Back to Journals » International Medical Case Reports Journal » Volume 13
Chemotherapy-Induced Central Retinal Artery Occlusion in Gestational Trophoblastic Neoplasia: Case Report
Authors Khadka S , Byanju R, Poon S
Received 17 June 2020
Accepted for publication 11 August 2020
Published 15 September 2020 Volume 2020:13 Pages 431—435
DOI https://doi.org/10.2147/IMCRJ.S266456
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Simanta Khadka, Raghunandan Byanju, Suchan Poon
Department of Vitreo-Retina, Bharatpur Eye Hospital, Bharatpur, Chitwan, Nepal
Correspondence: Simanta Khadka
Bharatpur Eye Hospital, Bharatpur, Chitwan Tel +977-9841572286
Fax +977-056-523333
Email [email protected]
Abstract: The use of anticancer chemotherapy (ACC) has resulted in longer patient survival but has also increased drug-related adverse effects. A 22-year-old female receiving cisplatin-based intravenous chemotherapy for high risk variant of gestational trophoblastic neoplasia (GTN) presented with complaints of sudden painless loss of vision in her right eye for a duration of 4 hours. Ocular findings were suggestive of central retinal artery occlusion (CRAO). After exclusion of other potential aetiological risk factors, the patient was diagnosed with CRAO associated with cisplatin. Cancer patients are prone to thromboembolic events (TEE) not only due to primary disease but also due to underlying comorbidities and treatment modalities. The high incidence of TEE in patients under cisplatin therapy mandates a high degree of suspicion among the treating physicians. This rare possibility of irreversible visual toxicity should also be considered among the patients under cisplatin chemotherapy.
Keywords: central retinal artery occlusion, chemotherapy, cisplatin, gestational trophoblastic neoplasia, thromboembolism
Introduction
Central retinal artery occlusion (CRAO) is considered to be an acute ischemic stroke of the eye that leads to painless and profound visual loss. CRAO occurs due to occlusion of the central retinal artery usually by a fibrin-platelet thrombus or embolus with resultant hypo-perfusion of the retina and optic nerve head which is frequently irreversible.1 Cerebrovascular and cardiovascular diseases are usually associated with acute retinal arterial occlusion.2
The increased use of chemotherapeutic agents in cancer patients has resulted in longer patient survival rate. Consequently adverse ocular side effects secondary to these anticancer agents are also reported.3 Moreover, neoplasm itself is an important risk factor for venous and arterial thromboembolic events (TEE). Cancer patients are prone to TEE due to primary disease, underlying comorbidities, and treatment modalities.4 The ocular toxicity induced by anticancer chemotherapy (ACC) includes a broad spectrum of disorders which signifies the unique anatomical, physiological and biochemical features of the eye.5 Chemotherapeutic agents may result in vascular complications such as veno-occlusive disease, venous thrombosis, and vascular ischemia.6
Pharmacological agents such as platinum-based compounds, vinca alkaloids, bleomycin, and tamoxifen are considered to be independent risk factors for vascular events and cause vaso-occlusive complications.7 However, retinal artery occlusions following ACC is a rare complication and is reported only in a few instances.
We describe a rare encounter of CRAO, which developed in a patient while undergoing treatment for gestational trophoblastic neoplasia (GTN) with intravenous ACC.
Case Description
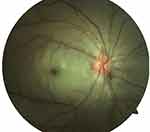
A 22-year-old female presented to the ocular emergency department with complaints of sudden painless loss of vision in her right eye for a duration of 4 hours. She was a diagnosed case of GTN and had been under a chemotherapeutic regimen for 12 months. There was no other significant history and she was a non-smoker and does not consume alcohol. Her vision at presentation was perception of light (PL) OD and 6/6 OS. Ocular examination OD revealed RAPD and dilated fundus examination showed presence of peripapillary axoplasmic ring with few peripapillary hemorrhages, white ischemic retina, attenuated arterioles and central cherry red spot. However, no thrombus or emboli was visible in the retinal vascular arcades (Figure 1). The anterior and posterior segment findings of the fellow eye were within normal limits.
Immediately following the ocular findings, possible measures recommended for CRAO were applied. Digital ocular massage along with carbogen inhalation therapy in a plastic bag was initiated and continued. Tablet acetazolamide 500mg and tablet ecospirin 75 mg stat. dose was also administered orally. Anterior chamber paracentesis was performed under aseptic precaution, but these efforts were to no avail. Internist consultation was advised to rule out other possible etiology leading to TEE.
