Back to Journals » Infection and Drug Resistance » Volume 11
Chlorhexidine-based body washing for colonization and infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an updated meta-analysis
Received 9 April 2018
Accepted for publication 11 June 2018
Published 13 September 2018 Volume 2018:11 Pages 1473—1481
DOI https://doi.org/10.2147/IDR.S170497
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Guibao Xiao,1,* Zhu Chen,2,* Xiaoju Lv1
1Center of Infectious Diseases, West China Hospital of Sichuan University, Chengdu, Sichuan Province, People’s Republic of China; 2Public Health Clinic Center of Chengdu, Chengdu, Sichuan Province, People’s Republic of China
*These authors contributed equally to this work
Background: The effects of chlorhexidine-based body washing (CHW) on health care-associated infections have been reported in numerous studies, while their findings remain conflicting. This study aims to update the evidence for the effects of CHW on the risk of colonization or infection with hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE).
Methods: Two independent authors searched PubMed, Embase, and Cochrane Library from inception through February 2018. We selected all observational studies or clinical trials for the effect of CHW on the risk of colonization and infection with hospital-acquired MRSA or VRE. Random-effects models were applied to calculate summary incidence rate ratios (IRRs) for the related associations.
Results: Of 140 records identified, we obtained data from 17 relevant articles for meta-analysis. Compared with patients without antiseptic bathing, patients with CHW had a significantly lower risk of MRSA colonization (IRR 0.61, 95% CI 0.48–0.77) and VRE colonization (IRR 0.58, 95% CI 0.42–0.80). Similarly, we also noted that patients with CHW had a significantly lower risk of MRSA infection (IRR 0.65, 95% CI 0.52–0.81). However, no significantly lower risk of VRE infection (IRR 0.61, 95% CI 0.30–1.25) was noted in patients with CHW. Sensitivity analyses or trim-and-fill method confirmed the robustness of the findings.
Conclusion: Current evidence supports that patients with CHW had a significantly lower risk of MRSA or VRE colonization and a lower risk of MRSA infection. More evidence should be accumulated to reinforce these findings, especially on the effect of CHW on the risk of VRE infection.
Keywords: chlorhexidine, methicillin-resistant Staphylococcus aureus, MRSA, vancomycin-resistant Enterococcus, VRE, bathing, meta-analysis
Introduction
Over the past few decades, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) have become two of the commonest causes of health care-associated infections (HAIs), occurring mostly among individuals with diagnosed health care-associated status such as hospitalization, surgical interventions (eg, central venous catheters), and dialysis. It is estimated that more than 100,000 HAIs occur in USA annually.1 These two kinds of infections frequently lead to increased length of hospital stay, patient morbidity and mortality, and substantial cost burden to the health care system.2
Chlorhexidine gluconate (CHG) has a broad-spectrum antibacterial activity, especially for Gram-positive bacteria such as MRSA and VRE. It has been reported that CHG can reduce the overall bioburden of multidrug-resistant Gram-positive organisms, thus reducing the incidence of HAIs and transmissions.3,4 Several epidemiological studies showed that daily use of CHG could reduce the rate of MRSA or VRE acquisition and bloodstream infections associated with these organisms5–8 in the intensive care units (ICUs) and general medicine units.9 However, several other studies have reported neutral findings that do not support using daily CHG bathing.10,11 There is also a lack of randomized clinical trials to provide direct evidence for the effect of CHG bathing on the risk of MRSA and VRE colonization or infection. With these dubious results, we aimed to reevaluate the existing uncertain evidence regarding this issue by updating the systematic review and meta-analysis of all published data.
