Back to Journals » Clinical Ophthalmology » Volume 11
Clinical and allergological analysis of ocular manifestations of sick building syndrome
Authors Saeki Y, Kadonosono K, Uchio E
Received 12 October 2016
Accepted for publication 17 December 2016
Published 14 March 2017 Volume 2017:11 Pages 517—522
DOI https://doi.org/10.2147/OPTH.S124500
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yusuke Saeki,1 Kazuaki Kadonosono,2 Eiichi Uchio1
1Department of Ophthalmology, Fukuoka University School of Medicine, Fukuoka, 2Department of Ophthalmology, Yokohama City University Medical Center, Yokohama, Japan
Purpose: The disease concept of sick building syndrome (SBS) is still unclear. Ocular mucous membrane irritation is one of the major symptoms of SBS. However, the immunological aspects of the ocular complications of SBS are not yet clarified. The clinical and allergological aspects of SBS cases with ocular disorders with special reference to allergic conjunctival diseases (ACD) were analyzed, especially with respect to local immunological features.
Methods: Twelve cases of SBS with ocular findings and 49 cases of ACD (allergic conjunctivitis [AC], atopic keratoconjunctivitis [AKC], and vernal keratoconjunctivitis [VKC]) for comparison were evaluated. The clinical findings in SBS and ACD were scored, and tear film breakup time (BUT) was measured. Cytokine (interferon-γ [IFN-γ], interleukin [IL]-2, IL-4, IL-5, IL-6, IL-8, and IL-13) concentrations in tears were analyzed by cytometric bead arrays. Eosinophil count in peripheral blood, total IgE in serum, and multiple allergen simultaneous test (MAST) for antigen-specific IgE were also measured.
Results: In SBS, conjunctival lesions were observed in all cases, and corneal abnormalities were found in two-thirds of the cases. Limbal lesions were observed in 2 pediatric cases. Mean serum total IgE level in SBS was significantly higher than that in AC; however, it was significantly lower than that in AKC and VKC. Eosinophil count in peripheral blood and number of positive allergens in MAST were significantly lower in SBS than in AKC and VKC. Significant elevation of tear IL-4 was observed in SBS and ACD. However, in contrast to ACD, elevation of other cytokines in tears was not observed in SBS. Mean tear BUT in SBS was in the normal range.
Conclusion: From these results, SBS is thought to be partially induced by an allergic response. However, clinical dissociation of the ocular clinical findings and local immunological features in tear cytokines may suggest that SBS belongs to a different entity from ACD.
Keywords: sick building syndrome, allergic conjunctival disease, cytokine, vernal keratoconjunctivitis, IgE
Introduction
In 1983, the World Health Organization (WHO) used the term “sick building syndrome (SBS)” for the first time to describe situations in which building occupants experience acute effects on health and comfort that appear to be linked to the time spent in a building,1 and SBS was divided into broad categories of mucous membrane irritation (eyes, nose, and throat irritation), neurotoxic effects (headaches, fatigue, and irritability), asthma and asthma-like symptoms (chest tightness and wheezing), skin dryness and irritation, gastrointestinal complaints, and others,2 but no specific illness or cause has been identified at present. The United States3 and Japanese4,5 governments have introduced environmental indexes for prevention of SBS, and clinical disorders associated with office buildings, houses, and schools have also been frequently recognized as SBS especially in Japan since around 2000.
Low quality of the indoor environment is associated with very non-specific symptoms affecting the eyes, nose, throat, and skin and general symptoms such as headaches and tiredness, sometimes denoted as SBS.6 Irritation and dryness of the eyes together with a blocked or runny nose and dry throat are especially considered an important part of the oculonasal mucosal symptoms of SBS.7 However, although symptoms of SBS occur only in specific buildings or dwellings, they disappear when the individual leaves the environment. Therefore, it is difficult to analyze the clinical and allergological characteristics of SBS, including ocular findings. Factors contributing to perceived indoor air quality include temperature, humidity, odors, air movement, ventilation, bioaerosols, and volatile organic compounds (VOC), such as formaldehyde, xylene, toluene, and methylene chloride.4,8
Several studies have reported the prevalence of ocular manifestations in SBS patients.9–13 In other studies, ocular disorders were analyzed in relation to nasal or respiratory symptoms, with special consideration of the mucosal characteristics of SBS.14–17 A few past studies focused especially on ocular findings in SBS.18,19 However, the immunological aspects of ocular complications of SBS have not been reported in detail.
