Back to Journals » Research and Reports in Neonatology » Volume 7
Chorioamnionitis and neonatal morbidity: current perspectives
Authors Galán Henríquez GM, García-Muñoz Rodrigo F
Received 29 June 2017
Accepted for publication 30 August 2017
Published 9 October 2017 Volume 2017:7 Pages 41—52
DOI https://doi.org/10.2147/RRN.S128751
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Robert Schelonka
Gloria Mercedes Galán Henríquez, Fermín García-Muñoz Rodrigo
Service of Neonatalogy, Complejo Hospital Universitario Insular Materno Infantil de Canarias, Las Palmas de Gran Canaria, Spain
Abstract: The term chorioamnionitis (CA) is commonly used to refer to different clinical or pathological conditions characterized by an infectious and/or inflammatory process that affects primarily the chorioamniotic membranes, but also the amniotic fluid, vessels of the chorionic plate, and, eventually, the umbilical cord (funisitis) and the fetus. Its incidence is higher at lower gestational ages, and the main mechanism is believed to be the ascending bacterial infection from the maternal genital tract. It can be diagnosed by clinical criteria, amniotic fluid examination for inflammatory mediators, and/or isolation of microorganisms, or by histopathological examination of the placenta. CA is an important cause of stillbirth and is related to an increased incidence of premature rupture of membranes, preterm delivery, and adverse maternal and neonatal outcomes such as early-onset neonatal sepsis and necrotizing enterocolitis. An independent causal association to other neonatal morbidities is more controversial. The heterogeneity in the diagnostic criteria and in the operative definitions for morbidity makes comparison of studies difficult, and results are inconsistent. In addition, the intensity and duration of the process are usually not considered. For all these reasons, evidence-based recommendations for the management of mother and infant under these circumstances are difficult to establish, and clinical practice varies widely.
Keywords: clinical chorioamnionitis, histological chorioamnionitis, stillbirth, prematurity, morbidity, mortality
Introduction
During the past several decades, intrauterine infection has been pointed out as one of the principal causes of premature rupture of membranes (PROM) and preterm birth,1,2 increasing the risk of fetal lesions, stillbirth, and morbidity and mortality after birth, including neonatal sepsis and pneumonia, respiratory distress syndrome (RDS), intraventricular hemorrhage, periventricular leucomalacia (PVL), and necrotizing enterocolitis (NEC), among others. These morbidities, in turn, have been linked to the development of chronic conditions such as bronchopulmonary dysplasia (BPD) and cerebral palsy (CP) and developmental delay.3,4 However, a direct causal relationship between CA and these prematurity-related morbidities is still controversial, with some studies showing no effect or even opposite results. The reason for such discrepancies could be the different definitions of CA used in the studies, and the diversity of criteria to include patients. In addition, many of these studies were retrospective, and they reported crude data without considering potential confounders.
Definitions
CA is an inflammatory condition of the intrauterine environment, frequently of infectious origin, that affects the chorion, amnion, or both.5,6 It is present in 0.1%–2% of all pregnancies,7,8 but in 25%–40% of preterm births,9 or even 40%–70% in cases of PROM or spontaneous labor.10 It is more frequent at the lowest gestational age (GA)10,11 (Table 1), but it also complicates 1%–13% of term births.6 The diagnosis of CA can be made on clinical, histopathological, biochemical, or microbiological grounds, but disagreement in the criteria for diagnosis is common. A recent survey of US obstetricians found that 26% diagnose CA based only on the presence of maternal fever without taking into account any other clinical signs or symptoms.12 Frequently the inflammation is subclinical, although histological, biochemical, or microbiological findings can be present. Edwards,13 in a case series of preterm deliveries, reported a 5%–10% prevalence of clinical CA (CCA), while the prevalence of histological CA (HCA) was >50%. Likewise, in a personal observation (data not published) we also showed a high discrepancy between clinical and histological diagnosis of CA (Table 2).