Methods
Literature search
This meta-analysis was conducted under the guidance of a 27-item checklist of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). We searched PubMed, Embase, and the Cochrane Library on February 1, 2018. The following words were searched as keywords and text words: (shower* OR bath* OR wash* OR cleans*) AND (chlorhexidine OR chlorohex* OR eludril* OR corsodyl* OR Periochip* OR CHX OR nolvasan* OR sebidin* OR tubulicid* OR Cervitec* OR Chlorzoin* OR hibitane*) AND (“methicillin-resistan* OR meticillin-resistan* OR MRSA OR EMRSA OR MDRO” OR “vancomycin resistant enterococc* OR VRE”). We did not restrict language or publication type. Gray literature including abstracts was also included. The bibliographies of relevant articles were manually searched for additional references that may have been missed in the database searches. The search strategies for the three databases are given in the “Supplementary materials”.
Study selection and eligibility criteria
Eligible studies were included if they satisfied the following inclusion criteria: 1) studies investigating the associations between the use of chlorhexidine-based body washing (CHW) and the risk of colonization or infection with hospital-acquired MRSA or VRE; 2) cluster-randomized trial, before-and-after study, quasi-experimental study, interrupted time series study, and sequential group single-arm clinical trial were applied as study designs; and 3) studies or trials reported incidence rate ratios (IRRs) and their 95% CIs or related data for the calculation of their IRRs. Studies were excluded if they did not satisfy the inclusion criteria. Two investigators (G.X. and Z.C.) independently conducted literature search and selection. We selected the largest studies with the most comprehensive data or analyses when overlapping studies were included.
Data extraction
Data extraction was carried out by two investigators (G.X. and Z.C.), independently using a Microsoft excel spreadsheet (2010 professional edition; Microsoft Corporation, Redmond, WA, USA). The extracted data were then cross-checked and determined by a senior investigator (X.L.). Data extracted included first author, publication year, study design, patient selection, study setting, major intervention, and control intervention. The corresponding authors of original studies were consulted for missing information if necessary.
Study bias assessment
Two authors (G.X. and Z.C.) independently assessed study bias using the Cochrane Risk of Bias Tool. The study was scored as low, unclear, or high risk of bias for randomized controlled trials based on random sequence generation, allocation concealment, blinding of participants, blinding of outcome assessment, incomplete data, selective reporting, and others.12 For nonrandomized studies, we used the Newcastle–Ottawa Scale (NOS) to assess the methodological bias, encompassing participant selection, comparability, and outcome assessment.13 A total of nine stars were assigned for each study with a score of ≥6 representing high quality.
Statistical analyses
IRR was set as the effect size measure. The summary effect size was pooled using a random-effects model because we considered that the different patients included in different regions during different periods with different study designs were very likely to have substantial heterogeneity. The Q test was applied to assess the existence of heterogeneity and I2 statistic to quantify the percentage of between-study heterogeneity, with a value being <0.10 considered as statistically significant.14 Funnel plot, Begg’s test, and Egger’s test were used to judge for publication bias.15,16 Furthermore, we also used the Duvall and Tweedle trim-and-fill model to adjust risk estimates,17 which imputes effect sizes until the error distribution closely approximates normality; such a procedure provides a more unbiased estimate of the effect size than does the observed estimate. All meta-analyses were conducted and figures were generated using Stata version 12.0 (StataCorp LP, College Station, TX, USA).
Results
Literature search
The database literature search yielded a total of 146 citations, and after removal of duplicates, 132 individual citations remained. After screening by title or abstract, 31 articles were identified for full text review. Finally, a total of 17 articles met the inclusion criteria. A manual reference search of included articles yielded no additional article that met inclusion criteria (Figure 1). Four articles identified in the original search were excluded because the data were insufficient for meta-analysis. We contacted the corresponding authors to request the original data; however, none of the primary data were available for meta-analysis.
