Back to Journals » Journal of Blood Medicine » Volume 7
Circulating hematopoietic progenitors and CD34+ cells predicted successful hematopoietic stem cell harvest in myeloma and lymphoma patients: experiences from a single institution
Authors Yu J, Cheng S, Yang Y, Chang K, Hwang W, Teng C
Received 3 September 2015
Accepted for publication 22 December 2015
Published 5 February 2016 Volume 2016:7 Pages 5—11
DOI https://doi.org/10.2147/JBM.S95679
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin Bluth
Jui-Ting Yu,1,2,* Shao-Bin Cheng,3,* Youngsen Yang,1 Kuang-Hsi Chang,4 Wen-Li Hwang,1 Chieh-Lin Jerry Teng,1,5,6
1Division of Hematology/Medical Oncology, Department of Medicine, Taichung Veterans General Hospital, 2Division of Hematology/Medical Oncology, Tungs' Taichung MetroHarbor Hospital, 3Division of General Surgery, Department of Surgery, 4Department of Medical Research and Education, Taichung Veterans General Hospital, 5Department of Life Science, Tunghai University, 6School of Medicine, Chung Shan Medical University, Taichung, Taiwan, Republic of China
*These authors contributed equally to this work
Background: Previous studies have shown that the numbers of both circulating hematopoietic progenitor cell (HPC) and CD34+ cell are positively correlated with CD34+ cell harvest yield. However, the minimal numbers of both circulating HPCs and CD34+ cells required for performing an efficient hematopoietic stem cell (HSC) harvest in lymphoma and myeloma patients have not been defined in our institution.
Patients and methods: Medical records of 50 lymphoma and myeloma patients undergoing peripheral blood HSC harvest in our institution were retrospectively reviewed. The minimal and optimal HSC harvest yield required for the treatment was considered to be ≥2×106 CD34+ cells/kg and ≥5×106 CD34+ cells/kg, respectively.
Results: The minimally required or optimal HSC yield obtained was not influenced by age (≥60 years), sex, underlying malignancies, disease status, multiple rounds of chemotherapy, or history of radiotherapy. The numbers of both circulating HPC and CD34+ cell were higher in patients with minimally required HSC yields (P=0.000 for HPC and P=0.000 for CD34+ cell) and also in patients with optimal HSC yields (P=0.011 for HPC and P=0.006 for CD34+ cell). The cell count cutoff for obtaining minimally required HSC harvest was determined to be 20/mm3 for HPCs and 10/mm3 for CD34+ cells. Furthermore, the cell count cutoff for obtaining optimal HSC harvest was determined to be 60/mm3 for HPCs and 35/mm3 for CD34+ cells.
Conclusion: A total of 60/mm3 of HPCs and 35/mm3 of CD34+ cells in peripheral blood predicted optimal HSC harvest in lymphoma and myeloma patients.
Keywords: hematopoietic progenitor cells, CD34+ cells, hematopoietic stem cells, myeloma, lymphoma
Introduction
Despite the rapid development in the treatments of hematological malignancies, autologous hematopoietic stem cell transplantation (auto-HSCT) remains one of the standard therapeutic modalities for treating myeloma and lymphoma.1,2 Auto-HSCT has been shown to improve the response rate and promote the complete remission in myeloma patients, especially in patients with at least very good partial responses before autografting.3 In addition, early auto-HSCT can improve progression-free survival of patients with high-intermediate-risk or high-risk aggressive non-Hodgkin’s lymphoma.4
Several factors, such as age, patients’ clinical performance, disease status, and possible alternative therapies, should be taken into consideration before performing auto-HSCT. Among all the factors that influence the effectiveness of auto-HSCT treatment for myeloma and lymphoma patients, adequate peripheral blood hematopoietic stem cell (HSC) mobilization and harvest play a crucial role. A harvest of >2×106 CD34+ cells/kg of body weight is generally considered to be the minimal requirement for stable hematological engraftment.5 Moreover, infusion of ≥5×106 CD34+ cells/kg, which is defined as an optimal HSC harvest, has been associated with a faster hematological recovery, especially recovery of the megakaryocytic lineage.6 A previous study has shown that lymphoma, advanced age, and high body weight are risk factors associated with poor peripheral blood HSC mobilization.7 Olivieri et al proposed that failure of previous mobilization, extensive radiotherapy to marrow-bearing tissue, and full courses of previous therapy are the major parameters associated with unsuccessful peripheral blood HSC harvest.8
In addition to the aforementioned clinical conditions, assessment of laboratory variables can provide useful information for determining an optimum time window to initiate HSC apheresis. Previous studies have shown that preapheresis circulating CD34+ cells significantly correlate with the number of CD34+ cells collected by leukapheresis.9 Additionally, the number of peripheral blood hematopoietic progenitor cell (HPC), an estimate of immature cells counted by the Sysmex-automated hematology analyzer, is considered to be an alternate surrogate for successful HSC harvest prediction.10 However, the predictive cell numbers of preapheresis circulating HPCs and CD34+ cells for successful HSC mobilization in myeloma or lymphoma patients have not been characterized. Moreover, the prediction accuracy of changes in the number of circulating HPCs or CD34+ cells in our institution has not been determined. Thus, the objective of this retrospective study was to determine the predictive number of circulating HPCs or CD34+ cells required to obtain an HSC harvest that is adequate for performing auto-HSCT for treating lymphoma and myeloma patients in our institution.