  | Table 1 Distribution of clinical and histological chorioamnionitis according to gestational age at delivery Notes: aData from García-Muñoz Rodrigo11; N=8,330 (in this study, only patients ≤32 weeks gestational age were included); bpersonal observation (data not published), N=86. |
  | Table 2 Correlation between clinical and histological chorioamnionitis in a series of 84 anatomopathological studies of placentas from gestations ≤34 weeks (personal observation) Notes: aAccording to Redline criteria;19 badapted from Gibbs criteria;14 sensitivity = 7/37 = 18.9%; specificity = 45/47 = 95.7%; positive predictive value (PPV) = 7/9 = 77.8%; negative predictive value (NPV) = 45/75 = 60%. |
Clinical diagnosis of CA is most frequently based on criteria adapted from Gibbs,14,15 consisting of maternal fever >38°C in at least two occasions separated by 1 hour, plus two or more of the following: uterine tenderness defined as pain referred by the mother on abdominal examination in the absence of uterine contractions, leukocytosis (>15,000 cells/mm3), maternal tachycardia (>100 bpm), fetal tachycardia (>160 bpm), or foul smelling vaginal discharge. However, the term CCA is mainly used to express clinical suspicion before there is any laboratory or histological confirmation of the inflammation/infection, and there is growing concern about the excessive use of this term. To improve decision-making in caring for mothers and newborns, and to unify definition criteria, in 2016 an expert panel of the American College of Obstetricians and Gynecologists (ACOG) proposed the use of the term “intrauterine inflammation or infection or both” (Triple I) with the following criteria, maternal fever with one or more of the following: 1) fetal tachycardia (>160 bpm for 10 minutes or longer); 2) maternal white blood cells (WBC) count >15,000 in absence of corticosteroids; 3) purulent fluid from the cervical os (cloudy or yellowish thick discharge confirmed visually on speculum exam to be coming from the cervical canal); and 4) biochemical or microbiological amniotic fluid (AF) results consistent with microbial invasion of the amniotic cavity (MIAC).5 When there is an objective finding of AF infection or histopathological evidence of infection/inflammation of the placenta, membranes, or the umbilical cord, Triple I is confirmed. Otherwise, it should be categorized as “suspected.” More recently, an ACOG Committee Opinion stated that “the diagnosis of suspected intraamniotic infection is made when the maternal temperature is greater than or equal to 39.0°C or when the maternal temperature is 38.0–38.9°C and one additional clinical risk factor is present,” and “isolated maternal fever is defined as any maternal temperature between 38.0°C and 38.9°C with no additional risk factors present, and with or without persistent temperature elevation.”16
The microbiological diagnosis is based on the isolation of the causative microorganism from culture of AF obtained by amniocentesis, but this procedure is seldom performed during labor, when symptomatology usually begins, and the results can be delayed for some days. This makes the microbiological diagnosis less useful for decision-making during labor. It is estimated that the frequency of positive AF cultures for bacteria in women with labor and intact membranes is 12.8%, and 37.5% of them develop CCA. In contrast, in cases of preterm PROM a positive AF is found in 32.4%, and CCA will develop in 29.7% of them.17 Another cause of underestimation of the rate of AF infections is that amniocentesis is less frequently carried out in women with oligohydramnios and in those admitted with labor, who usually have higher rates of infection in comparison to women admitted without labor.18
From a histological point of view, acute CA is characterized by diffuse infiltration of neutrophils into the chorioamniotic membranes19 as a result of intra-amniotic inflammation, although not always of bacterial origin. The process begins in the subchorionic fibrin and at the membranous choriodecidual interface, extends to the fibrous chorion and amnion, and finally necrosis of the amniotic epithelium occurs.20 Then, acute funisitis develops with involvement, in first instance, of the umbilical vein (phlebitis), which exhibits a higher expression of interleukin (IL)-8 mRNA and a pattern of gene expression suggestive of a greater predisposition to a proinflammatory response in comparison to arteries. Thereafter, polymorphonuclear (PMN) cells infiltrate arteries (arteritis), and the concentration of umbilical cord IL-6 increases, which is a marker of severity and is associated with a poorer infant prognosis. Finally, PMN cells infiltrate the Wharton’s jelly (funisitis), initially as multifocal rings that subsequently coalesce as the process advances.21 The origin of the WBC present in the AF of patients with CCA is mostly fetal.22 An in-depth discussion of the histological aspects and the gradation of the inflammatory process that occurs during chorioamnionitis are beyond the scope of the present work. We refer the reader to the comprehensive chorioamnionitis grading and staging system as proposed by Redline and colleagues.19 Interestingly, a recent study showed the correlation between MMP-8 concentration in the AF and the severity of HCA.23
