Back to Journals » Journal of Asthma and Allergy » Volume 9
Chronic sphenoid rhinosinusitis: management challenge
Authors Charakorn N, Snidvongs K
Received 27 July 2016
Accepted for publication 9 October 2016
Published 9 November 2016 Volume 2016:9 Pages 199—205
DOI https://doi.org/10.2147/JAA.S93023
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Amrita Dosanjh
Natamon Charakorn, Kornkiat Snidvongs
Department of Otolaryngology Head and Neck Surgery, Faculty of Medicine, Chulalongkorn University and King Chulalongkorn Memorial Hospital, Bangkok, Thailand
Abstract: Chronic sphenoid rhinosinusitis is a spectrum of inflammatory diseases in isolated sphenoid sinus which may persist over a period of 12 weeks. It is a different entity from other types of rhinosinusitis because clinical presentations include headache, visual loss or diplopia, and patients may or may not have nasal obstruction or nasal discharge. Nasal endoscopic examination is useful, and computed tomography is mandatory. The disease requires comprehensive knowledge and appropriate imaging technique for diagnosis. To treat patients with chronic sphenoid rhinosinusitis, surgical treatment with endoscopic transnasal sphenoidotomy is often required. As there are no recent updated reviews of chronic sphenoid rhinosinusitis, in this article, we review the anatomy of the sphenoid sinus and its clinical relationship with the clinical signs and symptoms of the disease, the imaging findings of each diagnosis and the comprehensive surgical techniques.
Keywords: sphenoid sinus, sphenoid sinusitis, chronic, rhinosinusitis, fungal rhinosinusitis, mucocele
Introduction
Rhinosinusitis is defined as inflammation of the nose and the paranasal sinuses characterized by clinical symptoms of either nasal obstruction or nasal discharge at times accompanied by facial pain or loss of smell. Endoscopic findings may reveal nasal polyposis or mucopurulent discharge or mucosal obstruction of the meatus. Mucosal changes within sinuses may also be observed by computed tomography (CT) scan.1 Chronic rhinosinusitis is defined as clinical symptoms that last for at least 12 weeks without complete resolution.1 Because there is no clear definition for chronic sphenoid rhinosinusitis, in this article, we define chronic sphenoid rhinosinusitis as a spectrum of either infective or inflammatory diseases occurring exclusively in sphenoid sinus, either in one or both sides, persisting for more than 12 weeks. This may include bacterial rhinosinusitis, fungal rhinosinusitis and mucocele.
Isolated sphenoid lesion is an uncommon condition with an increasing incidence.2 Patients may present with nonspecific symptoms such as headache, facial pain and fullness. Nasal symptoms are frequently absent. Among the isolated sphenoid diseases, inflammatory lesions are the most common with an incidence of over 50%.3–5 They are now more frequently diagnosed due to better recognition of the disease by the primary clinician as well as improved availability of diagnostic tools such as endoscopy and imaging techniques including CT and magnetic resonance imaging (MRI). Early diagnosis with appropriate radiological evaluation and timely management are important factors to decrease the likelihood of permanent neuro-ophthalmological sequelae.3 As there are no recent updated reviews of chronic sphenoid rhinosinusitis, in this article, we review the anatomy of the sphenoid sinus and its clinical relationship with the clinical signs and symptoms of the disease, the imaging findings of each diagnosis and the comprehensive surgical techniques.
Anatomy of sphenoid sinus
The sphenoid sinus develops after birth. The pneumatization progresses at the age of 6 years and is completed by the ninth to 12th year.6 The youngest known patient who was diagnosed and reported as having sphenoiditis was 10 years old.3 The sphenoid sinus lies posteriorly in the apex of the nasal cavity. The walls of this sinus adhere to the optic canal, dura mater, pituitary gland and cavernous sinus, which contain the internal carotid arteries, and the third, fourth, fifth and sixth cranial nerves. Infection of the cavity may directly spread to these structures due to the close proximity to the sinus cavity itself, often resulting in serious consequences.
