Back to Journals » Drug Design, Development and Therapy » Volume 9
Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin
Authors Peng X, Zhou Y, Han K, Qin L, Dian L, Li G, Pan X, Wu C
Received 10 April 2015
Accepted for publication 4 May 2015
Published 3 August 2015 Volume 2015:9 Pages 4209—4218
DOI https://doi.org/10.2147/DDDT.S86370
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Wei Duan
Xinsheng Peng,1,* Yanfang Zhou,1,* Ke Han,2,3 Lingzhen Qin,3 Linghui Dian,1 Ge Li,4 Xin Pan,3 Chuanbin Wu3
1Guangdong Medical University, Dongguan, 2The Second Affiliated Hospital of Guangzhou Medical University, 3School of Pharmaceutical Sciences, Sun Yat-Sen University, 4Guangzhou Neworld Pharmaceuticals Co. Ltd., Guangzhou, Guangdong, People’s Republic of China
*These authors contributed equally to this work
Abstract: Phytantriol- and glycerol monooleate-based cubosomes were produced and characterized as a targeted and sustained transdermal delivery system for capsaicin. The cubosomes were prepared by emulsification and homogenization of phytantriol (F1), glycerol monooleate (F2), and poloxamer dispersions, characterized for morphology and particle size distribution by transmission electron microscope and photon correlation spectroscopy. Their Im3m crystallographic space group was confirmed by small-angle X-ray scattering. An in vitro release study showed that the cubosomes provided a sustained release system for capsaicin. An in vitro diffusion study conducted using Franz diffusion cells indicated that the skin retention of capsaicin from cubosomes in the stratum corneum was much higher (2.75±0.22 µg versus 4.32±0.13 µg, respectively) than that of capsaicin cream (0.72±0.13 µg). The stress testing showed that the cubosome formulations were stable under strong light and high temperature for up to 10 days. After multiapplications on mouse skin, the irritation of capsaicin cubosomes and cream was light with the least amount of side effects. Overall, the present study demonstrated that cubosomes may be a suitable skin-targeted and sustained delivery system for the transdermal administration of capsaicin.
Keywords: cubosomes, skin-targeted delivery, capsaicin
Introduction
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide; Figure 1) is a natural alkaloid extracted from the fruits of the capsicum plant family. It exhibits broad bioactivities and has been used for the treatment of psoriasis, pruritus, apocrine chromhidrosis, and contact allergy.1,2 Because of its high degree of first-pass metabolism in the gastrointestinal tract and short half-life by intravenous administration (about 7 minutes),3 the transdermal delivery system seems to be more advantageous for capsaicin. Hydrogel, solution, ointment and cream, microemulsion, transfersome, noisome, and liposome preparations of capsaicin have been documented in the literature to treat pain-related disorders such as rheumatoid arthritis, osteoarthritis, diabetic neuropathy, and postoperative pain.4–9 Among them, transfersomes, niosomes, and liposomes are especially efficient to enhance the penetration of capsaicin through the skin and the absorption of the drug into the systemic circulation. Due to its short half-life, the transdermal application of capsaicin needs more frequent replacements in order to achieve satisfactory pain relief. However, the replacement of postoperation or postincision wound dressing is painful. In addition, overdosage of capsaicin adsorbed by systemic circulation may induce potential systemic side effects. So, skin-targeted delivery would be optimal for capsaicin, which could ensure that the drug would be mainly absorbed by the skin with less systemic absorption as compared with the conventional transdermal application.10–12 Therefore, the purpose of this study was to develop a novel delivery system that could enhance drug absorption to the skin and, at the same time, retard the systemic diffusion of the skin-absorbed drug.