Pathophysiology
The principal mechanism of CA is ascending bacterial infection after PROM, but it may also occur with intact membranes. Four stages have been described.18 In the first one, the normal vaginal and cervical microbial flora changes and/or pathological bacteria appear. Then (second stage) bacteria move toward the lower pole of the uterus, between the membranes and the chorion. Bacteria from the lower genital tract and their products are sensed by pattern recognition receptors, such as toll-like receptors (TLRs). Cervical dilatation and shortening (cervical incompetence) has also been associated with a higher rate of AF infections, but it is unclear whether such incompetence is the cause or consequence of the infection.24 In the third stage, bacteria invade the fetal vessels (choriovasculitis) or cross the amnion (amnionitis) into the amniotic cavity, stimulating the production of inflammatory mediators (prostaglandins and reactive oxygen radicals) and different proteases, like MMP. Finally, microorganisms reach the fetus through the respiratory or gastrointestinal tract, or through the mucous membranes (tympanic or conjunctiva). Other possible mechanisms, although less frequent, are hematogenous spread, invasion from the peritoneal cavity via the fallopian tubes, or accidental inoculation of bacteria during invasive procedures.
It is important to note that perinatal infection/inflammation, even if bacteria are not isolated, may lead to the fetal inflammatory response syndrome (FIRS), which is an endocrinological stress response with increased levels of fetal cortisol25 and fetal cellular and humoral immune activation in response to the exposure to microorganisms and/or their endotoxins, with release of cytokines and chemokines (IL-1, IL-6, IL-8, tumor necrosis factor [TNF]–α, MMP) and upregulation of leucocyte adhesion molecules (ICAM1, E-Selectin, VCAM-1).26,27 FIRS has been defined as a rise in fetal plasma concentrations of IL-6 >11 pg/mL.28 Patients with FIRS exhibit funisitis and chorionic vasculitis,29 and have an increased risk of neonatal sepsis,30 BPD, 31 and CP.32 FIRS also affects the hematopoietic system inducing the activation of the mononuclear phagocytic system with neutrophilia and neutrophil and monocyte activation. The elevation of IL-6 stimulates the increase in the number of nucleated red cells.33 An involution of the fetal/neonatal thymus has also been observed,34 even in cases of subclinical CA.35 The grade of thymic involution may be proportional to the duration of the inflammatory process,36 and it has been associated with lesions of the brain white matter.37 In addition, the bacterial products and the inflammatory mediators released during FIRS might induce myocardial depression in the fetus.38 Given its poor ability to maintain cardiac output, this leads to hypotension and cerebral hypoperfusion, which further contribute to brain damage.39 In the skin, FIRS leads to dermatitis with increased expression of TLR-2 in epidermal keratinocytes of fetuses whose mothers had CA.40
Microbiology
The AF is sterile under normal circumstances. When microbes are detected by culture or, more recently, by molecular techniques,41 the condition has been termed MIAC.17 The prevalence of MIAC varies widely according to the clinical condition (term or preterm birth, intact membranes or not, with or without labor, presence of intra-uterine device, etc.).42 In a study of women with CCA at term, the detection of microorganisms by conventional cultures and/or molecular techniques was 61%.43 In this study, intra-amniotic inflammation was defined as a concentration of IL-6 in AF ≥2.6 ng/mL. Although 54% of the AF cases studied showed both microorganisms detection and inflammation, interestingly, 24% had inflammation without bacterial isolation, 6.5% only bacterial detection without elevation of IL-6, and, finally, 15% neither bacteria nor inflammation. Of note, HCA was significantly more frequent in patients with microbial-associated intra-amniotic inflammation than in those without intra-amniotic inflammation (70.8% [17/24] vs 28.6% [2/7]; P=0.04).