  | Figure 1 Flow diagram of articles selected for inclusion in the meta-analysis. |
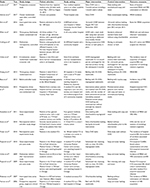
Seventeen individual articles (four cluster-randomized trials, four quasi-experimental studies, three before–after interventional studies and six nonrandomized controlled trials or observational studies) were included in this systematic review and meta-analysis.5,9–11,18–30 In total, 467,484 participants were analyzed, of whom 247,605 received intervention with CHW and 219,879 were not exposed to CHW intervention. Eight studies reported data from ICUs, and the others provided data from patients in mixed departments. Ten studies were conducted in multicentered institutions, and seven studies were carried out on single hospital sites. Details of the included studies are presented in Table 1. Generally, most of the nonrandomized trials had a low risk of bias with the NOS score ranging from 7 to 9, while most of the randomized trials have a high risk of bias, especially in the aspects of blinding method of participants and outcome assessment (refer the “Supplementary materials”).
Results of meta-analyses and publication bias assessment
CHW and MRSA colonization
Nine studies investigated the association between CHW and MRSA colonization, which included 438 events in the intervention group and 660 events in the control group among 322,053 participants. Meta-analysis showed that the summary IRR was 0.61 (95% CI 0.48–0.77, I2=60.9%, P<0.001 for heterogeneity; Figure 2A). There was no evidence of publication bias using the Begg’s (P=0.917) or Egger’s test (P=0.817). The results did not change after using the trim-and-fill method when no missing studies were added (Table 2).
CHW and MRSA infection
Ten studies investigated the association between CHW and MRSA infection, which included 137 events in the intervention group and 193 events in the control group among 370,422 participants. Summary estimates showed that the pooled IRR was 0.65 (95% CI 0.52–0.81, I2=0%, P=0.723 for heterogeneity; Figure 2B). There was no evidence of publication bias using the Begg’s test (P=0.592) or Egger’s test (P=0.896). The results did not change after using the trim-and-fill method when no missing studies were added (Table 2).
CHW and VRE colonization
Eight studies investigated the association between CHW and VRE colonization, which involved 195 events in the intervention group and 296 events in the control group among 201,556 participants. Meta-analysis showed that the pooled IRR was 0.58 (95% CI 0.42–0.80, I2=53.8%, P=0.034 for heterogeneity; Figure 2C). There was no evidence of publication bias using the Begg’s test (P=1.000) or Egger’s test (P=0.617). The results did not change after using the trim-and-fill method when no missing studies were added (Table 2).
CHW and VRE infection
Six studies investigated the association between CHW and VRE infection, which included 20 events in the intervention group and 37 events in the control group among 153,965 participants. Summary estimates showed that the pooled IRR was 0.61 (95% CI 0.30–1.25, I2=30.6%, P=0.206 for heterogeneity; Figure 2D). There was no evidence of publication bias using the Begg’s test (P=0.707) or Egger’s test (P=0.983). The results did not change after using the trim-and-fill method when no missing studies were added (Table 2).
Sensitivity analyses
Sensitivity analyses by excluding one study at a time from each analysis indicated that all the four meta-analysis results seemed to be robust to the influence of individual studies (Figure 3). The results were also not substantially altered when combining studies with the same study design (data not shown).
Discussion
In this meta-analysis of nonrandomized controlled studies, moderate to strong decreases in the risk of IRR of MRSA colonization, VRE colonization, and MRSA infection for individuals with CHW were observed. Although the result for VRE infection was not significant in the meta-analysis, the association appeared to have similar trend with MRSA infection.
Our findings are consistent with five previous meta-analyses of CHW and risk of HAIs31–35 but included a much larger sample size, more focused analyses on the two HAIs including MRSA and VRE, sensitivity and trim-and-fill method analyses, and analyses of incidence rate ratios. To our knowledge, this is the largest meta-analysis to comprehensively summarize results for the relationship between CHW and MRSA and VRE infections, not just focused on ICU patients. The null association for VRE infection might be because of the few studies involved in this outcome subset with a limited sample size, which should be further investigated.