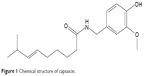  | Figure 1 Chemical structure of capsaicin. |
The cubic phase is an intriguing drug delivery system consisting of a curved continuous lipid bilayer extending in three dimensions and separating two congruent networks of water channels.13–16 It can enclose hydrophilic, amphiphilic, and hydrophobic substances ranging from small-molecular-weight drugs to proteins, peptides, amino acids, and nucleic acids.16 Typically, three forms of cubic phase have been used as drug delivery systems: cubic phase gel; cubic phase precursor; and cubosome. Cubic phase gels have been commonly used for mucosal, vaginal, periodontal, and transdermal drug delivery. Bender et al17 reported that lipid cubic phase gels significantly increased the delivery of δ-aminolevulinic acid hydrochloride and methyl δ-aminolevulinate hydrochloride through topical administration as compared with the normal ointments. However, the stiff and viscous nature of the cubic phase gel limits its potential use.15,16 Though low viscous cubic phase gel could be obtained by adding organic solvents (propylene glycol, ethanol, polyethylene glycol, and N-methyl-2-pyrrolidone),18,19 we found that the gel was not easy to spread and adhere to the skin. Cubosomes posses much lower viscosity than the bulk cubic phase,16 and they show better storing stability at room temperature and greater endurance of heat treatment as compared to liposomes.20–22 Besides, cubosomes could exist at almost any dilution level in water, and drug leakage was less of a concern as compared with liposomes.
Cubosomes and liposomes both have a bilipid membrane, and liposome gel could act in the skin-targeted transdermal drug delivery of hydrocortisone.23 Gan et al24 reported that dexamethasone-loaded cubosomes exhibited a permeability coefficient that was 4.5-fold greater than eye drops. Also, Morsi et al25 successfully developed silver sulfadiazine-loaded cubosome hydrogels for the topical treatment of burns with better patient compliance, excellent healing results, and the least amount of side effects. All of these properties are favorable for capsaicin delivery. Therefore, we hypothesized that cubosomes with such favorable properties might represent a promising vehicle for the effective transdermal delivery of capsaicin. As such, we performed the following experiments to test our hypothesis.
Materials and methods
Materials and reagents
Glycerol monooleate (GMO) (DIMODAN® MO/D KOSHER; catalog number: 116703) was kindly provided by Danisco Cultor (Brabrand, Denmark) and used as received. Poloxamer 407 (PEO98POP67PEO98) was a gift from BASF (Ludwigshafen, Germany). Phytantriol (PYT) was purchased from DSM (Basel, Switzerland). Capsaicin was purchased from Wuhan Hengshuo Technology Development Co. Ltd. (Wuhan, Hubei, People’s Republic of China). Capsaicin cream (Changchun Puhua Pharmaceutical Co., Ltd., Changchun, Jilin, People’s Republic of China) contained 2.5 mg/10 g of capsaicin. A dialysis bag with a molecular weight cutoff of 14,000 Da (Viskase Companies, Inc., Darien, IL, USA) was used. Milli-Q grade water purified through a Millipore system (ELGA LabWater, Sartorius; Reading, UK) was used throughout this study. All other reagents were of high-performance liquid chromatography (HPLC) or analytical grade and used as received. Animal experiments were carried out according to the protocol approved by the Experimental Animal Committee of Guangdong Medical University, Dongguan, Guangdong, People’s Republic of China.
