Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Chitinases and Chitinase-Like Proteins in Obstructive Lung Diseases – Current Concepts and Potential Applications
Authors Przysucha N, Górska K , Krenke R
Received 9 November 2019
Accepted for publication 10 March 2020
Published 23 April 2020 Volume 2020:15 Pages 885—899
DOI https://doi.org/10.2147/COPD.S236640
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Natalia Przysucha, Katarzyna Górska, Rafal Krenke
Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Warsaw, Poland
Correspondence: Katarzyna Górska
Department of Internal Medicine, Pulmonary Diseases and Allergy, Medical University of Warsaw, Banacha 1A, Warsaw 02-097 Email [email protected]
Abstract: Chitinases, enzymes that cleave chitin’s chain to low molecular weight chitooligomers, are widely distributed in nature. Mammalian chitinases belong to the 18-glycosyl-hydrolase family and can be divided into two groups: true chitinases with enzymatic activity (AMCase and chitotriosidase) and chitinase-like proteins (CLPs) molecules which can bind to chitin or chitooligosaccharides but lack enzymatic activity (eg, YKL-40). Chitinases are thought to be part of an innate immunity against chitin-containing parasites and fungal infections. Both groups of these hydrolases are lately evaluated also as chemical mediators or biomarkers involved in airway inflammation and fibrosis. The aim of this article is to present the current knowledge on the potential role of human chitinases and CLPs in the pathogenesis, diagnosis, and course of obstructive lung diseases. We also assessed the potential role of chitinase and CLPs inhibitors as therapeutic targets in chronic obstructive pulmonary disease and asthma.
Keywords: asthma, COPD, YKL-40, CHIT1, chitotriosidase, AMCase
Background
Chitin is the second (after cellulose) most abundant biopolymer on Earth,1 mainly forming various building block structures of fungi, mollusks, arthropods, and other invertebrates.2 Its major unique features include water insolubility and high resistance to mechanical stress and degradation. The utility of chitin-containing products has been recognized and, since many years, chitin has been widely used for commercial purposes: in agriculture, food industry, pharmacy or biomedical industry.3–5 Chitin is an enzymatic product of chitin synthases and requires specific enzymes – referred to as chitinases – for its degradation. Although it had previously been assumed that vertebral tissues contain no chitins and chitinases, in the light of recent studies, this assumption seems to be no longer valid.6 Endogenous chitin can be found in some vertebrates, including fish and amphibians.7 In general, human organisms are not able to produce chitin due to the absence of chitin synthases. However, minute amounts of chitin can be detected in humans. They either have external sources or may have the form of short-chain oligomers associated with hyaluronic acid synthesis.6,8 Thus, the lack of chitin synthases does not a priori exclude the presence of chitin in human tissues. It seems that under some circumstances even minute amounts of chitin or chitin-like polysaccharides can accumulate and contribute to human pathology, eg, in certain forms of Alzheimer’s disease.9 Considering the high prevalence of chitin and the fact that the immunological reaction to this polysaccharide is thought to be time- and dose-dependent, the above issue seems to have at least potential clinical implications.10 Nevertheless, the role of chitin in humans is still obscure.
Chitinases, enzymes that cleave chitin’s chain to low molecular weight chitooligomers, are widely distributed in nature. These enzymes are produced by bacteria, fungi, plants, actinomycetes, arthropods and vertebrates.11 Curiously, albeit the human genome is completely void of genes for chitin synthases, it does contain several genes encoding different human chitinases.12 Moreover, these genes are active and proteins with potent chitinolytic activity can be detected in different human tissues. This phenomenon is intriguing in the context of only a trace amount of chitin in human organisms. At least in part, chitinases probably represent a defensive mechanism against chitin-containing parasites and fungal infections.13 In addition, these enzymes may also play a role in destroying potent chitin antigens. Nonetheless, it must be admitted that our current knowledge on the place of chitinases in human physiology and pathophysiology is highly unsatisfactory.
Human chitinases belong to 18-glycosyl-hydrolases (GH18). Within the family of GH18, we can distinguish two groups based on their activity:14
- enzymatically active chitinases, represented by chitotriosidase (CHIT1) and acid mammalian chitinase (AMCase);
- chitinase-like proteins (CLPs), a group of several proteins which do not have hydrolytic activity but are still capable of binding chitin or chitin’s particles.15–17 The most important representatives of this group are:
- chitinase-3-like protein 1 (CHI3L1), also known as YKL-40 with animal homologue-BRP-39 (mouse breast regression protein 39);
- chitinase 3-like protein 2 (YKL-39);
- murine chitinase-like 3 protein (Ym1);
- stabilin-1-interacting chitinase-like protein (SI-CLP);
- oviductin.
Enzymatically active chitinases (true chitinases), ie, CHIT1 and AMCase, have been identified in different mammals including humans and laboratory animals (eg, mice). Humans and other mammals also produce YKL-40 (with mouse homologue BRP-39), oviductin and SI-CLP. Chitinase 3-like protein 2 (YKL-39) was found in humans but was absent in rodents.18 Animal studies indicate the role of eating behaviors in chitinase gene expression. Higher AMCase enzyme and mRNA levels were found in omnivores (eg, pigs, chickens, monkeys) than in herbivores (eg, bovines) and carnivores (eg, dogs).19,20 The highest levels of chitinase expression were reported in the lungs, stomach, kidney, and liver.19,20 It may be hypothesized that a higher exposure to chitin-containing organisms results in the overexpression of chitinase genes.21 It is worth to note, however, that chitinase genes are not always present in the genome (ie, according to the National Center for Biotechnology Information (NCBI) genome database, the CHIT1 gene is absent in the dog genome). The lack of chitinase gene in some species may lead to lower chitin digestibility and a reduced tolerance to overfeeding with chitin-containing food.19
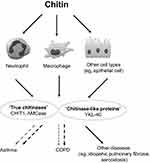
From the clinical perspective, CHIT1, AMCase and YKL-40 seem to play the most significant role in different human diseases. A common feature of the above enzymes is their potential involvement in the human immune response. Hence, the majority of studies have been focused on these molecules. These studies demonstrated various relationships between the level of CHIT1, AMCase or YKL-40 and different human diseases and pathologies, such as infections, cancer, chronic pulmonary disorders, arthritis, fibrosis, tissue remodeling, and others.12,14 The association of other CLPs with human diseases has been poorly addressed and both the number and the quality of data are limited, eg, YKL-39 was reported to be involved in osteoarthritis, whereas Sl-CLP probably plays a role in Th-2 induced allergies.14 Since chitinases and CLPs are believed to play an active role in human immune defense, their activity should be expressed mainly in locations with high exposure to microorganisms. This refers to the lungs which are exposed to approximately 10,000 liters of air contaminated with various potential pathogens per day.22,23 Moreover, the most common diseases of the lower respiratory tract such as asthma and chronic obstructive pulmonary disease (COPD) have a strong relationship with inhaled antigens and air pollution. Therefore, the respiratory system seems to be one of the major sites in which the presence and activity of chitinases should be considered. The possible role of chitin, chitinases and CLPs in pulmonary diseases is shown in Figure 1.6,18,24 The aim of this article is to present the current knowledge on the potential role of human chitinases and CLPs in the pathogenesis, diagnosis, and course of obstructive lung diseases. In particular, two major aspects that are critical from a clinical perspective will be reviewed: their role in pathogenesis as a potential target of therapeutic intervention and the role as biomarkers characterizing specific features of the disease.
Basic Characteristics of Selected Chitinases and CLPs Potentially Involved in Human Pathophysiology
Chitotriosidase
CHIT1 was the first true chitinase discovered in humans.25 This enzyme has a hydrolytic and trans-glycosylation activity.14 Elevated levels of CHIT1 have been first described in patients with Gaucher disease and later, in a variety of diseases (eg, acute and chronic infections, neurodegenerative diseases and other).14,25 In the human lung, CHIT1 is prominently expressed by alveolar macrophages and neutrophils and it is said to play an active role in the innate immune response.26–28 Under normal conditions, the importance of CHIT1 results from its role in the defense against chitin-containing human pathogens, including fungi and insects.28 CHIT1 may stimulate alveolar macrophages and enhance tissue inflammation.27 However, the exact mechanism of CHIT1 action has not been fully explained.
