment.
This study had several limitations. First, we did not have previous information on the included patients because they visited our hospital for the first time due to the injury. If we could have compared the BCVA and ECD before and after the injury, we could have more accurately assessed the effect of the G. physocarpus milky latex on the cornea. Second, the number of patients was small and it was difficult to perform statistical analysis. Because the patients’ clinical course varied, a future multicenter study is necessary to accurately investigate the clinical course of patients exposed to the plant toxin. Third, we did not evaluate the biological mechanism of the G. physocarpus milky latex penetrating the cornea and affecting the corneal endothelial cells. Further basic experimental study focusing on this mechanism is required.
Conclusions
In conclusion, we investigated the characteristics and prognosis of dysfunction of the corneal endothelium due to G. physocarpus milky latex-induced injury. When a patient presents with symptoms indicative of G. physocarpus milky latex-induced injury, it is important to ascertain the types of plants the patient has come in contact with to exclude other possible diagnoses, such as uveitis and corneal endotheliitis.
Abbreviations
BCVA, best-corrected visual acuity; CCT, central corneal thickness; ECD, endothelial cell density; IOP, intraocular pressure; logMAR, logarithm of the minimum angle of resolution; SPK, superficial punctate keratitis.
Disclosure
Dr Takashi Ono reports personal fees from Senju Pharmaceutical, Bayer, Kowa Pharmaceutical, and AbbVie GK, outside the submitted work. Dr Yosai Mori reports grants, personal fees from Alcon, J&J, HOYA, Senju Pharmaceutical, Santen pharmaceutical, and Novartis Pharma, personal fees from Kowa Pharmaceutical, grants from Kissei pharmaceutical, Wakamoto, and Shionogi, outside the submitted work. Dr Shiro Amano reports grants, personal fees from Santen Pharmaceutical, personal fees from Senju Pharmaceutical, Otsuka Pharmaceutical, Alcon, AMO, and HOYA, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Coombs G, Peter CI, Johnson SD. A test for Allee effects in the self-incompatible wasp-pollinated milkweed Gomphocarpus physocarpus. Austral Ecol. 2009;34(6):688–697. doi:10.1111/aec.2009.34.issue-6
2. Zhang RR, Tian HY, Tan YF, et al. Structures, chemotaxonomic significance, cytotoxic and Na(+),K(+)-ATPase inhibitory activities of new cardenolides from Asclepias curassavica. Org Biomol Chem. 2014;12(44):8919–8929. doi:10.1039/C4OB01545B
3. Zust T, Petschenka G, Hastings AP, Agrawal AA. Toxicity of milkweed leaves and latex: chromatographic quantification versus biological activity of cardenolides in 16 Asclepias species. J Chem Ecol. 2019;45(1):50–60. doi:10.1007/s10886-018-1040-3
4. Chakraborty S, Siegenthaler J, Buchi ER. Corneal edema due to Asclepias curassavica. Arch Ophthalmol. 1995;113(8):974–975. doi:10.1001/archopht.1995.01100080024013
5. Matsuura K, Hatta S, Terasaka Y, Inoue Y. Extensive bilateral corneal edema 6 weeks after cataract surgery: keratopathy due to Asclepias physocarpa: a case report. BMC Ophthalmol. 2017;17:5. doi:10.1186/s12886-017-0400-z
6. Pina S, Pedrosa C, Santos C, et al. Ocular toxicity secondary to Asclepias physocarpa: the balloon plant. Case Rep Ophthalmol Med. 2014;2014:829469.
7. Mikkelsen LH, Hamoudi H, Gul CA, Heegaard S. Corneal toxicity following exposure to Asclepias tuberosa. Open Ophthalmol J. 2017;11:1–4. doi:10.2174/1874364101711010001
8. Amiran MD, Lang Y, Yeung SN. Corneal endothelial toxicity secondary to Asclepias fruticosa. Eye (Lond). 2011;25(7):961–963. doi:10.1038/eye.2011.59
9. Roy MC, Chang FR, Huang HC, Chiang MY, Wu YC. Cytotoxic principles from the formosan milkweed, Asclepias curassavica. J Nat Prod. 2005;68(10):1494–1499. doi:10.1021/np0501740
10. Al-Mezaine HS, Al-Amry MA, Al-Assiri A, Fadel TS, Tabbara KF, Al-Rajhi AA. Corneal endothelial cytotoxicity of the Calotropis procera (ushaar) plant. Cornea. 2008;27(4):504–506. doi:10.1097/ICO.0b013e3181611c34
11. Hatou S, Yamada M, Mochizuki H, Shiraishi A, Joko T, Nishida T. The effects of dexamethasone on the Na,K-ATPase activity and pump function of corneal endothelial cells. Curr Eye Res. 2009;34(5):347–354. doi:10.1080/02713680902829624
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.