Clinical manifestation
Patients with isolated chronic sphenoid rhinosinusitis may present with nonspecific symptoms and signs. Headache is the most common presentation, presenting in over 80% of cases.4,7,8 Patients may have intermittent headaches for several years. The pain is described as either dull or sharp. It may commonly interfere with sleep and may not be relieved by analgesics.2 The typical location of headache is retro-orbital or vertex.7 Frontotemporal, fronto-orbital and hemicranial pain have been described as well.9 The mechanism of headache is believed to be the irritation of the first and second branches of the trigeminal nerve, via the nasociliary and sphenopalatine nerves, which innervate sphenoid sinus.9
Although cranial nerve involvements can be more commonly observed in sphenoid neoplasm, these presentations are not uncommon in sphenoiditis. Visual loss and other cranial nerve involvements were reported at an incidence of 12% in sphenoid rhinosinusitis presenting symptoms.10 Visual deterioration may be caused by either inflammation involving the optic nerve in the case of bacterial rhinosinusitis or mass effect in the case of fungal ball or allergic fungal rhinosinusitis.11,12
Diplopia is the result of palsy of the third, fourth or sixth nerves due to the nearby location of the sphenoid and cavernous sinuses. The abducens nerve is the most commonly affected cranial nerve in isolated sphenoid sinusitis, and this effect may occur in isolation or in combination with other cranial palsies.7,12,13
Physical examination
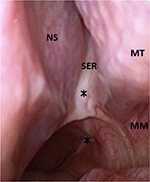
A rigid nasal endoscopy should be performed in every patient suspected of chronic sphenoiditis (Figure 1). Although some patients may not report nasal symptoms, endoscopic examination may reveal various signs of inflammatory sphenoid disease such as mucopurulent discharge, edema or polyps in the sphenoethmoidal recess.7 However, nasal endoscopy does not replace the necessity for imaging.12 It is known that a normal-looking sphenoethmoidal recess on nasal endoscopy does not exclude sphenoid pathology.7 On the other hand, the presence of mucopurulent discharge draining from the sphenoethmoidal recess should not be considered solely as bacterial infection. Advanced imaging study is still required to identify potential surgical cases.
Imaging studies
CT scan of the paranasal sinus is mandatory. It should always be performed to diagnose and provide a roadmap for sinus surgery.12 A CT scan may differentiate inflammatory disease from neoplasm and bacterial from fungal infections with the different characteristics of mucosal and bone appearance of each disease. MRI is not always necessary; it is only required when intracranial or orbital extension is suspected to delineate the interface with neighboring structures and to rule out any associated intracranial pathology.3 It may be useful in differentiating among mucoceles, benign tumors, encephaloceles and internal carotid artery aneurysm.10
Surgical management
Only a few cases of chronic sphenoid rhinosinusitis were reported in the literature as medically responsive,3 and the remaining required surgical intervention. Sphenoid sinus surgery is indicated when the diagnosis of bacterial sinusitis has been made with no clinical response to appropriate medical treatment during a certain period of 6–8 weeks and when the diagnosis of either fungal rhinosinusitis or mucocele has been made. Cases with cranial nerve involvements may require more urgent surgical treatment in order to timely restore or optimize all neural functions.
Endoscopic transnasal sphenoidotomy is acknowledged as the gold standard for surgical treatment of chronic sphenoid rhinosinusitis.3,14 In comparison to external approaches, endoscopic surgery provides better visualization and thus allows for faster healing, better esthetic outcome, lower morbidity and higher success rate.14 Sphenoid sinus can be opened with several techniques. The ostia can be directly approached. The sphenoid rostrum is approached between the middle and superior turbinate laterally and the septum medially. Unlike tumor surgery, resecting the inferior portion of the middle turbinate is usually not necessary. The sphenoid ostium is then widely opened and enlarged so that the infective or inflammatory content inside the sphenoid sinus cavity can be completely removed. Full inspection of the sphenoid sinus cavity is then performed to assure complete removal.