Acid Mammalian Chitinase
In 2001, a new enzyme with chitinolytic activity – AMCase – was discovered.29 It has been shown that AMCase has hydrolytic activity in the gastrointestinal tract and lungs and that it may play a role in digestion and/or immune defense.30 The enzyme is acid stable, has an optimal pH around 2 and an acidic environment is required to initiate AMCase enzymatic activity.31 This may suggest that the highest enzymatic activity can be found in gastric mucosa.20 AMCase is expressed by lung epithelial cells and macrophages. AMCase mRNA expression and the number of AMCase-positive cells are upregulated during Th2 inflammation. This phenomenon has been demonstrated mainly in asthma (in lung epithelial and subepithelial cells) but also in chronic rhinosinusitis with nasal polyps (in ethmoid mucosa).30,32 The above effects were found to be mediated mainly through IL-13.30,33,34 Although AMCase was detected in gastric mucosa, it did not correlate with the presence of H. pylori and inflammation.35 The role of AMCase in the human respiratory system has not been adequately investigated and some authors suggested that this enzyme could be inactive in human lungs.36
Chitinase-3-Like Protein 1
YKL-40, a 40 kDa plasma protein, is the best-known protein from the CLPs group. YKL-40 lacks chitinolytic activity due to the mutation of glutamic acid to leucine in the chitinase-3-like catalytic domain.27 The expression of YKL-40 was found in different mammalian organs and tissues (ie, humans, cows, mice, goats).37–40 The enzyme is secreted by macrophages, epithelial cells, neutrophils, chondrocytes, synoviocytes, vascular smooth muscle cells and cancer cells.41,42 Studies which used human lung specimens and bronchoalveolar lavage fluid (BALF) showed the expression of YKL-40 in the cytoplasm of neutrophils and macrophages both in healthy subjects and patients with obstructive airway diseases.43,44 Nevertheless, the role of this protein is somewhat controversial. It does not have any enzymatic activity but it is believed to be involved in the regulation of cell proliferation, adhesion, migration and activation.14 Higher concentrations of YKL-40 in synovial fluid from patients with osteoarthritis and inflammatory joint diseases were reported already in 1993.45 Later, elevated concentration of YKL-40 protein and mRNA were noted in many inflammatory diseases, malignancies and disorders associated with tissue remodeling (ie, pneumonia, inflammatory bowel disease, atherosclerosis, diabetes, liver fibrosis, and breast, liver, ovarian, colon or lung cancer).46–50 To date, YKL-40 has been the most extensively evaluated chitinase in patients with obstructive lung diseases.
The Role of Chitinases and CLPs in Obstructive Lung Diseases
The knowledge on the pathogenesis of obstructive airway diseases has significantly evolved in the last decades but the mechanisms of airway inflammation and obstruction are still not completely understood. The role of chitinases and CLPs in asthma and COPD has been evaluated with the perspective that better understanding of the complex relationships between different mechanisms involved in the pathogenesis of these two diseases may become a prerequisite for the development of more personalized treatment and improvement of its effectiveness.
The Role of Chitinases and CLPs in Asthma
Asthma is a common airway disease characterized by a relatively specific type of chronic airway inflammation, airway hyperresponsiveness, and variable, reversible airflow limitation. According to the Global Initiative for Asthma (GINA) 2019 report, 300 million people worldwide suffer from this disease.51 Untreated or inadequately treated asthma causes respiratory symptoms, reduces daily activity and quality of life, increases the risk and severity of exacerbations, including life-threatening events. Although the proportion of patients with severe asthma is rather small (usually reported between 3% and 8%), it is responsible for a significant proportion (20.6%) of total costs of treatment.52–54 Therefore, a better understanding of the phenotype and endotype of severe asthma may not only improve the efficacy of treatment but also significantly reduce its cost.55
The high prevalence of asthma and chronic cough among people exposed to chitin-containing compounds (eg, working in seafood or fungi manufactures) may suggest the importance of chitin in asthma pathogenesis.56,57 It is possible that chitin may act as an airborne endotoxin that triggers airway hyperresponsiveness. On the other hand, early exposition to chitin may also result in hypersensitization to chitin as an allergen.58 These observations are consistent with the hygiene hypothesis, which emphasizes the importance of early childhood exposure to infections, microorganisms, and parasites in the proper development of the immune system. Thus, it has been hypothesized that a reduced number of infections with chitin-containing pathogens could contribute to the exaggerated Th2 response of the host which involves such Th2 cytokines as IL-4, IL-5 and IL-13 and may provoke hyperresponsiveness of the airways.30,59
There has been a number of human and experimental studies on chitinases and chitinase-like proteins in asthma. Human studies revealed that the levels of these proteins in asthmatics are higher than in control subjects. In most of the studies, chitinase levels were measured in serum and induced sputum.60–62 Less commonly, BALF, and bronchial biopsies obtained by bronchoscopy or specimens from resected lung were used to measure chitinase activity and expression.36,64,65
Chitinases and CLPs in Animal Models of Asthma
Several studies showed that AMCase plays a role in animal models of asthma. The results of these studies imply that the enzyme can be an important mediator of IL-13-induced response in Th2-related conditions.30,64 A significant increase of AMCase mRNA and protein was detected in the lung of ovalbumin (OVA)-sensitized mice (aeroallergen asthma model).30 AMCase was significantly upregulated after 2 weeks of house dust mite (HDM) exposure in the murine model, while increased expression of this enzyme was not observed following cigarette smoke exposure.65 The above data suggest that AMCase can be regarded as a marker of allergic airway inflammation.65 HDM-induced mouse model of asthma was also used to evaluate the impact of selective AMCase inhibitor (compound 7f) on allergic airway inflammation. The study showed the effectiveness of this compound in reducing the number of inflammatory cells in BALF and total IgE concentration in serum. These changes correlated with the decrease of chitinolytic activity in BALF.66
Another selective AMCase inhibitor also reduced allergic inflammation (eosinophilia) but its application was associated with unexpected serious side effects, including neutrophilia, an increase in macrophage inflammatory protein-1 alpha (MIP-1α) secretion and alterations in airway remodeling genes (resulting in increased procollagen I, matrix-metalloprotease-12 (MMP-12), and Ym1 production).67 It has to be determined whether these serious adverse events were directly drug-induced or were the result of the altered eosinophil/neutrophil balance.