Preparation of capsaicin-loaded cubosomes
Capsaicin-loaded cubosomes were prepared through the fragmentation of GMO or PYT/poloxamer 407 bulk cubic phase gels. The formulations are shown in Table 1. GMO or PYT and poloxamer 407 were first completely melted at 60°C in a hot water bath, and then capsaicin was added to dissolve/blend under continuous stirring. Water (~6.7 mL) was then added gradually and the mixture was vortex-mixed to achieve a homogeneous state. After equilibration for 48 hours at room temperature, the cubic phase gel was formed. With the addition of approximately 20 mL of water, the cubic gel was disrupted under mechanical stirring. Subsequently, the crude dispersion of the gel was fragmented for 10 minutes by intermittent probe sonication (JYD-650; Shanghai Zhixin Instrument Co., Ltd., Shanghai, People’s Republic of China) at 400 W energy input with a pulse mode (9-second pulses interrupted by 18-second breaks) under cooling in a 20°C water bath. Afterward, the resulting milky coarse dispersion was homogenized using a high-pressure homogenizer (Em-C3; AVESTIN, Inc., Ottawa, ON, Canada) at certain high pressures and cycles to form an opalescent dispersion of the cubic nanoparticles. The final dispersion of liquid crystal nanoparticles was stored at room temperature for further studies.26
Determination of particle size of cubosomes
The size distribution of cubosomes was measured using a Zetasizer Nano ZS90 (Malvern Instruments, Malvern, UK). The samples were diluted 50-fold with water to 2% before measurement. Cubosomal size was analyzed by the Dispersion Technology Software provided by Malvern Instruments.
Transmission electron microscopy
Transmission electron microscopy (TEM) images were taken using a JEM 1400 JEOL instrument (JEOL, Tokyo, Japan). Specimens were prepared by drop casting of the cubosomes (10 μL) on a copper grid with 10% phosphotungstic acid. The size distribution was determined by examining an overview TEM image using software.
Small-angle X-ray scattering
Small-angle X-ray scattering (SAXS) measurements were carried out on a high-flux SAXS instrument (Anton Paar GmbH, Graz, Austria) operating in the line of collimation and equipped with an imaging plate (IP) as a detector. The IP with a pixel size of 42.3×42.3 μm2 was extended into a wide-angle range (the q range covered by the IP was up to 28 nm−1, q=[4πsinθ]/λ, where λ is the wavelength of 0.1542 nm and 2θ is the scattering angle). The liquid samples were carefully loaded into a quartz capillary with a diameter of 1 mm and exposed for 60 minutes.
Encapsulation efficiency
Encapsulation efficiency (EE) was evaluated using a membrane dialysis method, as described.27 The sample (2 mL) was dispersed in a dialysis bag, which was placed into a flask and filled with 50 mL of water. The flask was then put in a rotary incubator shaker with a setting of 100 rpm and 37°C. After 4 hours of shaking, the concentration of free capsaicin was determined by HPLC.28 The EE was calculated per the formula:
EE% = (Ctotal con - Cfree con)/Ctotal con ×100% | (1) |
Stability study
The physical stability of the cubosomes was studied by stress testing according Chinese Pharmacopoeia 2010 (part 2, Appendix XIX C). Namely, the cubosomal samples were deposited and sealed in glass sterile vials, and then the vials were placed in a drug stability test chamber (Chongqing Yongsheng Experiment Instrument Factory, Chongqing, People’s Republic of China) under strong light (4,500±500 lx) and high temperature (60°C), respectively, for 10 days. At the days 5 and 10, the appearance and particle size of the samples were checked.
In vitro release and evaluation of the release mechanism
The release of capsaicin from cubosomes in water at 37°C was evaluated by the dialysis method. The cubosomal sample (5 mL) was transferred into a dialysis bag, which was placed into a flask and filled with 250 mL of water. The flask was then put into a rotary incubator shaker at 50 rpm and 37°C. An aliquot of 5 mL was taken periodically and replaced with the same volume of water. The concentration of the released capsaicin was determined by HPLC on the basis of the standard curve. Different mathematical models of Higuchi, first-order, zero-order, Weibull, Hixson–Crowell, and Ritger–Peppas equations were used to explain the mechanism of capsaicin release from the cubosomes (data are shown in Table 2).29,30
In vitro percutaneous permeation
In vitro permeation procedures of the capsaicin-contained cubosome application were determined using vertical Franz diffusion cells with the epidermis facing the donor compartment. The abdominal skin of excised Sprague Dawley rats was used as the model membrane after the subcutaneous fat was maximally removed and the hair was shaved by an electric clipper.