Some surgeons may prefer to approach the sphenoid sinus from the posterior ethmoid sinus.15 Transethmoidal approach should be performed when patients have pathology in ethmoid sinus or extensive tumor. For cases in which the ostium cannot be identified, the use of a reliable landmark is essential. Orbital walls are fixed anatomical landmarks used when the sphenoid sinus ostium is not clearly seen.16 The level of orbital floor is always below the sphenoid roof, and this indicates the level of sphenoid ostium.16 The medial orbital wall indicates the lateral wall of the sphenoid sinus including the location of the optic nerve and the internal carotid artery. Traditionally, sphenoid sinus widening in an inferomedial fashion is widely suggested because it is safer when sphenoid face is resected away from vital structures. This may not be followed if the level of the sphenoid roof, optic nerve and internal carotid artery are clearly indicated by the inferior and medial orbital walls. Maximal opening of the sphenoid sinus can be achieved when it is widened superiorly and laterally.
Diseases of chronic sphenoid rhinosinusitis
Bacterial rhinosinusitis
Bacterial rhinosinusitis is the most common pathology in isolated inflammatory sphenoid lesion. Most studies reported in the literature do not clearly define their patient as having acute or chronic infection. It may be difficult to delineate the exact duration of the presenting symptom because the most common sphenoiditis presenting symptom of headache is nonspecific and may not be exactly recognizable. However, patients with chronic bacterial rhinosinusitis may look less severely ill than those with acute infection.2 The presenting symptoms, as well as physical and endoscopic findings, may be indistinguishable from fungal rhinosinusitis or mucocele (Figure 2). CT imaging may be the key to proper diagnosis. CT scan in chronic bacterial sphenoiditis may demonstrate abnormal findings of mucosal thickening, changes in air–fluid level and partial or complete opacification (Figure 3).
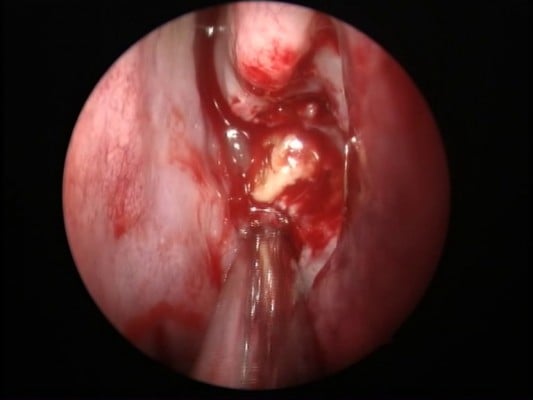
  | Figure 2 Endoscopic finding of bacterial sphenoiditis. Note: Purulent discharge from left sphenoethmoidal recess. |
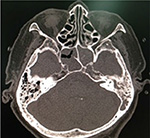  | Figure 3 CT finding of bacterial sphenoiditis. Note: The axial bone window CT demonstrates total opacification of both sphenoid sinuses. Abbreviation: CT, computed tomography. |
Once diagnosed, treatment with antibiotics for culture-based organisms is recommended.2 Staphylococci have been reported as the most common cultured pathogen. Other pathogens that have been reported include Streptococcus pneumoniae, aerobic Gram-negative bacilli (Pseudomonas aeruginosa, Klebsiella pneumoniae, Haemophilus influenzae, Escherichia coli) and anaerobes (Peptostreptococcus, Fusobacterium, Prevotella and Porphyromonas).2,4,7,17,18
Medical failure after comprehensive pharmacologic treatment with culture-based antibiotics, intranasal corticosteroids and topical decongestants within 6–8 weeks mandates surgery. However, surgical intervention may be required earlier in cases presenting with complications of cranial nerve involvements because early drainage of the sphenoid sinus may prevent persistent sequelae. Intraoperative findings of chronic bacterial sphenoiditis may include purulent discharge filled in the sinus cavity or mucosal thickening or polyps.