An animal study on the impact of CHIT1 on allergic asthmatic reaction led to some surprising conclusions.68 The CHIT1-deficient mice demonstrated the enhanced allergic Th2 inflammatory, cytokine and IgE responses to OVA or HDM allergen sensitization and challenge. A possible protective role of CHIT1 in the pathogenesis of allergic inflammation and asthmatic airway responses is considered to be mediated by regulating transforming growth factor-beta (TGF-β) expression and Foxp3+ regulatory T cells (Treg).68 BRP-39, an animal homologue of YKL-40, also plays an important role in the pathogenesis of IL-13-induced inflammation and tissue remodeling.69 The involvement of this CLP in multiple immunological responses includes antigen sensitization, immunoglobulin E induction, stimulation of dendritic cell accumulation and activation, induction of alternative macrophage activation, inhibition of eosinophil, T cell, and macrophage apoptosis.69
Chitinases and CLPs in Childhood Asthma
One study that included 322 children with asthma, 270 adult and 565 pediatric controls, demonstrated an association between polymorphism of genetic variants of AMCase and childhood asthma.70 In contrast, mutations in CHIT1 did not seem to significantly affect asthma prevalence and severity.71 Nevertheless, gene analysis of six children with severe asthma with fungal sensitization (SAFS) demonstrated that CHIT1 duplication may be a risk factor for SAFS, allergic bronchopulmonary aspergillosis and allergic fungal sinusitis.72 An association between the serological features of fungal infection (fungal-specific IgG and IgA reactivity) and higher BALF chitinase activity was reported in asthmatic children.73
Compared to non-asthmatics, children with asthma had increased YKL-40 levels in serum and BALF.61,73 Higher serum YKL-40 levels correlated with the fraction of exhaled nitric oxide, blood neutrophils, and bronchial wall thickening on high-resolution computed tomography.61 Konradsen et al suggested that YKL-40 might be a risk factor of a worse course of the disease and resistance to standard therapy.61 However, it must be stressed that other authors do not share this opinion.74
Chitinases and CLPs in Adult Asthma
Numerous studies analyzed the role of YKL-40 in adult asthma. In general, the results of these studies suggested a relationship between the level of this enzyme and asthma, its severity and the level of asthma control.44,61,75 Serum YKL-40 and CHIT1 were elevated in patients with asthma and their levels correlated with the degree of airway obstruction and the risk of exacerbation.60,76,77 There was also a weak but statistically significant correlation between serum YKL-40 level and CHIT1 activity in patients with obstructive airway diseases.60 Chupp et al found that patients with elevated serum levels of YKL-40 used significantly more rescue inhalers, required higher doses of oral corticosteroids and had a higher rate of hospitalization than patients with lower YKL-40 levels.78 These authors also demonstrated an increased local expression of YKL-40 in bronchial mucosa in patients with severe asthma compared to the healthy control. Importantly, local YKL-40 expression correlated with serum YKL-40 level.78 Other authors showed a significant increase in YKL-40 level in induced sputum (IS) after allergen bronchial provocation in patients with atopic asthma.62
An interesting study on the relationship between serum levels of YKL-40 and a specific asthma phenotype was published by Specjalski et al.44 The study included 167 patients with three different phenotypes of chronic asthma: early-onset atopic, late-onset non-atopic, and obesity-related asthma. The authors demonstrated significant differences between serum levels of YKL-40 in the three asthma phenotypes, with the highest YKL-40 levels in patients with the obesity-related disease. Serum levels of the enzyme were significantly higher in asthmatics with a high level of symptoms and exacerbations compared to patients with well-controlled disease as well as in atopic compared to non-atopic asthmatics.44 The positive correlation between serum and sputum concentrations of YKL-40 in asthmatic subjects and truncal adiposity was confirmed by another study.79 Moreover, the authors found that a high-fat diet augmented the pulmonary expression of YKL-40 (animal study). It was probably due to inflammatory, repair, and remodeling responses mediated by this CLP (probably partially mediated by interaction with Sirtuin 1).
A fascinating issue which may be, at least to some extent, linked to the relationship between severe asthma and high serum YKL-40 levels are the characteristics of inflammation associated with increased YKL-40 expression. Studies on sputum and serum YKL-40 level in asthma and COPD revealed a positive correlation with blood and sputum neutrophil count.43,80 At the same time, there were no correlations between chitinase concentrations (both YKL-40 and CHIT1) and blood eosinophils count either in asthma or in COPD patients.60 This pattern of YKL-40 correlation with the number of neutrophils but not the number of eosinophils may suggest that neutrophils are an important source of YKL-40 in asthma.43 On the other hand, as the correlation between the level of YKL-40 and some chemotactic factors for neutrophils (eg, IL-8) was reported, it might also be hypothesized that the increased number of neutrophils is an indirect effect of enhanced expression of YKL-40. Correlations between sputum YKL-40 and the levels of myeloperoxidase, IL-8, IL-6, and IL-5 in patients with severe asthma were observed by Hinks et al.81 Additionally, Tang et al showed that YKL-40 induced IL-8 expression in bronchial epithelium via a mitogen-activated protein kinase (MAPK: c-Jun N-terminal kinases – JNK and extracellular signal-regulated kinase – ERK) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) pathways, causing bronchial smooth muscle proliferation and migration.82 Considering the inability of corticosteroids to ameliorate neutrophilic asthma and neutrophil-generated overexpression of mediators aggravating airway remodeling [ie, TGF-β, IL-8, metalloproteinase 9 (MMP-9), elastase],83–85 the correlation between YKL-40 and neutrophil cells in asthma is still uncertain and intriguing. All the above data seem to be consistent with clinical observations linking YKL-40 with severe, therapy-resistant asthma. However, it cannot be excluded that YKL-40 is only a marker of extracellular tissue remodeling. Whether YKL-40 is only an independent marker of airway inflammation or it is truly involved in asthma pathogenesis still needs to be elucidated. This requires a more comprehensive explanation of the exact mechanism of YKL-40 stimulation and action.
Of the three discussed chitinases, the most controversial data on the involvement in asthma pathobiology refer to AMCase. Zhu et al found that AMCase is expressed in lung tissue samples from patients with asthma but seems to be inactive in human lungs.30,36 Despite the fact that airway pH in patients with acute asthma is lower compared to healthy control (pH 5.23 vs pH 7.65),86 the rate of airway acidification may be still insufficient for the optimum AMCase activity. The lack of AMCase bioactivity may, at least partially, result also from the increased pH in the airways during inhaled or systemic corticosteroid treatment.30,31,86,87 The lack or lower chitinolytic activity of human AMCase compared to the chitinolytic activity of mice AMCase may also result from evolutionary mutations of chitinase genes.88 These processes lead to amino acid substitutions (some specific amino acids in human AMCase are not present in mice AMCase) and, probably, synthesis of a nonfunctional enzyme.
The Role of Chitinases and CLPs in COPD
Chronic obstructive pulmonary disease is another common chronic lung disease with high morbidity and mortality worldwide. It has been estimated that 328 million people suffer from COPD and according to the newest WHO report, it is the third leading cause of death worldwide.89,90 Airway inflammation, obstruction and remodeling, as well as structural changes in lung parenchyma belong to the most important features of COPD. These processes produce clinical symptoms (ie, dyspnea, productive cough, exercise intolerance) which are directly associated with irreversible and progressive airflow limitation, manifested particularly during disease exacerbations. COPD significantly reduces the quality of life and leads to physical disability. The main cause of COPD is tobacco smoke exposure and air pollution. Despite a significant progress in the understanding of the complex COPD pathophysiology, there are still many unresolved questions (ie, why COPD develops only in some smokers). In consequence, the effectiveness of treatment seems to be highly unsatisfactory.
An interesting concept of the relationship between COPD, chitin, and chitinases was proposed by Seibold et al.36 These authors suggested that smoking is associated with higher airway exposure to chitin. This suggestion was based on the fact that tobacco leaves are commonly infected with fungal organisms.36 Some years earlier, Verveij et al showed that commercially available cigarettes and marijuana (semi-legally sold in the Netherlands) were heavily contaminated with fungal spores.91 Aspergillus fumigatus was the most frequently isolated organism from cigarettes, while Penicillium genus predominated in marijuana. Bearing in mind the above data, Seibold et al presented the hypothesis that polymeric chitin, present in cigarette smoke, might activate macrophages and airway epithelial cells and increase chitinase expression and secretion.36
Studies evaluating the role of chitinases in the pathophysiology of COPD have been conducted since 200892 and include both a human and animal models. However, it should be emphasized that the number of publications on COPD is significantly smaller than on asthma.
Chitinases and CLPs in Animal Models of COPD
A study in the murine model of COPD showed that cigarette smoke (CS) exposure did not change the level of AMCase in the murine lung.63 In contrast, an experimental study in the mouse model showed that exposure to CS led to higher YKL-40 accumulation in airway epithelial cells, alveolar macrophages, alveolar type II cells and an increase in YKL-40 mRNA concentration.93 Importantly, the authors documented that YKL-40 was induced by an IL-18-dependent pathway. An immunohistochemistry study demonstrated that the overexpression of CHIT1 in a mouse lung pretreated with an adenoviral vector carrying full-length CHIT1 was localized mainly in the bronchial and bronchiolar epithelium and tissue macrophages.27 CHIT1 overexpression promoted inflammation in the airways (as in COPD) manifested by a higher number of total cells, alveolar macrophages, and higher amounts of the murine homologue of IL-8 and monocyte chemoattractant protein 1 (MCP-1) in BALF.27 Interestingly, the study by Matsuura et al demonstrated the protective role of YKL-40 against CS-induced lung injury.93 The lack of this enzyme resulted in more pronounced CS-induced lung emphysema, mainly through the up-regulation of the CS-induced cell death response. Thus, the authors suggested that proper chitinase levels might play a protective role in preventing lung destruction.94 These pleiotropic effects of chitinases (tissue destruction as a consequence of inflammation and remodeling and, on the other hand, the protective role against apoptosis) should be taken into consideration when planning therapeutic interventions targeted at chitinases (application of potential chitinase inhibitors).