Patients and methods
Patients
This study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan. Patient informed consent was waived due to the retrospective study design. Medical records of 50 lymphoma or myeloma patients who received autologous peripheral blood HSC harvest at Taichung Veterans General Hospital, Taichung, Taiwan, from October 2010 to September 2013 were reviewed for this study. Granulocyte colony-stimulating factor (G-CSF; Kyowa Hakko Kirin, Tokyo, Japan) at a dosage of 5–10 μg/kg/d was routinely delivered to all patients before peripheral blood HSC collection. In addition to G-CSF, 33 of the 50 patients (66%) simultaneously received chemotherapeutic agents for peripheral blood HSC mobilization. Among these 33 patients who received chemomobilization, high-dose cyclophosphamide was the most common regimen, accounting for 69.70% (23 of the 33 patients). The minimally required HSC harvest yield for performing auto-HSCT was considered to be ≥2×106 CD34+ cells/kg. In addition, the optimal HSC harvest for performing auto-HSCT was considered to be ≥5×106 CD34+ cells/kg.6 Finally, minimally required harvest was obtained in 38 of the 50 patients (76%). However, optimal HSC harvest was obtained in only 18 of the 50 patients (36%).
Peripheral blood HSC collection
Peripheral blood HSCs were collected after 3 days of G-CSF administration in patients who did not receive chemotherapeutic mobilization. For patients mobilized by a combination of G-CSF and chemotherapeutic agents, peripheral blood HSC collection was not commenced until the white blood cell counts recovered from the nadir. Double lumen catheters were routinely placed for vascular access. A bolus of 10 μg/kg G-CSF was administered ~3 hours prior to each apheresis. COBE spectra continuous flow blood cell separator (Cobe Laboratories, Denver, CO, USA) was used for large-volume leukapheresis at a flow rate of 40–50 mL/min. The maximal processed volume was 2.5 times that of total body blood volume for each apheresis. After leukapheresis, light density-fractioned mononuclear cells were collected for CD34+ cell enumeration.
Preharvested circulating HPC analysis
The numbers of circulating HPCs were analyzed using the immature myeloid information channel of the Sysmex XE-2100 automated hematology analyzer (Sysmex Corporation, Kobe, Kansai, Japan) according to the manufacture’s protocol and as described in a previous study.10 Briefly, white blood cells in the immature myeloid information channel were analyzed using a radiofrequency and direct current. The data obtained were used to create a scattergram. The radiofrequency signal differentiates cells based on the intracellular contents, such as nuclear size and granules, whereas the direct current signal separates cells based on the size or volume. As the HPCs have fewer lipid molecules in the cell membrane, these cells are relatively refractory to surfactant treatment and are not easily lyzed by surfactants. HPCs that are not lyzed contain intact nucleus, have intracellular granules, appear in the lower area of the immature information scattergram, and can be expressed as an absolute number per cubic millimeter of peripheral blood.11
Circulating CD34+ cell enumeration
We used flow cytometry for quantifying CD34+ cells in both preharvest peripheral blood and resultant HSC product after leukapheresis by using the protocol specified by International Society of Hematotherapy and Graft Engineering.12 Briefly, phycoerythrin-conjugated anti-CD34 and fluorescein isothiocyanate-labeled anti-CD45 murine monoclonal antibodies were incubated with peripheral blood samples or HSCs collected by leukapheresis at room temperature for 15 minutes. Cell lysis buffer containing 7-aminoactinomycin D was then added. After incubating at room temperature for another 15 minutes, the stained samples were analyzed using a flow cytometer (FACSCanto II; Becton Dickinson, San Jose, CA, USA). Initially, cells positively stained for both CD45 and CD34 were differentiated from 7-aminoactinomycin D-negative cells and then isolated. The identity of CD34+ HSCs was further confirmed by the dim staining of CD45. Finally, CD34+ HSCs were expressed as an absolute number per kilogram of body weight.