Her complete blood count, renal-hepatic functions, acute phase reactants and lipid work up were normal. Fibrinogen, antiphospholipid antibodies, prothrombin time, activated thromboplastin time and serum protein screened for coagulopathies were also within normal limits. Echocardiography and neuro-imaging revealed no abnormality. However, carotid doppler ultrasonography revealed echogenic non-occluding thrombus in bilateral proximal common carotid artery without significant stenosis.
The past medical history of the patient was acquired as well. She was primigravida (G1) when she presented with a complaint of per vaginal bleeding at third month of her pregnancy. She underwent manual vacuum aspiration for high beta human chorionic gonadotropin hormone (β-HCG) level of 90,630 mIU/ml and the histopathological report confirmed complete hydatiform mole. She was started on a single agent methotrexate. Despite six doses of methotrexate, her β-HCG level was not regulated. Subsequently, combination chemotherapy including etoposide, methotrexate, actinomycin-D, cyclophosphamide and vincristine (EMA-CO) regimen was administered. She received six cycles of EMA-CO regimen and was stopped after three consecutive normal β-HCG levels. However, her β-HCG level started to peak again after 5 months of completion of her previous combination chemotherapeutic regimen and without pregnancy. Then she was started on etoposide, cisplatin, methotrexate, actinomycin-D (EMA-EP) regimen. During the course of EMA-EP regimen after the completion of her second cycle, she lost her vision in the right eye and presented to us on the same day 4 hours later. The regimen was stopped after consultation with the oncology department. She was then started on an alternative regimen consisting of paclitaxel.
She was under constant follow-up with the oncology department of the cancer hospital located in Bharatpur and under periodic follow-up for her ocular condition. Within a span of 1 year, her vision was hand movement OD and 6/6 OS. Ocular findings OD revealed RAPD and dilated fundus examination showed pale optic disc suggestive of optic atrophy (Figures 2 and 3). Gonioscopy was performed which revealed open angles in all the quadrants without any evidence of angle neovascularization.
 |
Figure 2 Fundus photograph of both eyes at the end of 1 year follow up. Optic atrophy with attenuated vessels is evident in the right eye whereas the fundus is within normal limits in the left eye. |
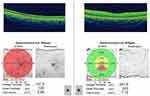 |
Figure 3 Optical coherence tomography (OCT) at the end of 1 year follow-up. (A) Gross retinal atrophy is visible in the right eye. (B) Normal OCT in the left eye. |
Discussion
Gestational trophoblastic neoplasia (GTN) arises from abnormal proliferation of placental trophoblasts and includes a group of interrelated lesions. GTN are generally highly responsive to ACC.8 The composite regimen of chemotherapeutic agents in our case unambiguously point towards the high risk and resistant variant of GTN.9
TEE are frequent complications of either primary disease and/or treatment modalities in oncology practice.4 The association between malignancy and thrombosis was first described in the 1860s and was termed as Trousseau syndrome.10 ACC has been associated with the development of several vascular complications in patients undergoing treatment.6 Moreover, chemotherapy itself is considered as an independent but significant risk factors for thrombosis.11 The possible mechanism of vascular toxicity by chemotherapeutic agents is due to endothelium damage, disequilibrium between pro-coagulant and anticoagulant molecules and induction of tumor/endothelial apoptosis.12 The endothelial damage is due to the release of cytokines like tumor necrosis factor (TNF) α, interleukins (IL)-1, and IL-6.13 Similarly, activation of platelets and factors XII and X due to interaction between tumor cells and macrophages leads to thrombin generation and thrombosis.14
There are numerous agents associated with increased risk of TEE in patients under chemotherapy. The patient in our scenario also received single agent methotrexate followed by EMA-CO and EMA-EP regimen when previous combination chemotherapeutic agents were not effective. There is no clear consensus on which specific drug led to the vision deteriorating CRAO. Though the history of the patient itself suggests that the event occurred only after administration of the combination regimen consisting of cisplatin. There are abundant literature that supports the evidence of arterial thromboembolism after combination therapeutic regimen inclusive of cisplatin.6,7,15–17 Furthermore, there was no other associated arteriopathic risk factors (history of smoking, hypertension or diabetes mellitus) in our case. Hence the authors believe that cisplatin probably contributed to CRAO in this particular patient.