Fungal rhinosinusitis
Fungal rhinosinusitis can be broadly classified as invasive and noninvasive fungal rhinosinusitis. The invasive fungal rhinosinusitis can be distinguished from noninvasive disease by the presence of fungal invasion in sinus tissue from the histopathological study. Fungal rhinosinusitis is thought to comprise at least five subtypes;19 however, only three subtypes might be diagnosed as chronic sphenoiditis which include chronic invasive fungal rhinosinusitis, allergic fungal rhinosinusitis and aspergilloma. The management of each subtype,
as well as its prognosis, is different.
Aspergilloma
Aspergilloma or fungal ball is a noninvasive type of fungal rhinosinusitis. It is diagnosed when there is a dense conglomeration of hyphae and cheesy or clay-like material within the sinus cavity and when histopathology reveals chronic inflammatory mucosal response without eosinophil predominance, granulomatous response and evidence of tissue or vascular invasion.12,20 Although literature has previously reported fungal ball of sphenoid sinus as a rare entity,7,13,21 it is now more commonly diagnosed with an incidence of 10–20% in more recent case series.4,8 The sphenoid sinus is acknowledged as the second most commonly involved sinus by fungal ball.14 Patients usually have normal immunologic status. In published reports, it was more commonly diagnosed in elderly patients (over 50 years old), and female preponderance was observed.22 Nasal endoscopic examinations should be performed; however, the examinations may not give any clue to the diagnosis as negative findings were reported for all 12 patients in the series by Lee et al.22
CT scan is an excellent tool for diagnosing sphenoid fungus ball. Typically, fungal ball appears as hyperattenuating in noncontrast CT due to dense-matted hyphae. The inflamed mucosal lining of the sinus is, however, hypoattenuating. Intrasinus metallic calcifications have been reported in 50% of cases.14 The bone wall of the sinus may be sclerotic and thickened or expanded and thinned with focal areas of erosion from pressure necrosis (Figure 4). Bone erosion has been reported in 63% of fungal ball cases and is not a sign of invasive fungal rhinosinusitis.23 MRI findings show the fungal ball as hypointense on T1-weighted and T2-weighted images. Calcifications also generate areas of signal void on T2-weighted images.24
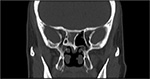  | Figure 4 CT finding of fungal ball sphenoiditis. Note: Bone window coronal CT demonstrates sclerotic bone change with intrasinus metallic calcification. Abbreviation: CT, computed tomography. |
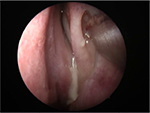
Medical treatment is not successful for aspergilloma. Surgical intervention is always indicated.12 Intraoperative findings may reveal fungal concrement with or without mucopurulent discharge (Figure 5). The mucosa of the sphenoid sinus should have normal color. Sinus mucosa should be sent for histopathology. Tissue or vascular invasion indicates invasive fungal rhinosinusitis.12
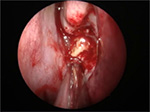  | Figure 5 Intraoperative finding of sphenoid fungal ball. Note: Fungal concrement in the sphenoid sinus. |
Allergic fungal sinusitis
Allergic fungal sinusitis (AFS) is another form of noninvasive fungal rhinosinusitis. Major criteria for diagnosis as proposed by Bent and Kuhn include the presence of nasal polyposis, characteristic sinus CT findings of heterogeneous hyperdensities that are often unilateral and asymmetric, type 1 hypersensitivity, eosinophilic mucin and positive fungal stain or culture.25 Patients are immunocompetent. AFS typically demonstrates low signal intensity of the sinus contents on T1-weighted images on MRI. The T2 signal void is also observed, due to a high concentration of various metals concentrated by the fungal organism, as well as a high protein and dry content of the allergic mucin.24 Bone erosion with bone remodeling is one of the most common clinical features of AFS which was reported in up to 50% of patients.26 Thus, AFS in sphenoid sinus can easily cause compressive symptoms of cranial neuropathies which surround the sphenoid sinus. It was reported that cranial neuropathies develop at 10% of the sphenoid AFS with bone erosion.26 Hyperprolactinemia and gynecomastia secondary to AFS compression of the pituitary gland were also reported as presenting symptoms of sphenoid AFS.27,28 To meet the criteria of diagnosis, nasal polyposis should be visible either when performing nasal endoscopic examination or during surgical treatment.