Variable definitions and statistical analysis
Briefly, day 0 indicated the first day of peripheral blood autologous HSC harvest. The ratios of absolute cell count on day 0 over that on day −1 were assessed. The slope indicated the net of absolute cell count between day −1 and day 0 over that on day −1. Chi-square and Mann–Whitney U tests were used wherever appropriate. Cell numbers for group comparisons were presented as mean ± standard error. Statistical significance was set at P<0.05. All the statistical analyses were performed using SPSS software, Version 20.0 (IBM Corporation, Armonk, NY, USA).
Results
Clinical characteristics of patients
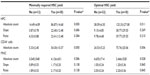
Clinical characteristics of patients were compared to determine which factors influenced minimally required or optimal HPC harvest yield (Table 1). Briefly, age ≥60 years (P=0.294 for minimally required yield and P=0.700 for optimal yield), sex (P=0.863 for minimally required yield and P=0.501 for optimal yield), underlying malignancies (P=0.741 for minimally required yield and P=0.141 for optimal yield), disease status (P=0.409 for minimally required yield and P=0.125 for optimal yield), if more than two lines of chemotherapy had been delivered (P=1.000 for minimally required yield and P=0.763 for optimal yield), and previous radiotherapy (P=1.000 for minimally required yield and P=1.000 for optimal yield) were not significantly different among patients with and without minimally required or optimal HSC collection. Optimal HSC yield was obtained from 94.4% (17/18) of patients mobilized by a combination of chemotherapeutic agents and G-CSF, whereas only 50.0% (16/32) of patients mobilized by G-CSF provided optimal HSC yield. Therefore, the probability of optimal yield was significantly higher with combination mobilization than with G-CSF mobilization alone (P=0.004). However, the probability of minimally required HSC yield was not significantly different between the patient group that was mobilized by using a combination of systemic chemotherapy and G-CSF and the patient group that was mobilized by G-CSF alone (P=0.294). The average apheresis time required for patients with and without minimally required HSC collection was 2.05 days and 2.50 days, respectively (P=0.077). However, the apheresis time required for patients with optimal HSC collection (1.78 days) was significantly fewer than that required for patients without optimal HSC collection (2.38 days; P=0.013).
HPC count in patients with minimally required or optimal HSC yield
For determining the number of circulating HPCs required for performing auto-HSCT, we analyzed the impact of absolute number, slope, and ratio of circulating HPC. The results are shown in Table 2. The absolute mean number of circulating HPC in patients with minimally required yield was 86.87±14.68/mm3, which was significantly higher than that of patients without minimally required yield (14.49±4.89/mm3; P=0.000). The mean number of circulating HPCs in patients with optimal HSC yield was 125.33±27.08/mm3, which was significantly higher than that of patients without optimal HSC yield (38.09±6.50/mm3; P=0.011).
We further analyzed whether the slope or ratio of the graphical data curve generated by analyzing the circulating HPC numbers could be used as indicators for successful autologous HSC harvest. The data analysis revealed that the values of these parameters were not significantly different among patients with and without minimally required or optimal HSC collection.
Patients with both minimally required and optimal HSC collection had more circulating CD34+ cells
The average number of circulating CD34+ cells in patients with minimally required HSC yield (54.30±10.37/mm3) was significantly higher (P=0.000) than that of patients without minimally required HSC yield (5.33±2.40/mm3). Similarly, the average number of circulating CD34+ cells in patients with optimal HSC yield (75.76±20.06/mm3) was significantly higher (P=0.006) than that of patients without optimal HSC yield (24.53±5.23/mm3). The data could not be analyzed for determining slope and ratio of circulating CD34+ cell due to missing data.