Chitinases and CLPs in Patients with COPD
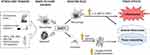
The involvement of chitinases in human COPD seems ambiguous. There were studies showing that AMCase probably plays no role in both patients with COPD and healthy smokers. The level of this chitinase in BALF was below the ELISA detection threshold.27 On the other hand, serum, induced sputum (IS) and BALF levels of CHIT1 and YKL-40, as well as the number of CHIT1 positive cells infiltrating the bronchial epithelium and submucosa, were significantly higher in smokers with COPD than in smokers without COPD or never smokers.27,61,62,94,95 It has been postulated that it could be related to higher macrophage and neutrophil CHIT1 and YKL-40 expression in COPD patients.27,36,96 Recent studies showed that the release of CHIT1 is stimulated by interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), granulocyte-macrophage colony-stimulating factor (GM-CSF), lipopolysaccharide (LPS) as well as toll-like receptor (TLR) and nucleotide-binding oligomerization domain (NOD)-2 activation.97–99 The exposure of alveolar macrophages from smokers with and without COPD to CHIT1 resulted in the production of IL-8, MMP-9 and MCP-1, cytokines said to be involved in tissue inflammation, alveolar destruction, and fibrosis.27 Moreover, CHIT1 increases the activity of TGF-β signaling in fibroblasts (eg, through the induction of TGF-β receptor expression, inhibition of its feedback inhibitor – SMAD7 and interacting with a chaperone protein TGF-β receptor associate protein 1 (TGFRAP1) and forkhead box O3 (FOXO3)) resulting in enhanced production of extracellular connective tissue (eg, collagen), extracellular matrix protein (ECM) accumulation that may lead to alveolar destruction and tissue remodeling.100–102 Possible CHIT1-related signaling pathways are shown in Figure 2.27,36,95,97-102 It should be mentioned, however, that the exact role of CHIT1 in the pathogenesis of lung diseases is still highly hypothetical.
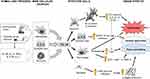
Similar pulmonary effect was demonstrated for YKL-40. Allergen and/or cigarette smoke (CS) exposure have been shown to trigger YKL-40 expression in several cell types (especially neutrophils, macrophages and epithelial cells).69,92,93,95,96 The induction of YKL-40 expression and secretion may be mediated by various mediators, including interleukin (IL)-1,-13,-17A,-18 and TNFα.65,69,95,104–106 YKL-40 induces the production of proinflammatory and profibrotic mediators, ultimately resulting in tissue inflammation, alveolar destruction and remodeling. This complex process involves different pathways, mechanisms and multiple stages of inflammatory and tissue reactions. These include: 1) the activation and accumulation of dendritic cells (DC),69 2) significant increase of IL-8 production from bronchial epithelial cells (dependent on MAPK and NF-κB pathway activation),82 3) prevention of macrophage apoptosis and induction of alternative (M2) macrophage differentiation69 with enhanced production of cytokines and chemokines, eg, IL-8, TGFβ, MCP-1, MIP-1α and MMP-9,92 4) increased collagen production from lung fibroblasts (dependent on ERK and p-38 pathway activation).96 Other inflammatory pathways may also play a role in modulation and aggravation of the YKL-40 response, eg, stimulation of TGFβ production and its activation by IL-13,103 as well as IL-8 mediated proliferation and migration of bronchial smooth muscle cells.82 The effects of YKL-40 on pulmonary structure and function are summarized in Figure 3.65,69,82,92,93,95,96,103-106
Higher levels of CHIT1 and YKL-40 in serum and BALF of COPD patients correlated with a faster decline of lung function, the degree of airflow obstruction, the degree of diffusion lung capacity impairment, and frequency of exacerbations.27,94,96 The results of other studies also emphasized a positive correlation between high serum YKL-40 concentrations and the severity of COPD (measured as the rate of decline in lung function, lower arterial oxygen pressure and desaturation during the 6-min walk test).104,107 Gumus et al showed a relationship between serum levels of YKL-40 and the low-attenuation area percentage (LAA%) in chest computed tomography in COPD patients, what may suggest the association between YKL-40 and development of emphysematous changes.107 Interestingly, some studies suggest a protective role of CHIT1 against lung injury.108,109 Aminuddin et al found that smokers without a functional isoform of CHIT1 developed a faster rate of FEV1 decline.108 This may indicate that an active form CHIT1 may play a protective role against rapid COPD progression. In fact, the relationship between CHIT1 and COPD refers rather to more profound mechanisms of the disease than to exposure to cigarette smoke and polymeric chitin, only. This might be supported by the observations on no relationship between the levels of CHIT1 chitinolytic activity in BALF and serum with smoking history (measured in pack-years).60,98
The possible protective role of chitinases in inflammation and lung injury may, at least partially, be related to anti-inflammatory effects of CHIT1 (mentioned earlier in the section on animal models of asthma).68 CHIT1 inhibits allergen-stimulated Th2 cytokine production while enhancing the expression of the anti-inflammatory cytokines (TGF-β1 and IL-10).68 Moreover, CHIT1 enhances TGF-β1–stimulated fibroblast proliferation and ECM accumulation in lungs.100 An optimal level of CHIT1 seems to be both necessary and sufficient for physiologic TGF-β1–stimulated fibrotic ECM deposition and tissue repair responses. Nevertheless, the protective and favorable role of chitinases in inflammation and lung injury seems to be somewhat controversial and the data supporting this view are uncertain.
The Impact of Sex and Age on Chitinase and CLP Levels in Various Biological Samples
The activity of chitinases and their levels should also be discussed in the context of sex and ageing. Several studies documented a relationship between serum level of YKL-40 as well as CHIT1 and age. These findings refer to both patients with obstructive airway diseases (asthma and COPD) and healthy individuals.60,110,111 The increase in chitinase levels and activity in the elderly population could be associated with age-related comorbidities. It is also possible that the higher serum chitinase activity in older patients is associated with the accumulation of lipid-laden macrophages during the progression of age-related atherosclerosis.111 In contrast to age, no differences between plasma YKL-40 levels in healthy men and women were found.110
The Polymorphisms of Chitinases/CLPs and the Course of Obstructive Airway Diseases
There were some studies aimed to evaluate whether the genetic variations of chitinase genes affect the course of obstructive diseases. Most of these studies analyzed the potential role of chitinase polymorphism (single-nucleotide polymorphisms – SNPs) in the expression of chitinase proteins and lung function.70,112,113 Some authors found that SNPs in AMCase and YKL-40 gene influence chitinase levels and have been associated with asthma status, atopy, IgE levels and impaired lung function.70,112,113 However, other studies showed no relationship between the polymorphism of chitinases and CLPs (AMCase, chitotriosidase and YKL-40) and asthma or allergy.71,114 One possible explanation for these discrepancies may be associated with differences in cohort characteristics included in various studies. It is possible that the influence of variation of these genes alone is weak and gene–environment interactions are essential to provoke allergic reactions. As mentioned previously, a 24-bp duplication in exon 10 in the CHIT1 gene results in producing a nonfunctional form of the protein.36 This polymorphism has been associated with reduced chitinase activity in the lungs of smokers. The results of the analysis of genetic variations of CHIT1 and AMCase genes in large cohort groups are inconsistent. Aminuddin et al demonstrated that polymorphisms of CHIT1 and AMCase were associated with the baseline FEV1 in a large cohort of COPD patients.108 Moreover, there was a relationship between CHIT1 rs2494303 and AMCase G339T genotypes and a faster rate of lung function decline. Due to the differences in demographic factors characterizing different study cohorts (ie, the LHD group was statistically younger than the subjects in other cohorts), the authors suggested that chitinase polymorphism probably plays a role in lung development or early impairment of lung function but its influence in later stages of COPD (eg, as an effect of multiple factors that influence the course of the disease) cannot be easily estimated.