Some studies have suggested an allergological background in SBS,20,21 but it has been pointed out that chemical, toxic, and neurological features are also important in the pathophysiology of SBS.22,23 In this study, we report the clinical and allergological aspects of SBS cases with ocular disorders with special reference to allergic conjunctival diseases (ACD: allergic conjunctivitis [AC], atopic keratoconjunctivitis [AKC], and vernal keratoconjunctivitis [VKC]), in order to analyze their similarities and differences, especially with respect to local immunological features.
Methods
Study population
This is a consecutive case series study of 12 patients (2 males and 10 females) who attended Yokohama City University Medical Center with SBS symptoms, especially ocular irritation in a specific building (Table 1). They were asked to complete a self-reporting questionnaire survey as previously proposed by Lu et al,24 and written informed consent was obtained from all patients. SBS symptoms were defined as one or more selected symptoms specified in the questionnaire for at least 1–3 days a week while at work in the office in the previous month, but which improved or disappeared after work or on days without work. The SBS symptoms were identified individually as the following groups: eyes (eye dryness and eye irritation), upper respiratory tract (nasal itching, runny nose, blocked nose, sneezing, and dry throat), lower respiratory tract (difficulty breathing), skin (skin dryness), and non-specific symptoms (headache, tiredness, difficulty concentrating, irritability, and dizziness).24 Those who had past history of medical treatment for systemic diseases, such as hypertension, cardiac diseases, and hyperthyroidism, were excluded from the study population.
  | Table 1 Cases of sick building syndrome |
For comparative evaluation with SBS, 49 patients with ACDs who also attended Yokohama City University Medical Center were included in this study. ACD was classified into AC, AKC, or VKC according to the guidelines for the diagnosis and treatment of conjunctivitis, and past reports.25,26 The ACD patients consisted of 14 with AC, 14 with AKC, and 21 with VKC. Normal (non-allergic) subjects were subjected to immunological testing for comparison. This study was approved by Yokohama City University Medical Center Review Committee of Clinical Research.
Clinical grading
Clinical evaluation of ocular findings was carried out according to the ocular clinical grading system reported previously.26 Ocular findings of slit lamp examination were recorded on the patients’ first visit to our outpatient clinic. Ten objective ocular clinical findings of conjunctival, limbal, and corneal lesions were graded on a 4-point scale (0= none, 1= mild, 2= moderate, and 3= severe; left and right eyes separately in each case). The total score of 10 findings, with a maximum of 30, taking the score of the more severe side in bilateral cases, was used as the clinical score.
Serological analysis
Serum total IgE level, eosinophil count in peripheral blood, and multiple allergen simultaneous test (MAST) for antigen-specific IgE were measured in each case.
Tear cytokine analysis
Tear fluid was extracted by the Schirmer method, as previously described, when the subjects attended our clinic.27 All samples were rapidly frozen at −20°C and maintained at −80°C until cytokine analysis. Tear fluid extraction was performed with 0.5 M NaCl and 0.5% Tween 20 in 0.01 M phosphate buffer (pH 7.2). The cytokine composition of tears was analyzed using a BD™ (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) Cytometric Bead Array system and a flow cytometer (BD™ FACS Canto II), according to the manufacturer’s instructions. Data were acquired and analyzed using FCAP Array™ software (Version 1.0.1; BD Biosciences, Tokyo, Japan). Standard curves were generated using the reference cytokine concentrations supplied by the manufacturer. The following 7 inflammatory cytokines were analyzed: interferon-γ (IFN-γ), interleukin (IL)-2, IL-4, IL-5, IL-6, IL-8, and IL-13.
Tear film breakup time (BUT)
The time from the conclusion of a blink to the occurrence of tear film fracture (BUT) was observed and recorded in SBS cases using a non-invasive method with slit lamp microscopy as reported previously.28
Statistical analysis
Since it has not been established whether the clinical score of ACD shows a normal distribution, non-parametric analysis was conducted in this study. The Mann–Whitney U test was used to identify differences among patient groups. A independent t-test for independent groups with unequal variance was also adopted. A P-value of <0.05 was accepted as statistically significant.
Results
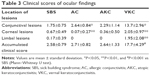
Demographic profiles of the SBS cases in this study are shown in Table 1. Middle-aged female patients comprised the majority of cases in this series. Most patients noticed their symptoms in closed spaces or poorly air-conditioned spaces. Conjunctival lesions were observed in all cases, and corneal abnormalities were found in two-thirds of cases. Two pediatric cases showed limbal proliferative change. SBS cases were older than ACD patients, with a significant difference between SBS and VKC (P<0.05 in independent t-test for independent groups with unequal variance; Table 2).