Frequently, MIAC is polymicrobial, with two or more bacteria isolated in the AF. Genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis) are the most frequent agents recovered. Other microorganisms include anaerobes (Gardnerella vaginallis and Bacteroides sp.), Fusobacteria sp., aerobes (Group B Stretpococcus), and Gram-negative rods (Escherichia coli). In the rare occasions where pregnancy takes place in women with intrauterine contraceptive devices, these have been pointed out as a risk for Candida albicans infection.44
Morbidity
Preterm birth
Infection is probably the only pathologic process clearly related to prematurity, and the molecular mechanisms involved have been widely studied.45 It is estimated that ~25%–40% of preterm births are related to intrauterine infections,2 and the earlier the gestational age at delivery, the higher the frequency of infection, with fetuses of 23 weeks GA colonized in up to 79% of cases in some studies.46 Furthermore, these figures may well underestimate the actual rate of intra-amniotic infections, since many of the pathogens involved, as mentioned before, do not grow in the usual cultures. The use of molecular microbiology techniques allows the isolation of DNA from bacteria in the AF of women with preterm labor in whom the conventional culture is negative.47
Preterm birth can also be triggered by changes in the normal vaginal flora (bacterial vaginosis), but although antimicrobial therapy can eradicate these bacteria, it has not proven to be useful to stop preterm birth,48 which supports its multifactorial origin.49 A mechanism of resistance of microorganisms (bacteria and fungi) to antibiotics was postulated by Donlan and Costerton50 consisting in the creation of biofilms in which they are encapsulated and protected from those. This type of biofilm has been described, among others, in the urinary epithelium and in the amniotic fluid,51 but its contribution to the onset of preterm birth is unknown. On the other hand, it is also important to point out that the presence of bacteria and/or inflammatory cells in the fetal membranes does not always cause premature labor.52
The inflammatory response associated with CA leads to the rupture of membranes or preterm labor by different mechanisms, including cytokine and MMP activation causing direct rupture of membranes, triggering apoptosis of the fetal membranes cells, activation of the extrinsic pathway in response to toxins and/or cytokines (TNF-α, IL-1ß, etc.), or activating cell mediators, such as prostaglandins, that directly induce uterine contractions. A comprehensive review of the role of inflammation and infection in preterm birth can be found in the paper by Romero et al.53
Infection-related complications
Most studies in this field have revealed a positive association between both CCA and HCA and the risk of an early-onset neonatal sepsis (EONS),11,54–60 and this association persists after adjusting for GA, birth weight (BW), and other confounders. Fetal infection is the most advanced stage of an ascending intrauterine infection, and bacteremia have been detected in 33% of fetuses with positive AF cultures, in contrast to only 4% of fetuses with AF negative cultures.61 It is estimated that about 1.5% of very low birth weight (VLBW) infants will be affected by an EONS, while 21% of them will develop at least an episode of late-onset neonatal sepis (LONS).62 Maternal infections transmitted before or during delivery seem to be the origin of EONS, while LONS are more frequently associated to pathogens acquired from nosocomial environment.63–65 In cases of PROM, a recent systematic review has shown that maternal administration of broad-spectrum antibiotics prolongs pregnancy, and decreases the risk of chorioamnionitis and neonatal infection and, potentially, its related adverse fetal and neonatal outcomes.66 Unfortunately, even with the administration of prenatal antibiotics, several studies showed that CA is still related to a 2- to 10-fold increase in the risk of EONS,11,59,64,65,67 and the risk seems to be higher when funisitis is present.30 In addition, the 7-year follow-up of children included in the ORACLE II trial found that the prescription of erythromycin for women in spontaneous preterm labor with intact membranes was associated with an increase in functional impairment among their children, and the risk of cerebral palsy was increased by both erythromycin and co-amoxiclav, although the overall risk was low.68
Interestingly and in the opposite direction, two recent studies on CCA and HCA, respectively, have shown a reduction in the risk of LONS.11,60 The authors speculated that early stimulation of the immune system from intrauterine exposure to less virulent pathogens, rather than to the more aggressive bacteria frequently associated with EONS, or the transfer of maternal cell mediators might be the possible explanation of this phenomenon. However, this hypothesis is now being challenged by the recent finding of an association between CA with aberrant neonatal gut colonization and a significant increase in the risk of the combined outcome of LONS and/or death.69
Regarding the future impact of perinatal infection in these patients, it is noteworthy that numerous epidemiological studies have demonstrated an association between systemic inflammation in the fetus/newborn of intrauterine or postnatal origin and brain damage,70–74 which will be discussed in more detail in the following section.