The Effect of Corticosteroids on Chitinase and CLP Levels
The impact of corticosteroids on the levels of chitinases and CLPs seems to be an important and interesting issue. This is not only because corticosteroids are commonly used to treat asthma and COPD but also because some intriguing data on the influence of systemic corticosteroids on chitinase levels in patients with different diseases have been reported. As early as in 1999, Johansen et al reported that prednisolone therapy reduced serum YKL-40 levels in patients with rheumatoid arthritis.115 On the other hand, the BIOAIR study showed that two-week oral prednisone course (0.5 mg/kg body weight/day) added to regular treatment did not change serum YKL-40 levels and chitotriosidase activity in patients with asthma.60 Surprisingly, the same therapeutic regimen in patients with COPD resulted in a reduction in serum chitotriosidase activity.60 Moreover, an in vitro study by Kunz et al revealed that dexamethasone strongly inhibited the release of YKL-40 from the population of proinflammatory macrophages that are characterized by classic mechanism of activation, secretion of pro-inflammatory cytokines (eg, IFN-γ, IL-1, IL-6, IL-12) and promotion of Th1 immunity.116 However, long-term treatment with ICS (30 months) did not significantly change sputum and serum YKL-40 levels in patients with COPD.116 The lack of change in serum YKL-40 level may result from a low circulating corticosteroid level during ICS treatment.117 In addition, there are data suggesting that lung macrophages from patients with COPD have reduced corticosteroid sensitivity compared with macrophages from smokers without COPD.118 Thus, it seems essential to elucidate whether YKL-40 and other chitinase gene expressions are directly controlled by corticosteroids.
Perspectives
To date, chitinases and CLPs were mainly evaluated as chemical mediators or biomarkers involved in airway inflammation and fibrosis (as shown in Table 1). Some data presented in the paper point to their role not only in airway immunopathology, including chronic inflammation and tissue remodeling,27,30,119 but also in the clinical course of asthma and COPD, ie, severity of airway obstruction, rate of exacerbations and response to steroid treatment.60,61 It might be supposed that future studies will be focused not only on the role of chitinases and CLPs in airway diseases but also on chitinases as a therapeutic target. Studies on chitinase inhibitors showed that methylxanthines (theophylline, caffeine, and pentoxifylline) are not only competitive inhibitors of fungal family 18 chitinases but are also active against human chitinases.120 It was also reported that another chitinase inhibitor – demethylallosamidin – reduces hyperresponsiveness (induced by IL-13) in OVA-induced asthma model and may have potential as a therapeutic agent for asthma.121 The results of recent studies that evaluated the efficacy of AMCase and CHIT1 inhibitors in animal models of asthma seem to be promising.66,122 A better understanding of the physiological activity of chitinases and chitinase-like proteins could accelerate the future development of chitinase-specific therapeutic agents in obstructive lung diseases.
 |
Table 1 Selected Information About Main Chitinases and Their Role in Humans |
Acknowledgments
The authors thank Dr Marta Maskey-Warzechowska for her assistance in the preparation of the final version of the manuscript. The authors alone are responsible for the content and writing of the paper.
Disclosure
KG reports fees for lectures and travel expenses from Boehringer Ingelheim, Chiesi, AstraZeneca, Polpharma and Roche, outside the submitted work. RK reports personal fees and non-financial support from Boehringer Ingelheim, Chiesi, and AstraZeneca; and personal fees from Polpharma, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Shahidi F, Abuzaytoun R. Chitin, chitosan, and co-products: chemistry, production, applications, and health effects. Adv Food Nutr Res. 2005;49:93–135.
2. Stern R. Go fly a chitin: the mystery of chitin and chitinases in vertebrate tissues. Front Biosci Landmark Ed. 2017;22:580–595. doi:10.2741/4504
3. Sharp RG. A review of the applications of chitin and its derivatives in agriculture to modify plant-microbial interactions and improve crop yields. Agronomy. 2013;3(4):757–793. doi:10.3390/agronomy3040757
4. Kato Y, Onishi H, Machida Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr Pharm Biotechnol. 2003;4(5):303–309. doi:10.2174/1389201033489748
5. Yang TL. Chitin-based materials in tissue engineering: applications in soft tissue and epithelial organ. Int J Mol Sci. 2011;12(3):1936–1963. doi:10.3390/ijms12031936
6. Ziatabar S, Zepf J, Rich S, Danielson BT, Bollyky PI, Stern R. Chitin, chitinases, and chitin lectins: emerging roles in human pathophysiology. Pathophysiology. 2018;25(4):253–262. doi:10.1016/j.pathophys.2018.02.005
7. Tang WJ, Fernandez J, Sohn JJ, Amemiya CT. Chitin is endogenously produced in vertebrates. Curr Biol CB. 2015;25(7):897–900. doi:10.1016/j.cub.2015.01.058
8. Weigel PH, Baggenstoss BA, Washburn JL. Hyaluronan synthase assembles hyaluronan on a [GlcNAc(β1,4)]n-GlcNAc(α1→)UDP primer and hyaluronan retains this residual chitin oligomer as a cap at the nonreducing end. Glycobiology. 2017;27(6):536–554. doi:10.1093/glycob/cwx012
9. Lomiguen C, Vidal L, Kozlowski P, Prancan A, Stern R. Possible role of chitin-like proteins in the etiology of alzheimer’s disease. J Alzheimers Dis. 2018;66(2):439–444. doi:10.3233/JAD-180326
10. Patel S, Goyal A. Chitin and chitinase: role in pathogenicity, allergenicity and health. Int J Biol Macromol. 2017;97:331–338. doi:10.1016/j.ijbiomac.2017.01.042
11. Bhattacharya D, Nagpure A, Gupta RK. Bacterial chitinases: properties and potential. Crit Rev Biotechnol. 2007;27(1):21–28. doi:10.1080/07388550601168223
12. Bussink AP, Speijer D, Aerts JM, Boot RG. Evolution of mammalian chitinase(-like) members of family 18 glycosyl hydrolases. Genetics. 2007;177(2):959–970. doi:10.1534/genetics.107.075846
13. Wiesner DL, Specht CA, Lee CK, et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 2015;11(3):e1004701. doi:10.1371/journal.ppat.1004701
14. Kzhyshkowska J, Gratchev A, Goerdt S. Human chitinases and chitinase-like proteins as indicators for inflammation and cancer. Biomark Insights. 2007;2:128–146. doi:10.1177/117727190700200023
15. Chang NC, Hung SI, Hwa KY, et al. A macrophage protein, Ym1, transiently expressed during inflammation is a novel mammalian lectin. J Biol Chem. 2001;276(20):17497–17506. doi:10.1074/jbc.M010417200
16. Meng G, Zhao Y, Bai X, et al. Structure of human stabilin-1 interacting chitinase-like protein (SI-CLP) reveals a saccharide-binding cleft with lower sugar-binding selectivity. J Biol Chem. 2010;285(51):39898–39904. doi:10.1074/jbc.M110.130781
17. Renkema GH, Boot RG, Au FL, et al. Chitotriosidase, a chitinase, and the 39-kDa human cartilage glycoprotein, a chitin-binding lectin, are homologues of family 18 glycosyl hydrolases secreted by human macrophages. Eur J Biochem. 1998;251(1–2):504–509. doi:10.1046/j.1432-1327.1998.2510504.x
18. Lee CG, Da Silva CA, Dela Cruz CS, et al. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi:10.1146/annurev-physiol-012110-142250
19. Tabata E, Kashimura A, Kikuchi A, et al. Chitin digestibility is dependent on feeding behaviors, which determine acidic chitinase mRNA levels in mammalian and poultry stomachs. Sci Rep. 2018;8(1):1461. doi:10.1038/s41598-018-19940-8
20. Uehara M, Tabata E, Ishii K, et al. Chitinase mRNA levels determined by QPCR in crab-eating monkey (Macaca fascicularis) tissues: species-specific expression of acidic mammalian chitinase and chitotriosidase. Genes. 2018;9(5):244. doi:10.3390/genes9050244
21. Malaguarnera L, Simporè J, Prodi DA, et al. A 24-bp duplication in exon 10 of human chitotriosidase gene from the sub-Saharan to the Mediterranean area: role of parasitic diseases and environmental conditions. Genes Immun. 2003;4(8):570–574. doi:10.1038/sj.gene.6364025