Mean clinical score of conjunctival changes in SBS was significantly lower than that in AC and VKC. Mean corneal score in SBS was significantly higher than that in AC, but significantly lower than that in VKC. Mean limbal lesion score in SBS was significantly lower than that in VKC. Mean total clinical score in SBS did not show a significant difference from that in AC and AKC, but that in VKC was higher than that in SBS (Mann–Whitney U test; Table 3). Adult cases of SBS showed similar clinical features of AC, thus comparison between AC type of SBS and AC was conducted; however, the difference was similar to that of whole SBS (data not shown).
Mean serum total IgE level in SBS was significantly higher than that in AC, but was significantly lower than that in AKC and VKC. Eosinophil count in peripheral blood and number of positive allergens in MAST were significantly lower in SBS than in AKC and VKC (Table 4).
Analysis of tear cytokine levels showed no statistically significant difference in IFN-γ and IL-2 between normal controls and any disease group. Tear IL-4 level was significantly elevated in all groups compared with controls. Tear IL-5, IL-6, and IL-8 levels in patients with VKC were significantly higher than those in normal controls, and that of IL-13 in AKC and VKC was significantly higher than that in normal controls, but such elevation was not observed in SBS (Table 5).
BUT in SBS cases was 12.1±3.1 seconds (mean ± standard deviation), meaning that SBS cases with ocular signs had normal lacrimation function. In these cases, the prevalence of SBS symptoms calculated based on a self-reporting questionnaire survey was as follows: 100% of participants had eye symptoms, 75% had upper respiratory symptoms, 33% had lower respiratory symptoms, and 67% had non-specific symptoms. Tiredness (42%), headache (58%), and difficulty concentrating (17%) were reported.
Discussion
This study showed that most cases were older than 40 years and were female, similar to past studies.10,13,14 It is basically recognized that symptoms of SBS are only observed in a specific building and that they disappear outside the building. However, some objective clinical findings can be observed in the clinic far away from the causal building. It is considered that some clinical changes in SBS might be irreversible due to pathological conditions. However, upon comparing the clinical ocular findings of SBS with those in ACD, conjunctival change was milder, and corneal change seemed to be more severe in the present study. It is difficult to explain the reason for these findings. From the allergological standpoint, it might be possible that the fact that the clinical and immunological surveys were conducted outside the specific building might have impaired the detection of early-phase reaction-induced changes in this study. In contrast to conjunctival findings, corneal and limbal lesions are considered to be affected by local invasion of eosinophils in the late phase reaction, thus explaining that the ocular findings observed in the clinic might have been induced by SBS.29 A VOC exposure study might be meaningful for direct clinical observation of ocular findings, but this type of experimental equipment is restricted in Japan at present.30 Experimental exposure to airborne office dust was conducted and analyzed in humans, and it was reported that irritative mucosal symptoms appeared.16 However, VOC were not evaluated in this study.16
The absence of an elevation of peripheral blood eosinophil count in SBS cases seems to be inconsistent with a previous study in which eosinophil count in peripheral blood was a predictor of SBS.31 However, the fact that a small proportion of patients exhibited serious systemic complaints in contrast to ocular changes might suggest that local ocular lesions hardly affected systemic eosinophil count in this study, and further investigation will be necessary. We observed 2 pediatric cases, who complained of irritative symptoms at school, and it is interesting that their findings included limbal proliferative change, which is specific to VKC. The reason why pediatric cases exhibited more serious ocular changes than those in adult cases is unclear, but a recent study revealed age-related changes in conjunctival structure,32 leading to the assumption that an immature conjunctival structure might induce those specific clinical characteristics that resemble VKC. However, it is not denied that pediatric SBS patients with ocular symptoms are complicated with VKC. Limbal type of VKC is a minor spectrum in VKC whose pathological mechanism is not clarified at present, and further investigation is also needed.