Cisplatin is one of the widely used anticancer chemotherapeutic agents which is effective against several human malignancies.18 The pathogenesis of thrombosis induced by cisplatin is obscure, although few possible mechanisms are suggested. Cisplatin induces endothelial injury and exposes the sub endothelial structures releasing the procoagulant micro particles. It also activates the platelets and upregulates the prothrombotic von Willebrand factor.19 Hypomagnesemia and autonomic dysfunction leading to vasospasm has also been implicated to thrombogenicity.20 This pathway involves both venous as well as arterial systems, contrary to which the majority of thrombotic risk factors affects venous compartments only.17 Furthermore, a histopathological examination of the blood vessel after intravenous administration of cisplatin showed vascular intimal edema, pyknosis of the endothelial cells and thrombus formation.20 The incidence of thromboembolic phenomenon was found to be higher (1.92%) in patients treated with cisplatin-based chemotherapy compared to those treated with non cisplatin-based regimens (0.79%). Similarly, patients under cisplatin-based chemotherapy had a significant risk of venous thrombosis with a relative risk of 1.67.21
Though limited, a few other reported ophthalmic complications of cisplatin chemotherapy include retinal pigment abnormality, retinal ischemia with neovascularization, retinal microvascular complications, subclinical retinal dysfunction, cone dysfunction, optic neuropathy, and cortical blindness.22,23 The stated timing of thromboembolic event tends to occur with a median interval of 52 days,24 or during the first two courses of cisplatin therapy.25 In accordance, the circumstance of ocular morbidity was similar in our case which occurred after the completion of the second cycle during the course of EMA-EP regimen.
The discontinuation of cisplatin therapy has even resulted in reversal of visual symptoms in a case of branch retinal artery occlusion.22 Nonetheless, the prime determinant in visual recovery in CRAO is considered to be the time factor. The longer the duration of ischemia, the more extensive the damage. The retina can withstand ischemia without detectable damage up to 97 minutes. But CRAO lasting for approximately 240 minutes suffers irreversible damage.26 The patient in our report presented 4 hours after the ocular event, and even with our best efforts the vision was not recovered and the sequelae of retinal ischemia was evident with optic and retinal atrophy. There is a high chance of development of ocular neovascularization following CRAO. The reported prevalence is 18.2% with an average duration of 8.5 weeks.27 The reason behind our patient without ocular neovascularization after a follow-up of 1 year, might be because of timely abolishment of thrombogenic trigger after the occurrence of CRAO and absence of other arteriopathic risk factors.
To the best of our knowledge, this could be the first case of CRAO in GTN under cisplatin therapy. The ocular complications induced by ACC are often underestimated and neglected, which is due to the priority given to other life-threatening adverse events. But deterioration of vision ultimately affects the quality of life in cancer patients.28 The high incidence of TEE in patients under cisplatin therapy mandates a high degree of suspicion among the treating physicians. Clinicians acquainted with the administration of cisplatin should be aware of this rare complication, and should work in co-ordination with ophthalmologists to prevent irreversible visual toxicities induced by chemotherapeutic agents.
Abbreviations
ACC, anticancer chemotherapy; CRAO, central retinal artery occlusion; GTN, gestational trophoblastic neoplasia; PL, perception of light; OD, oculus dexter; OS, oculus sinister; β-HCG, beta human chorionic gonadotropin hormone; EMA-CO, etoposide, methotrexate, actinomycin-D, cyclophosphamide and vincristine; EMA-EP, etoposide, cisplatin, methotrexate, actinomycin-D; IL, interleukins; OCT, optical coherence tomography; TEE, thromboembolic events; TNF, tumor necrosis factor.
Ethical Consideration
The identity of the patient has been anonymized throughout the text. Written and informed consent has been obtained from the patient for the publication of the case details and accompanying images. Institutional approval was not required to publish case details.
Acknowledgment
Dr Jayaram Adhikari, MD, Medical Oncology Department, BP Koirala Memorial Cancer Hospital, Bharatpur, Chitwan.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Chen CS, Lee AW. Management of acute central retinal artery occlusion. Nat Clin Pract Neurol. 2008;4(7):376–383. doi:10.1038/ncpneuro0811
2. Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmol. 2000;11(6):462–467. doi:10.1097/00055735-200012000-00013
3. Fraunfelder F, Meyer SM. Ocular toxicity of antineoplastic agents. Ophthalmology. 1983;90(1):1–3. doi:10.1016/S0161-6420(83)34600-5
4. Alkan A, Talaz S. Cilioretinal artery occlusion associated with cisplatin. J Oncol Pharm Pract. 2019;25(4):969–971. doi:10.1177/1078155218759805
5. Omoti AE, Omoti CE. Ocular toxicity of systemic anticancer chemotherapy. Pharm Pract. 2006;4(2):55. doi:10.4321/S1885-642X2006000200001
6. Cool RM, Herrington JD, Wong L. Recurrent peripheral arterial thrombosis induced by cisplatin and etoposide. Pharmacotherapy. 2002;22(9):1200–1204. doi:10.1592/phco.22.13.1200.33524
7. Oshitari T. Central retinal artery occlusion during cisplatin and etoposide chemotherapy for small cell lung cancer. Int J Ophthalmol Eye Res. 2015;3(4):107–109.