This meta-analysis has several strengths. First, it is strengthened by applying a comprehensive search strategy, making literature screening and eligibility criteria rigorous, and reporting the findings transparently. Second, the three major databases were thoroughly searched without language or publication date limits, making the risk of missing ublications less possible, which could minimize publication bias. Third, at least two authors selected studies and cross-checked and identified the final included studies. In order to perform the meta-analysis more objectively and minimize the selection bias to the greatest extent, all the authors jointly developed a data abstract form through discussion.
There are some limitations for this meta-analysis. First, most of the studies have difference in study design such as cluster-randomized trials, quasi-experimental studies, and before–after interventional studies, which is one source of inter-study heterogeneity. In fact, most of the studies were observational and retrospective, with some having limited capacity for adjustment, and thus were at a high risk of selection bias and residual confounding. Second, since there were a small number of studies in each outcome subset, we had to interpret the results with caution, although no evidence of publication bias in the analysis of all four outcome subsets was noted. Third, heterogeneity was rather high in two of the four analyses (I2>50%), but this appeared to partly attribute to differences in the size of the risk estimates between studies rather than a lack of association. Fourth, study patients had wide variation in baseline features, and were from different kinds of units such as ICUs,5,10,20,23,25,27,28,30 general medicine units, tertiary care hospital units,24,29 and inpatient medical units,19 potentially leading to significant heterogeneity in outcomes, which limited the capacity for pooled analyses.
Conclusion
Current evidence to some extent supports the hypothesis that patients with application of CHW had significantly lower MRSA colonization and infection, as well as VRE colonization. More evidence should be accumulated to reinforce these findings, especially on the effect of CHW on VRE infection.
Disclosure
The authors report no conflicts of interest in this work.
References
Magill SS, Edwards JR, Bamberg W, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370(13):1198–1208. | ||
Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in health care settings, 2006. Am J Infect Control. 2007;35(10 Suppl 2):S165–S193. | ||
Supple L, Kumaraswami M, Kundrapu S, et al. Chlorhexidine Only Works If Applied Correctly: Use of a Simple Colorimetric Assay to Provide Monitoring and Feedback on Effectiveness of Chlorhexidine Application. Infect Control Hosp Epidemiol. 2015;36(9):1095–1097. | ||
Cassir N, Papazian L, Fournier PE, Raoult D, La Scola B. Insights into bacterial colonization of intensive care patients’ skin: the effect of chlorhexidine daily bathing. Eur J Clin Microbiol Infect Dis. 2015;34(5):999–1004. | ||
Climo MW, Yokoe DS, Warren DK, et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533–542. | ||
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265. | ||
Milstone AM, Elward A, Song X, et al. Daily chlorhexidine bathing to reduce bacteraemia in critically ill children: a multicentre, cluster-randomised, crossover trial. Lancet. 2013;381(9872):1099–1106. | ||
O’Horo JC, Silva GL, Munoz-Price LS, Safdar N. The efficacy of daily bathing with chlorhexidine for reducing healthcare-associated bloodstream infections: a meta-analysis. Infect Control Hosp Epidemiol. 2012;33(3):257–267. | ||
Kassakian SZ, Mermel LA, Jefferson JA, Parenteau SL, Machan JT. Impact of chlorhexidine bathing on hospital-acquired infections among general medical patients. Infect Control Hosp Epidemiol. 2011;32(3):238–243. | ||
Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Daily skin cleansing with chlorhexidine did not reduce the rate of central-line associated bloodstream infection in a surgical intensive care unit. Intensive Care Med. 2010;36(5):854–858. | ||
Bass P, Karki S, Rhodes D, et al. Impact of chlorhexidine-impregnated washcloths on reducing incidence of vancomycin-resistant enterococci colonization in hematology-oncology patients. Am J Infect Control. 2013;41(4):345–348. | ||
Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. | ||
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. | ||
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. | ||
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. | ||
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. | ||
Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–463. | ||
Lowe CF, Lloyd-Smith E, Sidhu B, et al. Reduction in hospital-associated methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus with daily chlorhexidine gluconate bathing for medical inpatients. Am J Infect Control. 2017;45(3):255–259. | ||
Amirov CM, Binns MA, Jacob LE, Candon HL. Impact of chlorhexidine bathing on methicillin-resistant Staphylococcus aureus incidence in an endemic chronic care setting: A randomized controlled trial. Am J Infect Control. 2017;45(3):298–300. | ||
Kim JS, Chung YK, Lee SS, et al. Effect of daily chlorhexidine bathing on the acquisition of methicillin-resistant Staphylococcus aureus in a medical intensive care unit with methicillin-resistant S aureus endemicity. Am J Infect Control. 2016;44(12):1520–1525. | ||
Millar EV, Chen WJ, Schlett CD, et al. Frequent use of chlorhexidine-based body wash associated with a reduction in methicillin-resistant Staphylococcus aureus nasal colonization among military trainees. Antimicrob Agents Chemother. 2015;59(2):943–949. | ||
Colling K, Statz C, Glover J, Banton K, Beilman G. Pre-operative antiseptic shower and bath policy decreases the rate of S. aureus and methicillin-resistant S. aureus surgical site infections in patients undergoing joint arthroplasty. Surg Infect. 2015;16(2):124–132. | ||
Huang SS, Septimus E, Kleinman K, et al. Targeted versus universal decolonization to prevent ICU infection. N Engl J Med. 2013;368(24):2255–2265. | ||
Montecalvo MA, Mckenna D, Yarrish R, et al. Chlorhexidine bathing to reduce central venous catheter-associated bloodstream infection: impact and sustainability. Am J Med. 2012;125(5):505–511. | ||
Fraser TG, Fatica C, Scarpelli M, et al. Decrease in Staphylococcus aureus colonization and hospital-acquired infection in a medical intensive care unit after institution of an active surveillance and decolonization program. Infect Control Hosp Epidemiol. 2010;31(8):779–783. | ||
Evans HL, Dellit TH, Chan J, Nathens AB, Maier RV, Cuschieri J. Effect of chlorhexidine whole-body bathing on hospital-acquired infections among trauma patients. Arch Surg. 2010;145(3):240–246. | ||
Popovich KJ, Hota B, Hayes R, Weinstein RA, Hayden MK. Effectiveness of routine patient cleansing with chlorhexidine gluconate for infection prevention in the medical intensive care unit. Infect Control Hosp Epidemiol. 2009;30(10):959–963. | ||
Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37(6):1858–1865. | ||
Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28(10):1155–1161. | ||
Vernon MO, Hayden MK, Trick WE, et al. Chlorhexidine gluconate to cleanse patients in a medical intensive care unit: the effectiveness of source control to reduce the bioburden of vancomycin-resistant enterococci. Arch Intern Med. 2006;166(3):306–312. | ||
Kim HY, Lee WK, Na S, Roh YH, Shin CS, Kim J. The effects of chlorhexidine gluconate bathing on health care-associated infection in intensive care units: A meta-analysis. J Crit Care. 2016;32:126–137. | ||
Huang HP, Chen B, Wang HY, He M. The efficacy of daily chlorhexidine bathing for preventing healthcare-associated infections in adult intensive care units. Korean J Intern Med. 2016;31(6):1159–1170. | ||
Frost SA, Alogso MC, Metcalfe L, et al. Chlorhexidine bathing and health care-associated infections among adult intensive care patients: a systematic review and meta-analysis. Crit Care. 2016;20(1):379. | ||
Derde LP, Dautzenberg MJ, Bonten MJ. Chlorhexidine body washing to control antimicrobial-resistant bacteria in intensive care units: a systematic review. Intensive Care Med. 2012;38(6):931–939. | ||
Chen W, Li S, Li L, Wu X, Zhang W. Effects of daily bathing with chlorhexidine and acquired infection of methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: a meta-analysis. J Thorac Dis. 2013;5(4):518–524. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.