Treatment of AFS is surgery. Endoscopic sphenoidotomy with total removal of the thick mucin and fungal debris is the treatment of choice. It is essential that all sinuses involved be widely opened. In case of isolated sphenoid AFS, the sphenoid sinus should be maximally widened to optimize postoperative care and topical steroid therapy.29,30 Traditional endoscopic transnasal sphenoidotomy and traditional endoscopic sinus surgery with small-hole concept may not achieve this aim. Postoperative systemic steroids can be tapered off early if aggressive topical steroid therapy is effectively given. Histopathology should be performed with both specimens of sinus mucosa and mucin to confirm diagnosis of AFS. Immunotherapy is recommended postoperatively.
Chronic invasive fungal rhinosinusitis
Invasive fungal sinusitis is defined as fungal sinusitis in which mycotic organisms infiltrate sinus mucosa. Chronic invasive fungal sinusitis of isolated sphenoid sinus is rare. In most cases, the immunological status of patients is normal or only mildly abnormal. Presenting symptoms are often unspecific. Diagnosis should be suspected when patients have disease invasion to adjacent structures such as cranial nerve palsies or pituitary involvement. The disease can be difficult to differentiate from fungal ball by CT or MRI if there is no bone involvement. Focal or diffuse bone destruction is commonly observed. On the other hand, sclerotic bone changes are commonly noted as well.31
Diagnosis can be made from material obtained intraoperatively from the sphenoid sinus and histopathological confirmation of fungal invasion within sinus mucosa. Aspergillus sp. is reported as the most common causative agent. The gold standard for treatment is radical surgical debridement with long-term use of systemic antifungal agents. As the sphenoid sinus is adjacent to several vital structures, extensive sphenoidectomy with aggressive mucosa removal may not be easily performed. Wide sphenoidotomy with long-term use of antifungal agents is an acceptable surgical management with a satisfying result.32 Amphotericin B and voriconazole are both commonly prescribed for the treatment of invasive fungal disease.32 Intraoperative findings may not be distinguished from fungal ball; however, intraoperative mucosal necrosis may be observed as a clue for invasive nature.31 The presence of fungus ball suggests the potential of a previous fungal ball transformation into invasive fungal sphenoiditis.31 The prognosis of chronic invasive fungal rhinosinusitis is rather poor with a report of 25% mortality rate.31
Mucocele
Mucoceles are benign, encapsulated lesions filled with mucus and lined by epithelium. They are expansile and locally destructive with the ability to resorb bone, causing erosions of the bony walls of the sinus. Sphenoid sinus mucocele is quite common. It represents up to 20% of all paranasal sinus mucoceles.33 The pathophysiology of this lesion is still unknown but is believed to be caused by obstruction of the sinus ostium, cystic dilatation of glandular structures or cystic development from embryonic epithelial residues.34 Radiation exposure at head and neck area has also been postulated.33
Sphenoid sinus mucocele may be asymptomatic. It is commonly discovered accidentally after CT or MRI of the head and neck region.35 Patients with sphenoid sinus mucocele may become symptomatic when the mucocele compresses or displaces structures around sphenoid sinus. Clinical symptoms may mimic neoplasm. Thus, visual loss is more common in sphenoid sinus mucocele than in other inflammatory sphenoid diseases.