The test formulations (1 g in 1 mL) were applied on the diffusion surface of the skin (2.8 cm2 available diffusion area) in the donor compartment. The receiver compartment was filled with 6.5±0.5 mL of 20% ethanol solution, stirred at 300 rpm, and maintained at 32°C±0.5°C using a circulating water bath. Ethanol was added to increase the solubility of capsaicin to reach the sink condition. Skin permeation studies were performed for 24 hours under unocclusive conditions, and 1 mL of the samples were withdrawn from the receptor at predetermined intervals and immediately replaced by an equal volume of fresh receptor solution. The samples were assayed by HPLC, as described previously (20 μL of the sample was injected into a Kromasil C18 at room temperature). The mobile phase was methanol:water =70:30 with a flow rate of 1.0 mL/minute; the detection wavelength was 280 nm.28 Each sample was performed in triplicate. Cumulative permeated amounts were plotted against time. The permeation rate of capsaicin at steady state,
(Js = dQ/dt, μg·cm−2·hour−1) | (2) |
through the rat skin was calculated by linear regression.31,32
Skin retention of capsaicin
The skin retention of capsaicin was determined as described previously.33 At 24 hours postapplication, the treated skin surfaces were thoroughly washed with distilled water and wiped with cotton swabs to remove the excess formulation. To separate the stratum corneum (SC) from the remaining epidermis (E) and dermis (D), the skin sections were subject to tape stripping. The skin was stripped with 15 pieces of adhesive tape; the first one was discarded, and the other tapes containing SC were immersed in 5 mL of methanol, vortex stirred for 2 minutes, and sonicated with probe sonication for 10 minutes. The remaining (E + D) was cut into small pieces, vortex stirred for 2 minutes in 10 mL of methanol, followed by probe sonication for 10 minutes. All samples were centrifuged at 15,000 rpm for 10 minutes; the supernatant was collected and the extract was analyzed for capsaicin content using HPLC.
Evaluation of skin irritation
Histological changes in Kunming mouse skin were examined after treatment with different capsaicin applications for 7 days to evaluate the possible skin irritation caused by the formulations. Capsaicin formulations (cubosomes, cream; 500 mL each) were applied topically on a limited area (2×2 cm2) of skin on the back of the mouse twice a day for 1 week. On the 7th day, the mice were killed and the treated skin area was dissected. After fixation, the skin samples were dehydrated and embedded in paraffin, and then 5 μm sections of skin were stained with hematoxylin and eosin for observation under microscope. The skin of the untreated mouse was used as the control.
Statistical analysis
All experimental data were expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS 11.5 software with one-way analysis of variance or Student’s t-test. Differences were considered significant at P<0.05.
Results and discussion
Particle size and morphology of capsaicin-loaded cubosomes
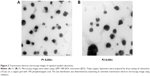
The average particle size of capsaicin-loaded cubosomes F1 and F2 was 251 nm and 215 nm, respectively, with a small polydispersity index (<0.1), indicating homogeneous micelle size. Figure 2 showed the TEM images of capsaicin-loaded cubosomes, which demonstrated that the cubosomes of irregular hexagonal shape were well dispersed as individual particles. In addition, the particle size observed in Figure 2 was approximately 200 nm, consistent with that determined by the particle detector.