Since circulating mononuclear cell count is one of the conventional parameters used for determining the HSC collection period,13 we also investigated whether the cell counts could be used for determining the optimal HSC collection periods in myeloma and lymphoma patients. Our study results showed that the absolute count, slope, and ratios of mononuclear cells were not different among patients with and without minimally required HSC collection. Additionally, the absolute numbers of mononuclear cells in patients with optimal HSC yields (2,466±528/mm3) were significantly lower (P=0.028) than that of patients without optimal HSC yields (4,655±714/mm3).
Cutoff of circulating HPC and CD34+ cell for predicting successful HSC harvest
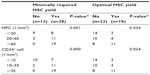
For further understanding of the cutoff values of circulating HPCs and CD34+ cells that were predictive of successful autologous HSC harvest in patients with myeloma and lymphoma, we stratified patients into three groups on the basis of similar patient numbers in each group. According to this stratification, data comparisons among patient groups showed statistical significance (Table 3). Briefly, when the absolute HPC count was >20/mm3, 90.91% (30/33) of the patients had minimally required HSC yield. Additionally, if optimal HSC collection was the target, >60/mm3 circulating HPCs were required because ≥5×106/kg CD34+ cells were collected in only 22.58% (7/31) of patients with circulating HPCs of ≤60/mm3 on day 0.
Similarly, when the circulating CD34+ cell counts were ≥10/mm3, minimally required HPC collection could be achieved in 93.75% (30/32) of the patients. On the other hand, only 20.00% (6/30) of the patients with optimal HSC yields had circulating CD34+ cell counts of ≤35/mm3 on day 0. However, optimal HSC could be harvested in 57.89% (11/19) of the patients if the circulating CD34+ cell counts were >35/mm2.
Discussion
Adequate peripheral blood autologous HSC harvest remains one of the most important steps for successful auto-HSCT. In our study cohort, the majority of the myeloma and lymphoma patients (76%, 38/50) had minimally required HSC yield. However, only 36% (18/50) of the patients achieved optimal HSC harvest. Previous studies have shown that failed mobilization attempts, extensive radiotherapy to marrow-bearing tissue, refractory diseases, and age >65 years were associated with poor mobilization in myeloma and lymphoma patients.14 However, a study conducted by Costa et al15 demonstrates that the premobilization clinical characteristics should not be used to determine the mobilization strategy for myeloma patients. The findings of our study supported the results reported by Costa et al, and our data indicated that the age ≥60 years, disease status, and previous chemotherapy or radiotherapy did not significantly correlate with the type of yield (minimally required or optimal HSC yield) obtained in myeloma and lymphoma patients. However, patients mobilized by a combination of chemotherapeutic agents and G-CSF were more likely to yield optimal HSC harvest than those who were mobilized by G-CSF alone (P=0.004). Similar findings have been reported by Milone et al.16 In terms of apheresis procedures, patients with minimally required HSC collection did not need more apheresis procedures (P=0.077). Interestingly, fewer apheresis procedures were observed in patients with optimal CD34+ cell harvest than those without optimal CD34+ cell harvest (P=0.013), indicating that the window for efficient autologous HSC collection could be narrow. Once the optimal collection window was missed, successful autologous HSC harvest was difficult in myeloma and lymphoma patients.
In addition to clinical characteristics, several laboratory parameters have been proposed to predict the optimal timing for initiating the peripheral blood HSC apheresis. In the past, the number of circulating mononuclear cells was considered as an easily assessable and effective laboratory variable.17 However, in contrast to the data reported by Schwella et al,17 our data demonstrated that myeloma and lymphoma patients with optimal HSC yields had a lower circulating mononuclear cell count than that of patients without optimal HSC yield (P=0.028). A study by Suzuya et al18 showed that donor’s age, body mass index, and white blood cell count before mobilization were significantly correlated with the yield of both mononuclear and CD34+ cells in healthy peripheral blood HSC donors. However, no positive correlation between the numbers of mononuclear and CD34+ cells was shown in this study. With the support of our result, we hypothesize that the number of mononuclear cells could possibly increase ahead in the surge of CD34+ cells. Further studies are required for this hypothesis.