CT imaging is required to diagnose and to define the exact boundaries of the mucocele. Mucoceles may show various imaging features, depending on their contents. They often have low attenuation on CT, a low signal on T1-weighted MRI and a high signal on T2-weighted MRI, due to their watery content. There is usually no enhancement, or at most marginal enhancement, on CT and T1-weighted MRI, whereas many of the lesions in the differential diagnosis show contrast enhancement. In mucoceles with high protein content, T1-weighted MRI shows a lesion which is homogeneously hyperintense centrally with a surrounding rim of hypointense mucosa.33
Medical treatment is not a successful option for mucocele. Asymptomatic mucocele does not mandate surgical treatment and can be followed up. Mucocele requires surgical intervention when it is symptomatic, shows rapid growth or has extensive involvement of the orbit and brain.
Conclusion
The condition discussed, key diagnostic findings, recommended imaging and treatment are summarized in Table 1. Comprehensive history taking, physical examination, endoscopic examination and advanced imaging studies provide appropriate clinical diagnosis. Histopathology and microbiology are important for definite diagnosis. Endoscopic sphenoidotomy is the treatment of choice and should be performed in a timely manner to prevent permanent sequelae.
  | Table 1 Summary of chronic sphenoid rhinosinusitis Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging. |
Disclosure
The authors report no conflicts of interest in this work.
References
Fokkens WJ, Lund VJ, Mullol J, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology. 2012;50(1):1–12. | ||
Lew D, Southwick FS, Montgomery WW, Weber AL, Baker AS. Sphenoid sinusitis. A review of 30 cases. N Eng J Med. 1983;309(19):1149–1154. | ||
Nour YA, Al-Madani A, El-Daly A, Gaafar A. Isolated sphenoid sinus pathology: spectrum of diagnostic and treatment modalities. Auris Nasus Larynx. 2008;35(4):500–508. | ||
Friedman A, Batra PS, Fakhri S, Citardi MJ, Lanza DC. Isolated sphenoid sinus disease: etiology and management. Otolaryngol Head Neck Surg. 2005;133(4):544–550. | ||
Ng YH, Sethi DS. Isolated sphenoid sinus disease: differential diagnosis and management. Curr Opin Otolaryngol Head Neck Surg. 2011;19(1):16–20. | ||
Som PM, Lawson W, Fatterpekar GM, Zinreich SJ. Embryology, anatomy, physiology, and imaging of the sinonasal cavities. In: Som PM, Curtin HD, editors. Head and Neck Imaging. 5th ed. St Louis: Elsevier Health Sciences; 2011:102–165. | ||
Sethi DS. Isolated sphenoid lesions: diagnosis and management. Otolaryngol Head Neck Surg. 1999;120(5):730–736. | ||
Martin TJ, Smith TL, Smith MM, Loehrl TA. Evaluation and surgical management of isolated sphenoid sinus disease. Arch Otolaryngol Head Neck Surg. 2002;128(12):1413–1419. | ||
Leroux E, Valade D, Guichard JP, Herman P. Sphenoid fungus balls: clinical presentation and long-term follow-up in 24 patients. Cephalalgia. 2009;29(11):1218–1223. | ||
Lawson W, Reino AJ. Isolated sphenoid sinus disease: an analysis of 132 cases. Laryngoscope. 1997;107(12 Pt 1):1590–1595. | ||
Wang JK, Lin SY, Lai PC, Jou JR. Compressive optic neuropathy secondary to sphenoid sinus aspergillosis. J Neuroophthalmol. 2005;29(2):77–80. | ||
Charakorn N, Snidvongs K. Isolated oculomotor nerve palsy caused by fungal ball rhinosinusitis: a case report and literature review. Asian Rhinol J. 2016;3(1):57–60. | ||
Wyllie JW 3rd, Kern EB, Djalilian M. Isolated sphenoid sinus lesions. Laryngoscope. 1973;83(8):1252–1265. | ||