Necrotizing enterocolitis
NEC is less common than other complications in the preterm newborn, but remains a relevant problem for the VLBW infant due to its potential severity and its adverse consequences in both the short and long term. Although believed to be multifactorial, the exact mechanism of its development is not fully understood, and its incidence has not changed substantially over time.75 It affects around 7% of neonates with BW 500–1,500 g, and 11.5% in the subgroup of ≤750 g.76 Mortality varies between 20% and 30%, and it is higher in patients requiring surgery.77,78 Evidence relating CA to NEC is not consistent, but several studies, summarized in the meta-analysis of Been et al,79 support that CCA is significantly associated with NEC (odds ratio [OR] 1.24, 95% CI 1.01–1.52; P=0.04), as well as HCA with fetal involvement (OR, 3.29; 95% CI, 1.87–5.78; P≤0.001). In addition, a recent study showed that, after adjusting for GA, a high grade of fetal inflammation was significantly associated with chronic lung disease and necrotizing enterocolitis.80 Of note, in the context of PROM and subclinical infection, it is well known that maternal antibiotic therapy might lessen infectious morbidity and delay labor, but co-amoxiclav should be avoided because of the increased risk of NEC (risk ratio [RR] 4.60, 95% CI 1.98–10.72).81
NEC basically consists of ischemic necrosis of the intestinal mucosa with important inflammation and invasion by gas-forming microorganisms, which can dissect the muscular layer of the mucosa and reach the portal venous system. However, the clinical spectrum varies from this “classic” form to focal intestinal perforations with less inflammatory component that probably have a different etiopathogenesis.82 The lack of consistent diagnostic criteria makes it difficult to compare results among studies. However, the gradation system of Bell et al in 1978,83 with some subsequent adaptations84 despite some possible limitations, has been very useful for decision-making with patients and to compare results between different centers. NEC is the first cause of short bowel syndrome in infancy. Apart from the potentially devastating local effects, the intense inflammatory processes that accompany its course have systemic repercussion, with damage to distant organs such as the brain. This adds to an increased risk of neurodevelopment impairment in this already vulnerable group of patients.85,86 Martin et al recently reported an increased risk of neurodevelopmental dysfunction and microcephaly in patients with surgical NEC, especially when associated with late bacteremia.87 Finally, a systematic review aimed at characterizing the neurodevelopment of extremely-low-birth-weight infants (<1,000 g) with NEC and the possible relationship between NEC stage and the neurosensory outcomes found 10 studies comparing patients of the same GA with and without NEC and an average follow-up of 20 months.88 Globally, 45% of infants with NEC had some form of neurodevelopment impairment. Table 3 summarizes the main results of this study.
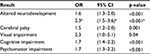  | Table 3 Risk of neurodevelopment and neurosensorial impairment in VLBW infants with NEC in comparison to patients of the same GA without NEC Note: Data from Rees et al;88 ain patients with Bell’s stage 3. Abbreviations: VLBW, very low birth weight; NEC, necrotizing enterocolitis; GA, gestational age; OR, odds ratio. |
Respiratory morbidity
Again, the relationship between CA and respiratory morbidity in the preterm infants, although extensively studied, remains controversial. Fetuses make breathing movements in utero and may aspirate AF that reaches the distal airway and the alveoli. In Watterberg et al’s study,89 infants prenatally exposed to HCA had greater concentrations of IL-1β in tracheal aspirates from the first day of intubation and exhibited less respiratory distréss syndrome (RDS), but thereafter they developed more frequently BPD. In animal experimentation, a maturative effect of intra-amniotic administration of IL-1α has also been observed,90 with increased expression of surfactant proteins A and B, and lipids. Likewise, Ghezzi et al studied the exposure of fetuses to subclinical intrauterine inflammation and found elevated concentrations of IL-8 in AF of mothers of 24–28 weeks who subsequently developed BPD.91 All these studies support the hypothesis that HCA could stimulate lung maturation, reducing the incidence of acute RDS but, at the same time, increasing the lung susceptibility to postnatal damage, which finally leads to BPD. The maturational effect of prenatal inflammation may be accompanied not only by structural changes in the lungs,92 but also by an alteration in the response to exogenous surfactant and the need of prolonged mechanical ventilation.93 Nevertheless, it is important to remember that prematurity itself is characterized by lungs with a smaller number of alveoli, a slow microvascular development, and thickening of the arteriolar walls. Other mechanisms, such as a neonatal leukemoid reaction induced by CA, have also been suggested as important risk factor for BDP.94,95 In contrast to HCA, Alexander et al reported an increased incidence of RDS among preterm babies exposed to CCA. Of note, many of these studies were conducted prior to the widespread use of prenatal corticosteroids for fetal lung maturation, exogenous surfactant administration, and/or noninvasive ventilation, which are currently considered standard practices with proven benefit for patients and improved respiratory outcomes.96 In summary, although unadjusted and adjusted analyses showed that CA was significantly associated with BPD, a recent systematic review and meta-analysis by Hartling et al concluded that CA cannot be definitively considered a risk factor for BPD due to the strong evidence of publication bias with potential overestimation of the association between them97 (Table 4).