Previous studies have shown that cell counts of both circulating HPCs and CD34+ cells are good predictors for successful HSC apheresis.19,20 The findings of this study also corroborated the previous findings. As compared to the circulating cell counts of both HPCs and CD34+ cells of patients with low yields, the cell counts were significantly higher in myeloma and lymphoma patients with either minimally required yield (P=0.000 for HPC and P=0.000 for CD34+ cell) or optimal yield (P=0.011 for HPC and P=0.006 for CD34+ cell). However, the data analysis indicated that increase in HPC count, either by slope or by ratio, was not a reliable indicator of efficient autologous HSC harvest, although a trend was found. These findings were in contrast to those reported in a previous study by Jo et al,21 which shows that >2/mm3 a day increase in HPC count is associated with fewer number of apheresis procedures required for obtaining optimal autologous HSC harvest in myeloma patients. The amount of increase in CD34+ cell count required for efficient autologous HSC harvest could not be analyzed in this study due to missing data.
One of the important findings of this study was the determination of cutoff values of circulating HPC and CD34+ cell counts for predicting successful autologous HSC harvest under apheresis condition in our institution. Our results showed that myeloma and lymphoma patients with ≥20/mm3 circulating HPCs had 90% or higher probability of providing minimally required HSC yield. This result was partially supported by the study by Villa et al,22 showing that an HPC count of 20/mm3 or higher is a good indicator of adequate initial CD34+ cell yields. Our data also indicated that circulating CD34+ cell counts of ≥10/mm3 and >35/mm3 could consistently predict whether minimally required yield and optimal HSC yield, respectively, could be obtained from adult myeloma and lymphoma patients, which was partially supported by a study conducted by Rujkijyanont et al,23 which showed the cutoff values >35/mm3 of CD34+ cells in patients younger than 15 years or the cutoff values >45/mm3 of CD34+ cells in patients older than 15 years are good predictors of an adequate autologous HSC collection in one apheresis session. Furthermore, Sinha et al24 reported that circulating CD34+ cell counts of <6/mm3 and <15/mm3 on days 4 and 5 predict failure to achieve a target collection of 2×106/kg CD34+ cells and 4×106/kg CD34+ cells, respectively, in myeloma patients. Thus, different cutoff values of circulating CD34+ cells for the prediction of successful autologous HSC harvest are needed under different apheresis procedure settings.
The major limitations of this study are that the study was retrospective with a relatively small patient number. In addition, patients with minimally required HSC yield after one apheresis procedure might not undergo further apheresis. This bias may have resulted in overestimation of cutoff counts of both circulating HPCs and CD34+ cells for indicating optimal HSC harvest. Studies with prospective and randomized control design are required to further corroborate these data.
Conclusion
Our study determined the absolute number of both circulating HPCs and CD34+ cells required to predict successful autologous HSC harvest in myeloma and lymphoma patients in our institution. The cutoff values were determined to be 20/mm3 for circulating HPC and 10/mm3 for CD34+ cell to predict successful minimally required HSC harvest, which is the basic requirement to perform one auto-HSCT in patients with myeloma or lymphoma. Furthermore, 60/mm3 of circulating HPCs and 35/mm3 of CD34+ cells were determined to be the possible cutoff cell counts for predicting optimal HSC collection.
Disclosure
The authors report no conflicts of interest in this work.
References
Attal M, Harousseau JL, Facon T, et al; InterGroupe Francophone du Myélome. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349(26):2495–2502. | |
Oliansky DM, Czuczman M, Fisher RI, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the treatment of diffuse large B cell lymphoma: update of the 2001 evidence-based review. Biol Blood Marrow Transplant. 2011;17(1):20–47.e30. | |
Harousseau JL, Avet-Loiseau H, Attal M, et al. Achievement of at least very good partial response is a simple and robust prognostic factor in patients with multiple myeloma treated with high-dose therapy: long-term analysis of the IFM 99-02 and 99-04 trials. J Clin Oncol. 2009;27(34):5720–5726. | |