22. Carroll RG. Elsevier's Integrated Physiology. Philadelphia: Mosby Elsevier; 2007:99–115.
23. Tipton MJ, Harper A, Paton JFR, Costello JT. The human ventilatory response to stress: rate or depth? J Physiol. 2017;595(17):5729–5752. doi:10.1113/JP274596
24. Mack I, Hector A, Ballbach M, et al. The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol Cell Pediatr. 2015;2(1):3. doi:10.1186/s40348-015-0014-6
25. Hollak CE, van Weely S, van Oers MH, Aerts JM. Marked elevation of plasma chitotriosidase activity. A novel hallmark of Gaucher disease. J Clin Invest. 1994;93(3):1288–1292. doi:10.1172/JCI117084
26. Boot RG, Bussink AP, Verhoek M, de Boer PA, Moornan AF, Aerts JM. Marked differences in tissue-specific expression of chitinases in mouse and man. J Histochem Cytochem. 2005;53(10):1283–1292. doi:10.1369/jhc.4A6547.2005
27. Létuvé S, Kozhich A, Humbles A, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. Am J Pathol. 2010;176(2):638–649. doi:10.2353/ajpath.2010.090455
28. Kanneganti M, Kamba A, Mizoguchi E. Role of chitotriosidase (chitinase 1) under normal and disease conditions. J Epithel Biol Pharmacol. 2012;5:1–9. doi:10.2174/1875044301205010001
29. Boot RG, Blommaart EF, Swart E, et al. Identification of a novel acidic mammalian chitinase distinct from chitotriosidase. J Biol Chem. 2001;276(2):6770–6778. doi:10.1074/jbc.M009886200
30. Zhu Z, Zheng T, Homer RJ, et al. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science. 2004;304(5677):1678–1682. doi:10.1126/science.1095336
31. Wakita S, Kimura M, Kato N, et al. Improved fluorescent labeling of chitin oligomers: chitinolytic properties of acidic mammalian chitinase under somatic tissue pH conditions. Carbohydr Polym. 2017;164:145–153. doi:10.1016/j.carbpol.2017.01.095
32. Ramanathan M, Lee W-K, Lane AP. Increased expression of acidic mammalian chitinase in chronic rhinosinusitis with nasal polyps. Am J Rhinol. 2006;20(3):330–335. doi:10.2500/ajr.2006.20.2869
33. Sutherland TE, Maizels RM, Allen JE. Chitinases and chitinase-like proteins: potential therapeutic targets for the treatment of T-helper type 2 allergies. Clin Exp Allergy. 2009;39(7):943–955. doi:10.1111/cea.2009.39.issue-7
34. Homer RJ, Zhu Z, Cohn L, et al. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;291(3):L502–511. doi:10.1152/ajplung.00364.2005
35. Cozzarini E, Bellin M, Norberto L, et al. CHIT1 and AMCase expression in human gastric mucosa: correlation with inflammation and Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2009;21(10):1119–1126. doi:10.1097/MEG.0b013e328329742a
36. Seibold MA, Donnelly S, Solon M, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008;122(5):944–950. doi:10.1016/j.jaci.2008.08.023
37. Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268(34):25803–25810.
38. Rejman JJ, Hurley WL. Isolation and characterization of a novel 39 kilodalton whey protein from bovine mammary secretions collected during the nonlactating period. Biochem Biophys Res Commun. 1988;150(1):329–334. doi:10.1016/0006-291X(88)90524-4
39. Lian Z, De Luca P, Di Cristofano A. Gene expression analysis reveals a signature of estrogen receptor activation upon loss of Pten in a mouse model of endometrial cancer. J Cell Physiol. 2006;208(2):255–266. doi:10.1002/(ISSN)1097-4652
40. Mohanty AK, Singh G, Paramasivam M, et al. Crystal structure of a novel regulatory 40-kDa mammary gland protein (MGP-40) secreted during involution. J Biol Chem. 2003;278(16):14451–14460. doi:10.1074/jbc.M208967200
41. Ringsholt M, EVS H, Johansen JS, Price PA, Christentsen LH. YKL-40 protein expression in normal adult human tissues–an immunohistochemical study. J Mol Histol. 2007;38(1):33–43. doi:10.1007/s10735-006-9075-0
42. Nyirkos P, Golds EE. Human synovial cells secrete a 39 kDa protein similar to a bovine mammary protein expressed during the non-lactating period. Biochem J. 1990;269(1):265–268. doi:10.1042/bj2690265
43. Otsuka K, Matsumoto H, Niimi A, et al. Sputum YKL-40 levels and pathophysiology of asthma and chronic obstructive pulmonary disease. Respir Int Rev Thorac Dis. 2012;83(6):507–519.
44. Specjalski K, Chełmińska M, Jassem E. YKL-40 protein correlates with the phenotype of asthma. Lung. 2015;193(2):189–194. doi:10.1007/s00408-015-9693-y
45. Johansen JS, Jensen HS, Price PA. A new biochemical marker for joint injury. Analysis of YKL-40 in serum and synovial fluid. Br J Rheumatol. 1993;32(11):949–955. doi:10.1093/rheumatology/32.11.949
46. Rathcke CN, Vestergaard H. YKL-40, a new inflammatory marker with relation to insulin resistance and with a role in endothelial dysfunction and atherosclerosis. Inflamm Res. 2006;55(6):221–227.
47. Spoorenberg SMC, Vestjens SMT, Rijkers GT, et al. YKL-40, CCL18 and SP-D predict mortality in patients hospitalized with community-acquired pneumonia. Respirol Carlton Vic. 2017;22(3):542–550. doi:10.1111/resp.12924
48. Kzhyshkowska J, Yin S, Liu T, Riabov V, Mitrofanova I. Role of chitinase-like proteins in cancer. Biol Chem. 2016;397(3):231–247. doi:10.1515/hsz-2015-0269
49. Vind I, Johansen JS, Price PA, Munkholm P. Serum YKL-40, a potential new marker of disease activity in patients with inflammatory bowel disease. Scand J Gastroenterol. 2003;38(6):599–605. doi:10.1080/00365520310000537
50. Nøjgaard C, Johansen JS, Christensen E, et al. Serum levels of YKL-40 and PIIINP as prognostic markers in patients with alcoholic liver disease. J Hepatol. 2003;39(2):179–186. doi:10.1016/S0168-8278(03)00184-3
51. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention; 2019.
52. Hekking -P-PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi:10.1016/j.jaci.2014.08.042
53. Larsson K, Ställberg B, Lisspers K, et al. Prevalence and management of severe asthma in primary care: an observational cohort study in Sweden (PACEHR). Respir Res. 2018;19(1):12. doi:10.1186/s12931-018-0719-x
54. Antonicelli L, Bucca C, Neri M, et al. Asthma severity and medical resource utilisation. Eur Respir J. 2004;23(5):723–729. doi:10.1183/09031936.04.00004904
55. Wenzel S. Severe asthma: from characteristics to phenotypes to endotypes. Clin Exp Allergy. 2012;42(5):650–658. doi:10.1111/j.1365-2222.2011.03929.x
56. Cartier A, Lehrer SB, Horth-Susin L, et al. Prevalence of crab asthma in crab plant workers in Newfoundland and Labrador. Int J Circumpolar Health. 2004;63(2):333–336. doi:10.3402/ijch.v63i0.17930
57. Tanaka H, Saikai T, Sugawara H, et al. Workplace-related chronic cough on a mushroom farm. Chest. 2002;122(3):1080–1085. doi:10.1378/chest.122.3.1080
58. Brinchmann BC, Bayat M, Brøgger T, Muttuvelu DV, Tjønneland A, Sigsgaard T. A possible role of chitin in the pathogenesis of asthma and allergy. Ann Agric Environ Med. 2011;18(1):7–12.
59. Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol. 2010;10(12):838–848. doi:10.1038/nri2870
60. James AJ, Reinius LE, Verhoek M, et al. Increased YKL-40 and chitotriosidase in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(2):131–142. doi:10.1164/rccm.201504-0760OC
61. Konradsen JR, James A, Nordlund B, et al. The chitinase-like protein YKL-40: a possible biomarker of inflammation and airway remodeling in severe pediatric asthma. J Allergy Clin Immunol. 2013;132(2):328–335. doi:10.1016/j.jaci.2013.03.003
62. Lee JH, Park KH, Park JW, Hong CS. YKL-40 in induced sputum after allergen bronchial provocation in atopic asthma. J Investig Allergol Clin Immunol. 2012;22(7):501–507.
63. Bara I, Ozier A, Girodet P-O, et al. Role of YKL-40 in bronchial smooth muscle remodeling in asthma. Am J Respir Crit Care Med. 2012;185(7):715–722. doi:10.1164/rccm.201105-0915OC
64. Shen C-R, Juang -H-H, Chen H-S, et al. The correlation between chitin and acidic mammalian chitinase in animal models of allergic Asthma. Int J Mol Sci. 2015;16(11):27371–27377. doi:10.3390/ijms161126033
65. Nikota JK, Botelho FM, Bauer CM, et al. Differential expression and function of breast regression protein 39 (BRP-39) in murine models of subacute cigarette smoke exposure and allergic airway inflammation. Respir Res. 2011;12:39. doi:10.1186/1465-9921-12-39
66. Mazur M, Olczak J, Olejniczak S, et al. Targeting acidic mammalian chitinase is effective in animal model of asthma. J Med Chem. 2018;61(3):695–710. doi:10.1021/acs.jmedchem.7b01051
67. Sutherland TE, Andersen OA, Betou M, et al. Analyzing airway inflammation with chemical biology: dissection of acidic mammalian chitinase function with a selective drug-like inhibitor. Chem Biol. 2011;18(5):569–579. doi:10.1016/j.chembiol.2011.02.017
68. Hong JY, Kim M, Sol IS, et al. Chitotriosidase inhibits allergic asthmatic airways via regulation of TGF-β expression and Foxp3+ Treg cells. Allergy. 2018;73(8):1686–1699. doi:10.1111/all.2018.73.issue-8
69. Lee CG, Hartl D, Lee GR, et al. Role of breast regression protein 39 (BRP-39)/chitinase 3-like-1 in Th2 and IL-13-induced tissue responses and apoptosis. J Exp Med. 2009;206(5):1149–1166. doi:10.1084/jem.20081271
70. Bierbaum S, Nickel R, Koch A, et al. Polymorphisms and haplotypes of acid mammalian chitinase are associated with bronchial asthma. Am J Respir Crit Care Med. 2005;172(12):1505–1509. doi:10.1164/rccm.200506-890OC
71. Bierbaum S, Superti-Furga A, Heinzmann A. Genetic polymorphisms of chitotriosidase in Caucasian children with bronchial asthma. Int J Immunogenet. 2006;33(3):201–204. doi:10.1111/eji.2006.33.issue-3
72. Vicencio AG, Chupp GL, Tsirilakis K, et al. CHIT1 mutations: genetic risk factor for severe asthma with fungal sensitization? Pediatrics. 2010;126(4):e982–e985. doi:10.1542/peds.2010-0321
73. Goldman DL, Li X, Tsirilakis K, Andrade C, Casadevall A, Vicencio AG. Increased chitinase expression and fungal-specific antibodies in the bronchoalveolar lavage fluid of asthmatic children. Clin Exp Allergy. 2012;42(4):523–530. doi:10.1111/j.1365-2222.2011.03886.x
74. Santos CB, Davidson J, Covar RA, et al. The chitinase-like protein YKL-40 is not a useful biomarker for severe persistent asthma in children. Ann Allergy Asthma Immunol. 2014;113(3):263–266. doi:10.1016/j.anai.2014.05.024
75. Lai T, Chen M, Deng Z, et al. YKL-40 is correlated with FEV1 and the asthma control test (ACT) in asthmatic patients: influence of treatment. BMC Pulm Med. 2015;15:1. doi:10.1186/1471-2466-15-1
76. Gomez JL, Yan X, Holm CT, et al. Characterisation of asthma subgroups associated with circulating YKL-40 levels. Eur Respir J. 2017;50(4):1700800. doi:10.1183/13993003.00800-2017
77. Saba M, Sharif MR, Akbari H, Nikoueinejad H, Ramazani Jolfaii M. YKL-40 in asthma and its correlation with different clinical parameters. Iran J Allergy Asthma Immunol. 2014;13(4):271–277.
78. Chupp GL, Lee CG, Jarjour N, et al. A chitinase-like protein in the lung and circulation of patients with severe asthma. N Engl J Med. 2007;357(20):2016–2027. doi:10.1056/NEJMoa073600
79. Ahangari F, Sood A, Ma B, et al. Chitinase 3-like-1 regulates both visceral fat accumulation and asthma-like Th2 inflammation. Am J Respir Crit Care Med. 2015;191(7):746–757. doi:10.1164/rccm.201405-0796OC
80. James A, Stenberg Hammar K, Reinius L, et al. A longitudinal assessment of circulating YKL-40 levels in preschool children with wheeze. Pediatr Allergy Immunol. 2017;28(1):79–85. doi:10.1111/pai.2017.28.issue-1
81. Hinks TSC, Brown T, Lau LCK, et al. Multidimensional endotyping in patients with severe asthma reveals inflammatory heterogeneity in matrix metalloproteinases and chitinase 3-like protein 1. J Allergy Clin Immunol. 2016;138(1):61–75. doi:10.1016/j.jaci.2015.11.020
82. Tang H, Sun Y, Shi Z, et al. YKL-40 induces IL-8 expression from bronchial epithelium via MAPK (JNK and ERK) and NF-κB pathways, causing bronchial smooth muscle proliferation and migration. J Immunol. 2013;190(1):438–446. doi:10.4049/jimmunol.1201827
83. Louis R, Lau LC, Bron AO, Roldaan AC, Radermecker M. Djukanović R The relationship between airways inflammation and asthma severity. Am J Respir Crit Care Med. 2000;161(1):9–16. doi:10.1164/ajrccm.161.1.9802048
84. Fukakusa M, Bergeron C, Tulic MK, et al. Corticosteroids decrease eosinophil and CC chemokine expression but increase neutrophil, IL-8, and IFN-gamma-inducible protein 10 expression in asthmatic airway mucosa. J Allergy Clin Immunol. 2005;115(2):280–286. doi:10.1016/j.jaci.2004.10.036
85. Ray A, Kolls JK. Neutrophilic inflammation in asthma and association with disease severity. Trends Immunol. 2017;38(12):942–954. doi:10.1016/j.it.2017.07.003
86. Hunt JF, Fang K, Malik R, et al. Endogenous airway acidification. Implications for asthma pathophysiology. Am J Respir Crit Care Med. 2000;161(3):694–699. doi:10.1164/ajrccm.161.3.9911005
87. Tesse R, Fiore F, Sillecchia O, et al. Effects of inhaled corticosteroids on exhaled breath condensate (EBC) pH and cytokines levels in children with asthma and atopic dermatitis (AD). J Allergy Clin Immunol. 2006;117(2):S281. doi:10.1016/j.jaci.2005.12.1163
88. Okawa K, Ohno M, Kashimura A, et al. Loss and gain of human acidic mammalian chitinase activity by nonsynonymous SNPs. Mol Biol Evol. 2016;33(12):3183–3193. doi:10.1093/molbev/msw198
89. Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi:10.1016/S0140-6736(12)61729-2