From the evaluation of tear cytokine levels, the significant elevation of IL-4 (a Th2-type cytokine) and the absence of significant change in IL-2 or IFN-γ (Th1-type cytokines) levels in SBS strongly reflect the presence of allergic reaction in the ocular surface of SBS patients. However, considering the lower levels of serum total IgE and inflammatory tear cytokines in SBS compared to ACD, and the fact that limbal proliferative change is found only in pediatric cases of SBS, the ocular lesions in SBS can hardly be categorized in the same spectrum as ACD, because in our past study, we reported that the ocular clinical grading system had sufficient sensitivity to differentiate each disease within ACD, such as AC vs VKC.26
Regarding BUT in SBS cases, no significant change was observed in our study. Several studies have reported a significant decrease in BUT in SBS cases,16,17 but BUT was also reported to increase only at higher night air temperatures in another study.9 Because a significant decrease in BUT was observed in an experimental air-borne exposure study in which office dust was used,16 BUT value might be affected by different test circumstances. From these controversial results, it is considered that multiple elements and air status affect the result of BUT. In this study, BUT was carried out only in SBS cases due to the study design, and recent studies mentioned that the incidence of dry eyes has increased in patients with ACD.33,34 It is estimated that chronic pathological change seems to affect the ocular surface, leading to abnormalities in tear film stability, epithelial cells integrity, and corneal nerve function in ACD,31 whereas this pathophysiological feature cannot be applied to SBS because of its basically reversible nature.
The limitations of this study were as follows: this study was designed from the standpoint of ophthalmological aspects of SBS; thus, the study population was small, and unlike in cross-sectional studies based on populations in office buildings, multiple logistic regression analysis was impossible in our study. Measurement of environmental parameters using calibrated instruments for each patient was not done because of the restriction of laboratory equipment and considerable diversity in the specific location of each patient. The causal elements might vary in each case, such as VOC, odors, dust, and bioaerosols. Systemic features were only collected from self-reporting questionnaires, and medical examination was not carried out because most patients only suffered ocular symptoms that did not require systemic medical treatment.
Conclusion
Few studies have investigated the ocular symptoms in SBS, and little is known about the clinical and pathological properties of SBS, especially its ocular lesions. We report that the ocular changes in SBS have allergological characteristics, but the existence of several findings that are not similar to conventional ACD also suggest that further investigation is needed for proper orientation of SBS with allergic diseases.
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement of Scientists (15K10911) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Dr W Gray for editing this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
World Health Organization. Indoor Air Pollutants, Exposure and Health Effects Assessment. Euro-Reports and Studies No 78. Copenhagen: World Health Organization Regional Office for Europe; 1983. | ||
Chang CC, Ruhl RA, Halpern GM, Gershwin ME. The sick building syndrome. I. Definition and epidemiological considerations. J Asthma. 1993;30(4):285–295. | ||
Popay J, Bartley M, Owen C. Gender inequalities in health: social position, affective disorders and minor physical morbidity. Soc Sci Med. 1993;36(1):21–32. | ||
Saijo Y, Kishi R, Sata F, et al. Symptoms in relation to chemicals and dampness in newly built dwellings. Int Arch Occup Environ Health. 2004;77(7):461–470. | ||
Takigawa T, Horike T, Ohashi Y, Kataoka H, Wang DH, Kira S. Were volatile organic compounds the inducing factors for subjective symptoms of employees working in newly constructed hospitals? Environ Toxicol. 2004;19(4):280–290. | ||
Burge PS. Sick building syndrome. Occup Environ Med. 2004;61(2):185–190. | ||
Editorials. Sick building syndrome. Lancet. 1991;338(8781):1493–1494. | ||
Apter A, Bracker A, Hodgson M, Sidman J, Leung WY. Epidemiology of the sick building syndrome. J Allergy Clin Immunol. 1994;94(2 pt 2):277–288. | ||
Bakke JV, Norbäck D, Wieslander G, et al. Symptoms, complaints, ocular and nasal physiological signs in university staff in relation to indoor environment – temperature and gender interactions. Indoor Air. 2008;18(2):131–143. | ||