  | Table 4 Characteristics of studies evaluating the association between chorioamnionitis and neonatal morbidity Notes: aData available only for CA cases. Abbreviations: CCA, clinical chorioamnionitis; HCA, histological chorioamnionitis; NEC, necrotizing enterocolitis; BPD, bronchopulmonary dysplasia; PDA, patent ductus arteriosus; CP, cerebral palsy; ROP, retinopathy of prematurity; EONS, early-onset neonatal sepsis; LONS, late-onset neonatal sepsis; GA, gestational age; CPR, cardiopulmonary resuscitation; RDS, respiratory distress syndrome; IVH, intraventricular hemorrhage; PVL, periventricular leukomalacia; VLBW, very low birth weight; I2 and P, heterogeneity; RR; risk ratio; OR, odds ratio; CRIB I, Clinical Rosk Index for Babies I. |
Patent ductus arteriosus
The relationship between the exposure to CCA or HCA and patent ductus arteriosus (PDA) is again conflicting. Most studies seem to show a positive relationship when crude data are analyzed.98 In contrast, in our large multicenter study, after adjusting for confounders, we found that the risk of PDA was significantly lower among infants exposed to CCA (adjusted OR 0.831, 95% CI 0.711–0.971; P=0.02).11 However, a recent meta-regression analysis by Behbodi et al99 concluded that the differences in GA or BW between the CA-exposed and nonexposed groups were significantly correlated with the effect size of the association between CA and PDA and, consequently, CA appears not to be a risk factor for PDA in preterm infants (Table 4).
Brain injury
In 1955, Eastman Eastman and Deleon first observed that intrapartum maternal fever increased seven times the risk of CP.100 Subsequently, this same phenomenon was observed in VLBW infants born to mothers with CA.101 Since then, numerous studies have tried to delve into the mechanisms by which the infection/inflammation produces brain damage. However, neither clinical trials nor large meta-analyses have yielded homogeneous results.102 Although germinal matrix HIV is the most frequently acquired brain lesion in VLBW infants diagnosed by cerebral ultrasound (CUS), evaluation at discharge with magnetic resonance imaging (MRI) has shown that brain white matter damage (WMD) is much more common, affecting at least 50% of very preterm infants.103,104 This injury is probably the leading cause of deficits observed in ~90% of survivors, including CP (5%–10%) and cognitive, attention, and behavioral disorders (about 50%).105,106 Although other lesions, such as periventricular hemorrhagic infarction, post-hemorrhagic dilatation, or cerebellar lesion, may coexist, WMD seems to be the predominant one.107 Two major factors are involved in the etiology of WMD, ischemia and infection/inflammation, along with the marked vulnerability of oligodendroglia to oxidative stress. The cerebral vasculature of the preterm infant is characterized by “borderline” zones,108 where cerebral blood flow (CBF) is more vulnerable to decreases in blood pressure. In addition, sick preterm infants have a lower capacity for CBF autoregulation, making the brain more susceptible to diffuse ischemic damage.109,110 Hypoxia-ischemia can lead to cytokine elevation even in the absence of infection.111 Infection causes damage or death of oligodendroglial precursors,107 mediated by proinflammatory cytokines, especially interferon-γ and TNF-α,112 and by the activation of the microglia in the immature brain white matter, with the subsequent production of free radicals.113
Some clinical and epidemiological studies have shown an association between maternal-fetal infection and PVL (detected by CUS), CP,114,115 and/or sensorineural involvement (visual impairment, hearing loss, and/or speech delay) during follow-up.116,117 Among extremely low GA neonates (<27 weeks), Pappas et al showed that infants exposed to CA had a lower GA, and that HCA alone not, but HCA plus CCA increased the risk of cognitive impairment at 18–22 months’ corrected age (adjusted OR 2.00, 95% CI 1.10–3.64).118 However, as stated before, CA is strongly associated with preterm birth and, after adjusting for GA, the association between CA and PVL decreases or disappears. For this reason, a causal relationship between them has been questioned by some.119 Yliloki et al recently reported the lack of association between CCA and neurodevelopmental problems in very preterm infants.120 In contrast, HCA was associated with a slightly less optimal performance at 5 years of age, although, in their opinion, further studies are needed to verify the clinical significance of these findings. Previously, in a systematic review by the same group that included 84 original papers, they concluded that the majority of the studies did not support the hypothesis that CA poses a direct risk on the central nervous system of preterm infants.121 Similar conclusions seem to be derived from most recent reviews.122,123