90. World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva: World Health Organization; 2018.
91. Verweij PE, Kerremans JJ, Voss A, Meis JF. Fungal contamination of tobacco and Marijuana. JAMA. 2000;284(22):2875. doi:10.1001/jama.284.22.2869
92. Létuvé S, Kozhich A, Arouche N, et al. YKL-40 is elevated in patients with chronic obstructive pulmonary disease and activates alveolar macrophages. J Immunol. 2008;181(7):5167–5173. doi:10.4049/jimmunol.181.7.5167
93. Matsuura H, Hartl D, Kang M-J, et al. Role of breast regression protein-39 in the pathogenesis of cigarette smoke-induced inflammation and emphysema. Am J Respir Cell Mol Biol. 2011;44(6):777–786. doi:10.1165/rcmb.2010-0081OC
94. Guerra S, Halonen M, Sherrill DL, et al. The relation of circulating YKL-40 to levels and decline of lung function in adult life. Respir Med. 2013;107(12):1923–1930. doi:10.1016/j.rmed.2013.07.013
95. Majewski S, Tworek D, Szewczyk K, et al. Overexpression of chitotriosidase and YKL-40 in peripheral blood and sputum of healthy smokers and patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2019;14:1611–1631.
96. Lai T, Wu D, Chen M, et al. YKL-40 expression in chronic obstructive pulmonary disease: relation to acute exacerbations and airway remodeling. Respir Res. 2016;17:31. doi:10.1186/s12931-016-0338-3
97. van Eijk M, van Roomen CPAA, Renkema GH, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17(11):1505–1512. doi:10.1093/intimm/dxh328
98. van Eijk M, Scheij SS, van Roomen CP, Speijer D, Boot RG, Aerts JM. TLR- and NOD2-dependent regulation of human phagocyte-specific chitotriosidase. FEBS Lett. 2007;581(28):5389–5395. doi:10.1016/j.febslet.2007.10.039
99. Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J Clin Lab Anal. 2005;19(3):128–132. doi:10.1002/(ISSN)1098-2825
100. Cho SJ, Weiden MD, Lee CG. Chitotriosidase in the pathogenesis of inflammation, interstitial lung diseases and COPD. Allergy Asthma Immunol Res. 2015;7(1):14–21. doi:10.4168/aair.2015.7.1.14
101. Lee CM, He CH, Park JW, et al. Chitinase 1 regulates pulmonary fibrosis by modulating TGF-β/SMAD7 pathway via TGFBRAP1 and FOXO3. Life Sci Alliance. 2019;2(3):e201900350.
102. Lee CG, Herzog EL, Ahangari F, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β1 signaling. J Immunol. 2012;189(5):2635–2644. doi:10.4049/jimmunol.1201115
103. Lee CG, Homer RJ, Zhu Z, et al. Interleukin-13 induces tissue fibrosis by selectively stimulating and activating transforming growth factor beta(1). J Exp Med. 2001;194(6):809–821. doi:10.1084/jem.194.6.809
104. Sakazaki Y, Hoshino T, Takei S, et al. Overexpression of chitinase 3-like 1/YKL-40 in lung-specific IL-18-transgenic mice, smokers and COPD. PLoS One. 2011;6(9):e24177. doi:10.1371/journal.pone.0024177
105. Kang MJ, Yoon CM, Nam M, et al. Role of chitinase 3-Like-1 in Interleukin-18-induced pulmonary type 1, type 2, and type 17 inflammation; alveolar destruction; and airway fibrosis in the murine lung. Am J Respir Cell Mol Biol. 2015;53(6):863–871. doi:10.1165/rcmb.2014-0366OC
106. Kang MJ, Homer RJ, Gallo A, et al. IL-18 is induced and IL-18 receptor alpha plays a critical role in the pathogenesis of cigarette smoke-induced pulmonary emphysema and inflammation. J Immunol. 2007;178(3):1948–1959. doi:10.4049/jimmunol.178.3.1948
107. Gumus A, Kayhan S, Cinarka H, et al. High serum YKL-40 level in patients with COPD is related to hypoxemia and disease severity. Tohoku J Exp Med. 2013;29(2):163–170. doi:10.1620/tjem.229.163
108. Aminuddin F, Akhabir L, Stefanowicz D, et al. Genetic association between human chitinases and lung function in COPD. Hum Genet. 2012;131(7):1105–1114. doi:10.1007/s00439-011-1127-1
109. Cho SJ, Nolan A, Echevarria GC, et al. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013;33(6):1134–1142. doi:10.1007/s10875-013-9913-2
110. Bojesen SE, Johansen JS, Nordestgaard BG. Plasma YKL-40 levels in healthy subjects from the general population. Clin Chim Acta. 2011;412(9–10):709–712. doi:10.1016/j.cca.2011.01.022
111. Kurt I, Abasli D, Cihan M, et al. Chitotriosidase levels in healthy elderly subjects. Ann N Y Acad Sci. 2007;1100:185–188. doi:10.1196/annals.1395.017
112. Chatterjee R, Batra J, Das S, Sharma SK, Ghosh B. Genetic association of acidic mammalian chitinase with atopic asthma and serum total IgE levels. J Allergy Clin Immunol. 2008;122(1):202–208. doi:10.1016/j.jaci.2008.04.030
113. Ober C, Tan Z, Sun Y, et al. Effect of variation in CHI3L1 on serum YKL-40 level, risk of asthma, and lung function. N Engl J Med. 2008;358(16):1682–1691. doi:10.1056/NEJMoa0708801
114. Wu AC, Lasky-Su J, Rogers CA, Klanderman BJ, Litonjua A. Polymorphisms of chitinases are not associated with asthma. J Allergy Clin Immunol. 2010;125(3):754–757. doi:10.1016/j.jaci.2009.12.995
115. Johansen JS, Stoltenberg M, Hansen M, et al. Serum YKL-40 concentrations in patients with rheumatoid arthritis: relation to disease activity. Rheumatol Oxf Engl. 1999;38(7):618–626. doi:10.1093/rheumatology/38.7.618
116. Kunz LIZ, Van’t Wout EFA, van Schadewijk A, et al. Regulation of YKL-40 expression by corticosteroids: effect on pro-inflammatory macrophages in vitro and its modulation in COPD in vivo. Respir Res. 2015;16:154. doi:10.1186/s12931-015-0314-3
117. Falcoz C, Oliver R, McDowall JE, Ventresca P, Bye A, Daley-Yates PT. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39(1):9–15. doi:10.2165/00003088-200039001-00002
118. Culpitt SV, Rogers DF, Shah P, et al. Impaired inhibition by dexamethasone of cytokine release by alveolar macrophages from patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(1):24–31. doi:10.1164/rccm.200204-298OC
119. Agapov E, Battaile JT, Tidwell R, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41(4):379–384. doi:10.1165/2009-0122R
120. Rao FV, Andersen OA, Vora KA, Demartino JA, van Aalten DM. Methylxanthine drugs are chitinase inhibitors: investigation of inhibition and binding modes. Chem Biol. 2005;12(9):973–980. doi:10.1016/j.chembiol.2005.07.009
121. Matsumoto T, Inoue H, Sato Y, et al. Demethylallosamidin, a chitinase inhibitor, suppresses airway inflammation and hyperresponsiveness. Biochem Biophys Res Commun. 2009;390(1):103–108. doi:10.1016/j.bbrc.2009.09.075
122. Mazur M, Bartoszewicz A, Dymek B, et al. Discovery of selective, orally bioavailable inhibitor of mouse chitotriosidase. Bioorg Med Chem Lett. 2018;28(3):310–314. doi:10.1016/j.bmcl.2017.12.047
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.