Pagella F, Matti E, De Bernardi F, et al. Paranasal sinus fungus ball: diagnosis and management. Mycoses. 2007;50(6):451–456. | ||
Stankiewicz JA. The endoscopic approach to the sphenoid sinus. Laryngoscope. 1989;99(2):218–221. | ||
Wuttiwongsanon C, Chaowanapanja P, Harvey RJ, et al. The orbital floor is a surgical landmark for the Asian anterior skull base. Am J Rhinol Allergy. 2015;29(6):e216–e219. | ||
Socher JA, Cassano M, Filheiro CA, Cassano P, Felippu A. Diagnosis and treatment of isolated sphenoid sinus disease: a review of 109 cases. Acta Otolaryngol. 2008;128(9):1004–1010. | ||
Brook I. Bacteriology of acute and chronic sphenoid sinusitis. Ann Otol Rhinol Laryngol. 2002;111(11):1002–1004. | ||
deShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337(4):254–259. | ||
deShazo RD, O’Brien M, Chapin K, et al. Criteria for the diagnosis of sinus mycetoma. J Allergy Clin Immunol. 1997;99(4):475–485. | ||
Ruoppi P, Seppa J, Pukkila M, Nuutinen J. Isolated sphenoid sinus diseases: report of 39 cases. Arch Otolaryngol Head Neck Surg. 2000;126(6):777–781. | ||
Lee TJ, Huang SF, Chang PH. Characteristics of isolated sphenoid sinus aspergilloma: report of twelve cases and literature review. Ann Otol Rhinol Laryngol. 2009;118(3):211–217. | ||
Panda NK, Balaji P, Chakrabarti A, Sharma SC, Reddy CE. Paranasal sinus aspergillosis: its categorization to develop a treatment protocol. Mycoses. 2004;47(7):277–283. | ||
Aribandi M, McCoy VA, Bazan C 3rd. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007;27(5):1283–1296. | ||
Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg. 1994;111(5):580–588. | ||
Illing EA, Dunlap Q, Woodworth BA. Outcomes of pressure-induced cranial neuropathies from allergic fungal rhinosinusitis. Otolaryngol Head Neck Surg. 2015;152(3):541–545. | ||
Menendez JY, Woodworth BA, Johnston JM Jr. Gynecomastia and hyperprolactinemia secondary to advanced allergic fungal rhinosinusitis in a pediatric patient. Turk Neurosurg. 2016;26(1):166–168. | ||
Chapurin N, Wang C, Steinberg DM, Jang DW. Hyperprolactinemia secondary to allergic fungal sinusitis compressing the pituitary gland. Case Rep Otolaryngol. 2016;2016:7260707. | ||
Snidvongs K, Kalish L, Sacks R, Sivasubramaniam R, Cope D, Harvey RJ. Sinus surgery and delivery method influence the effectiveness of topical corticosteroids for chronic rhinosinusitis: systematic review and meta-analysis. Am J Rhinol Allergy. 2013;27(3):221–233. | ||
Snidvongs K, Pratt E, Chin D, Sacks R, Earls P, Harvey RJ. Corticosteroid nasal irrigations after endoscopic sinus surgery in the management of chronic rhinosinusitis. Int Forum Allergy Rhinol. 2012;2(5):415–421. | ||
Lee DH, Yoon TM, Lee JK, Joo YE, Park KH, Lim SC. Invasive fungal sinusitis of the sphenoid sinus. Clin Exp Otorhinolaryngol. 2014;7(3):181–187. | ||
Baumann A, Zimmerli S, Hausler R, Caversaccio M. Invasive sphenoidal aspergillosis: successful treatment with sphenoidotomy and voriconazole. ORL J Otorhinolaryngol Relat Spec. 2007;69(2):121–126. | ||
Soon SR, Lim CM, Singh H, Sethi DS. Sphenoid sinus mucocele: 10 cases and literature review. J Laryngol Otol. 2010;124(1):44–47. | ||
Kosling S, Hintner M, Brandt S, Schulz T, Bloching M. Mucoceles of the sphenoid sinus. Eur J Radiol. 2004;51(1):1–5. | ||
Giovannetti F, Filiaci F, Ramieri V, Ungari C. Isolated sphenoid sinus mucocele: etiology and management. J Craniofac Surg. 2008;19(5):1381–1384. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.