Stiff PJ, Unger JM, Cook JR, et al. Autologous transplantation as consolidation for aggressive non-Hodgkin’s lymphoma. N Engl J Med. 2013;369(18):1681–1690. | |
Allan DS, Keeney M, Howson-Jan K, et al. Number of viable CD34(+) cells reinfused predicts engraftment in autologous hematopoietic stem cell transplantation. Bone Marrow Transplant. 2002;29(12):967–972. | |
Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86(10):3961–3969. | |
Donmez A, Yilmaz F, Gokmen N, Tombuloglu M. Risk factors for a poor hematopoietic stem cell mobilization. Transfus Apher Sci. 2013;49(3):485–488. | |
Olivieri A, Marchetti M, Lemoli R, et al; Italian Group for Stem Cell Transplantation. Proposed definition of ‘poor mobilizer’ in lymphoma and multiple myeloma: an analytic hierarchy process by ad hoc working group Gruppo ItalianoTrapianto di Midollo Osseo. Bone Marrow Transplant. 2012;47(3):342–351. | |
Yu J, Leisenring W, Bensinger WI, Holmberg LA, Rowley SD. The predictive value of white cell or CD34+ cell count in the peripheral blood for timing apheresis and maximizing yield. Transfusion. 1999;39(5):442–450. | |
Yang SH, Wang TF, Tsai HH, Lin TY, Wen SH, Chen SH. Preharvest hematopoietic progenitor cell counts predict CD34+ cell yields in granulocyte-colony-stimulating factor-mobilized peripheral blood stem cell harvest in healthy donors. Transfusion. 2010;50(5):1088–1095. | |
Peng L, Yang J, Yang H, Peng Z, Xu C, Liu T. Determination of peripheral blood stem cells by the Sysmex SE-9500. Clin Lab Haematol. 2001;23(4):231–236. | |
Sutherland DR, Anderson L, Keeney M, Nayar R, Chin-Yee I. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. International Society of Hematotherapy and Graft Engineering. J Hematother. 1996;5(3):213–226. | |
Webb IJ, Eickhoff CE, Elias AD, et al. Kinetics of peripheral blood mononuclear cell mobilization with chemotherapy and/or granulocyte-colony-stimulating factor: implications for yield of hematopoietic progenitor cell collections. Transfusion. 1996;36(2):160–167. | |
Piccirillo N, Vacca M, Lanti A, et al. Poor mobilizer: a retrospective study on proven and predicted incidence according to GITMO criteria. Transfus Apher Sci. 2012;47(2):217–221. | |
Costa LJ, Nista EJ, Buadi FK, et al. Prediction of poor mobilization of autologous CD34+ cells with growth factor in multiple myeloma patients: implications for risk-stratification. Biol Blood Marrow Transplant. 2014;20(2):222–228. | |
Milone G, Leotta S, Indelicato F, et al. G-CSF alone vs cyclophosphamide plus G-CSF in PBPC mobilization of patients with lymphoma: results depend on degree of previous pretreatment. Bone Marrow Transplant. 2003;31(9):747–754. | |
Schwella N, Beyer J, Schwaner I, et al. Impact of preleukapheresis cell counts on collection results and correlation of progenitor-cell dose with engraftment after high-dose chemotherapy in patients with germ cell cancer. J Clin Oncol. 1996;14(4):1114–1121. | |
Suzuya H, Watanabe T, Nakagawa R, et al. Factors associated with granulocyte colony-stimulating factor-induced peripheral blood stem cell yield in healthy donors. Vox Sang. 2005;89(4):229–235. | |
Mitani N, Yujiri T, Tanaka Y, et al. Hematopoietic progenitor cell count, but not immature platelet fraction value, predicts successful harvest of autologous peripheral blood stem cells. J Clin Apher. 2011;26(3):105–110. | |
Schots R, Van Riet I, Damiaens S, et al. The absolute number of circulating CD34+ cells predicts the number of hematopoietic stem cells that can be collected by apheresis. Bone Marrow Transplant. 1996;17(4):509–515. | |
Jo JC, Yoon DH, Kim S, et al. Increment of hematopoietic progenitor cell count as an indicator of efficient autologs stem cell harvest in patients with multiple myeloma. J Clin Apher. 2012;27(5):229–234. | |
Villa CH, Shore T, Van Besien K, Cushing M. Addition of plerixafor to mobilization regimens in autologous peripheral blood stem cell transplants does not affect the correlation of preharvest hematopoietic precursor cell enumeration with first-harvest CD34+ stem cell yield. Biol Blood Marrow Transplant. 2012;18(12):1867–1875. | |
Rujkijyanont P, Hipps J, Gan K, et al. Prediction of CD34(+) cell yield in hematopoietic cell products from children by peripheral blood CD34(+) cell counts. Cytotherapy. 2012;14(4):473–482. | |
Sinha S, Gastineau D, Micallef I, et al. Predicting PBSC harvest failure using circulating CD34 levels: developing target-based cutoff points for early intervention. Bone Marrow Transplant. 2011;46(7):943–949. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.