An additional problem when evaluating the relationship between CA and WMD is that most of the initial studies, especially epidemiological ones, referred to cystic PVL, which is currently estimated to contribute to less than 10% of WMD cases. Modern technologies could provide new insight into the subject. In a recent study, magnetic resonance spectroscopy in healthy term newborns exposed to maternal CA showed evidence of occult neuroinflammation, and the author suggested that this finding might be associated to neurodevelopmental outcomes at 12 months.124 Another study with MRI in patients exposed to CA revealed long-term alterations in brain morphology in areas related to cognitive and motor functions, leading to psychiatric and neurodevelopmental disorders, although this study was limited by the small sample size.125 In summary, to date, the relationship between CA and neurodevelopmental outcomes is not completely understood, and the studies’ results might be influenced by variables that covariate with or mediate the effect of the other.
Mortality
Perinatal mortality related to maternal CA has been addressed in several studies, but results are still conflicting. Elimian et al found that HCA was independently associated with neonatal death, although in exposed infants the administration of antenatal steroids significantly reduced morbidity and mortality.126 The risk of neonatal mortality seems to be higher when there is evidence of fetal inflammation (funisitis and/or fetal vessel angiitis).127 In a population-based study of full-term infants born in the United States during 2008, the prevalence of CCA was 9.7 per 1,000 live births, and the neonatal mortality rate was higher among exposed infants (OR 1.72, 95% CI 1.20–2.45).7 In contrast, several studies on CCA or HCA in VLBW infants, after correcting for confounders, showed no effect on mortality11,60 or, on the contrary, they showed a protective effect.128,129 In the study by Lahra et al, both HCA and a histological fetal response to CA were observed to be more common in preterm survivors of the neonatal period.130 In the same direction, Pappas et al found that HCA was associated with a reduced risk of death or neurodevelopmental impairment (adjusted OR 0.72, 95% CI 0.56–0.93]).118
Conclusion
Although some experimental evidence in animal models and the results of human research have demonstrated or suggest adverse effects of intra-amniotic infection/inflammation in the fetus/newborn, controversy still persists regarding a direct relationship between maternal CA and most of the neonatal outcomes studied. Table 4 summarizes the most relevant systematic reviews and meta-analyses. To date, the association between CA and preterm birth and EONS and NEC seems to be clear. Prematurity itself is a major risk factor for many different morbidities known to be of multifactorial origin. Prenatal infection/inflammation or both could have a modulating effect over them in both directions, sometimes increasing the final risk while sometimes reducing it, as it seems to be the case for RDS, for instance. The heterogeneity of the studies and the variety of clinical situations and management of patients in a changing field of knowledge (prenatal steroids, maternal antibiotics, postnatal surfactant administration, noninvasive ventilator support, etc.) precludes establishing a definitive relationship between CA and a particular morbidity. In the opinion of the authors, as new diagnostic tools develop and as new strategies of patient management are implemented, new associations could be detected or, on the contrary, some current beliefs will vanish.
Disclosure
The authors report no conflicts of interest in this work.
References
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