8. May T, Goldstein DP, Berkowitz RS. Current chemotherapeutic management of patients with gestational trophoblastic neoplasia. Chemother Res Pract. 2011;2011:1–12. doi:10.1155/2011/806256
9. Bower M, Newlands E, Holden L, et al. EMA/CO for high-risk gestational trophoblastic tumors: results from a cohort of 272 patients. J Clin Oncol. 1997;15(9):3168. doi:10.1200/JCO.1997.15.9.3168
10. Trousseau A. Phlegmasia alba dolens. Lect Clin Med. 1865;5:281–332.
11. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000;160(6):809–815. doi:10.1001/archinte.160.6.809
12. Haddad TC, Greeno EW. Chemotherapy-induced thrombosis. Thromb Res. 2006;118(5):555–568. doi:10.1016/j.thromres.2005.10.015
13. Bick RL. Cancer-associated thrombosis. N Engl J Med. 2003;349(2):109–111. doi:10.1056/NEJMp030086
14. Cyriac S, Sagar T, Mahajan V. Choriocarcinoma with arterial and venous thrombosis. Neurol India. 2009;57(4):505–507. doi:10.4103/0028-3886.55586
15. Conti JA, Scher HI. Acute arterial thrombosis after escalated‐dose methotrexate, vinblastine, doxorubicin, and cisplatin chemotherapy with recombinant granulocyte colony‐stimulating factor: a possible new recombinant granulocyte colony‐stimulating factor toxicity. Cancer. 1992;70(11):2699–2702. doi:10.1002/1097-0142(19921201)70:11<2699::AID-CNCR2820701122>3.0.CO;2-C
16. Molloy R, Welch G, Drury J, Abel B. Arterial thrombosis after chemotherapy with cisplatin, vinblastine and methotrexate. Br J Clin Pract. 1995;49(1):50–51.
17. Moore RA, Adel N, Riedel E, et al. High incidence of thromboembolic events in patients treated with cisplatin-based chemotherapy: a large retrospective analysis. J Clin Oncol. 2011;29(25):3466. doi:10.1200/JCO.2011.35.5669
18. Hanigan MH, Devarajan P. Cisplatin nephrotoxicity: molecular mechanisms. Cancer Ther. 2003;1:47.
19. Licciardello JT, Moake JL, Rudy CK, Karp DD, Hong WK. Elevated plasma von Willebrand factor levels and arterial occlusive complications associated with cisplatin-based chemotherapy. Oncology. 1985;42(5):296–300. doi:10.1159/000226049
20. Icli F, Karaoguz H, Dincol D, et al. Severe vascular toxicity associated with cisplatin-based chemotherapy. Cancer. 1993;72(2):587–593. doi:10.1002/1097-0142(19930715)72:2<587::AID-CNCR2820720242>3.0.CO;2-V
21. Seng S, Liu Z, Chiu SK, et al. Risk of venous thromboembolism in patients with cancer treated with cisplatin: a systematic review and meta-analysis. J Clin Oncol. 2012;30(35):4416–4426. doi:10.1200/JCO.2012.42.4358
22. Mitra A, Edmunds MR, Walji N, Fernando IN, Scott RA, Good P. Reversible branch retinal artery occlusion following intravenous cisplatin chemotherapy for cervical carcinoma. Int Ophthalmol. 2011;31(5):429–432. doi:10.1007/s10792-011-9476-2
23. Kwan AS, Sahu A, Palexes G. Retinal ischemia with neovascularization in cisplatin related retinal toxicity. Am J Ophthalmol. 2006;141(1):196–197. doi:10.1016/j.ajo.2005.07.046
24. Weijl NI, Rutten MF, Zwinderman AH, et al. Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. J Clin Oncol. 2000;18(10):2169–2178. doi:10.1200/JCO.2000.18.10.2169
25. Numico G, Garrone O, Dongiovanni V, et al. Prospective evaluation of major vascular events in patients with nonsmall cell lung carcinoma treated with cisplatin and gemcitabine. Cancer. 2005;103(5):994–999. doi:10.1002/cncr.20893
26. Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140(3):
27. Rudkin AK, Lee AW, Chen CS. Ocular neovascularization following central retinal artery occlusion: prevalence and timing of onset. Eur J Ophthalmol. 2010;20(6):1042–1046. doi:10.1177/112067211002000603
28. Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51(1):19–40. doi:10.1016/j.survophthal.2005.11.001
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.