Brasche S, Bullinger M, Bronisch M, Bischof W. Eye- and skin symptoms in German office workers – subjective perception vs objective medical screening. Int J Hyg Environ Health. 2001;203(4):311–316. | ||
Engvall K, Norrby C, Norbäck D. Ocular, airway, and dermal symptoms related to building dampness and odors in dwellings. Arch Environ Health. 2002;57(4):304–310. | ||
Finnegan MJ, Pickering CA, Burge PS. The sick building syndrome: prevalence studies. Br Med J (Clin Res Ed). 1984;289(6458):1573–1575. | ||
Engvall K, Norrby C, Norbäck D. Ocular, nasal, dermal and respiratory symptoms in relation to heating, ventilation, energy conservation, and reconstruction of older multi-family houses. Indoor Air. 2003;13(3):206–211. | ||
Muzi G, dell’Omo M, Abbritti G, Accattoli P, Fiore MC, Gabrielli AR. Objective assessment of ocular and respiratory alterations in employees in a sick building. Am J Ind Med. 1998;34(1):79–88. | ||
Muzi G, Abbritti G, Accattoli MP, dell’Omo M. Prevalence of irritative symptoms in a nonproblem air-conditioned office building. Int Arch Occup Environ Health. 1998;71(6):372–378. | ||
Pan Z, Mølhave L, Kjaergaard SK. Effects on eyes and nose in humans after experimental exposure to airborne office dust. Indoor Air. 2000;10(4):237–245. | ||
Bakke JV, Wieslander G, Norbäck D, Moen BE. Atopy, symptoms and indoor environmental perceptions, tear film stability, nasal patency and lavage biomarkers in university staff. Int Arch Occup Environ Health. 2008;81(7):861–872. | ||
Franck C, Bach E, Skov P. Prevalence of objective eye manifestations in people working in office buildings with different prevalences of the sick building syndrome compared with the general population. Int Arch Occup Environ Health. 1993;65(1):65–69. | ||
Kjaergaard S, Pedersen OF, Mølhave L. Sensitivity of the eyes to airborne irritant stimuli: influence of individual characteristics. Arch Environ Health. 1992;47(1):45–50. | ||
Nakazawa H, Ikeda H, Yamashita T, et al. A case of sick building syndrome in a Japanese office worker. Ind Health. 2005;43(2):341–345. | ||
Edmondson DA, Nordness ME, Zacharisen MC, Kurup VP, Fink JN. Allergy and “toxic mold syndrome”. Ann Allergy Asthma Immunol. 2005;94(2):234–239. | ||
Campbell AW, Thrasher JD, Madison RA, Vojdani A, Gray MR, Johnson A. Neural autoantibodies and neurophysiologic abnormalities in patients exposed to molds in water-damaged buildings. Arch Environ Health. 2003;58(8):464–474. | ||
Rea WJ, Didriksen N, Simon TR, Pan Y, Fenyves EJ, Griffiths B. Effects of toxic exposure to molds and mycotoxins in building-related illnesses. Arch Environ Health. 2003;58(7):399–405. | ||
Lu CY, Lin JM, Chen YY, Chen YC. Building-related symptoms among office employees associated with indoor carbon dioxide and total volatile organic compounds. Int J Environ Res Public Health. 2015;12(6):5833–5845. | ||
BenEzra D. Guidelines on the diagnosis and treatment of conjunctivitis. Ocul Immunol Inflamm. 1994;2(suppl):17–26. | ||
Uchio E, Kimura R, Migita H, Kozawa M, Kadonosono K. Demographic aspects of allergic conjunctival diseases and evaluation of new criteria for clinical assessment of ocular allergy. Graefes Arch Clin Exp Ophthalmol. 2008;246(2):291–296. | ||
Shoji J, Kitazawa M, Inada N, et al. Efficacy of tear eosinophil cationic protein level measurement using filter paper for diagnosing allergic conjunctival disorders. Jpn J Ophthalmol. 2003;47(1):64–68. | ||
American Academy of Ophthalmology. Basic and Clinical Science Course Section 7 2002–2003: Orbit, Eyelids, and Lacrimal System (Basic & Clinical Science Course). San Francisco, 2002:244–245. | ||
Vichyanond P, Pacharn P, Pleyer U, Leonardi A. Vernal keratoconjunctivitis: a severe allergic eye disease with remodeling changes. Pediatr Allergy Immunol. 2014;25(4):314–322. | ||
Brouwer DH, de Pater NA, Zomer C, Lurvink MW, van Hemmen JJ. An experimental study to investigate the feasibility to classify paints according to neurotoxicological risks: occupational air requirement (OAR) and indoor use of alkyd paints. Ann Occup Hyg. 2005;49(5):443–451. | ||
Sahlberg B, Norbäck D, Wieslander G, Gislason T, Janson C. Onset of mucosal, dermal, and general symptoms in relation to biomarkers and exposures in the dwelling: a cohort study from 1992 to 2002. Indoor Air. 2012;22(4):331–338. | ||
Zhang X, Li Q, Xiang M, et al. Bulbar conjunctival thickness measurements with optical coherence tomography in healthy Chinese subjects. Invest Ophthalmol Vis Sci. 2013;54(7):4705–4709. | ||
Akil H, Celik F, Ulas F, Kara IS. Dry eye syndrome and allergic conjunctivitis in the pediatric population. Middle East Afr J Ophthalmol. 2015;22(4):467–471. | ||
Villani E, Strologo MD, Pichi F, et al. Dry eye in vernal keratoconjunctivitis: a cross-sectional comparative study. Medicine (Baltimore). 2015;94(42):e1648. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.