SAXS
The SAXS experiment was carried out to investigate the internal structure of the cubosome. The values of scattering vector q corresponding to the scattering peaks for cubosomes F1 and F2 (Figure 3) appeared in the ratio of q1:q2:q3 =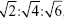 , which corresponded to the Miller indices, hkℓ =110, hkℓ =200, hkℓ =211, respectively. The results fit the characteristic peaks of the Im3m crystallographic space group (Q229), which contains a primitive cube phase.16
, which corresponded to the Miller indices, hkℓ =110, hkℓ =200, hkℓ =211, respectively. The results fit the characteristic peaks of the Im3m crystallographic space group (Q229), which contains a primitive cube phase.16
Encapsulation efficiency
To determine the dialysis time of free drug, 2 mL of capsaicin solution was firstly sealed into the dialysis bag, and dialysis balance was reached within 4 hours (Figure 4). So, 2 mL of cubosome particles were placed in dialysis bags according to the process detailed earlier for 4 hours of testing. Calculated as the formula, the EEs of F1 and F2 were calculated as 97.14%±0.54% and 97.58%±0.53%, respectively (n=3). Such high EE could be attributed to the unique structure of the cubic phase and the property of capsaicin. Hydrophobic capsaicin can dissolve in GMO or PYT and distribute in the lipid bilayers.30
In vitro release study
The capsaicin release profiles from the cubosome particles (Figure 5) showed that the drug released faster from formulation F1 than from formulation F2, as about 41% and 33% of capsaicin was released from F1 and F2 at 36 hours, respectively. Obviously, cubosomes could provide a sustained release system for capsaicin. Table 2 summarizes the results of fitting the release data for cubosomes. A linear relationship was found between capsaicin release and the square root of time (R2>0.99), indicating that the release is under diffusion control following Higuchi’s equation.34
Though our previous study showed that the cubic phase gel also possessed diffusion-controlled sustained release properties with approximately 34% capsaicin released after 108 hours,30 the drug release rate from cubosomes was much faster than from the cubic phase gel. As cubosomes are discrete, submicron, or nanostructured particles formed from fragmentation of the cubic phase gels, their huge specific surface area provides more chances for capsaicin to diffuse from the lipid bilayers, resulting in faster release.30
Comparison of permeation from cubosomes and cream
The results of permeation experiments (Figure 6) showed that the permeation of capsaicin was linear with the permeating time for cubosomes and cream. The capsaicin permeation rates of F1, F2, and cream at steady state (Js) obtained from the slope of the linear portion of the cumulative amount that permeated through the rat skins per unit area versus time plot were calculated as follows: 0.32±0.05 μg·cm−2·hour−1; 0.18±0.02 μg·cm−2·hour−1; and 0.28±0.15 μg·cm−2·hour−1, respectively. Both PYT cubosomes and cream exhibited higher permeation (0.32±0.05 μg·cm−2·hour−1 and 0.28±0.15 μg·cm−2·hour−1) than did the GMO-cubosomes (0.18±0.02 μg·cm−2·hour−1). Furthermore, the permeation rate of capsaicin from the PYT cubosome (0.32±0.05 μg·cm−2·hour−1) was similar to that from cream (0.28±0.15 μg·cm−2·hour−1). Obviously, cubosomes formed from different liquid crystal materials possess significantly different permeation characteristics.
Lopes et al33 reported that the cubic phase formed by GMO showed enhancement in penetration and retention in the SC and (E + D) of cyclosporin A. Steluti et al35 also reported that GMO significantly increased the in vitro skin permeation/retention of 5-aminolevulinic acid as compared with the control without GMO. In addition, with respect to GMO, the permeation rate and retention of the drug increased with the increase of GMO in the preparation. But the permeation and retention decreased as the GMO concentration reached 30% and 20%, respectively.
Figure 7 shows the retention of capsaicin in SC and (E + D). The incorporation of capsaicin in cubosomes led to an increase of drug skin retention 24 hours postapplication. For the capsaicin-loaded cream formulation, 0.72±0.13 μg and 1.52±0.11 μg of the drug was retained in SC and (E + D), respectively. For capsaicin-loaded cubosomes, the drug retention in SC and (E + D) was enhanced approximately four to six times (P<0.01) and two to five times (P<0.01), respectively.36 Contrary to previous reports, which suggested that cubosomes could enhance the percutaneous penetration of drugs, the cubosomes in this study reduced the skin absorption of capsaicin as compared to the conventional cream formulation. The amount of capsaicin absorbed from the GMO-based cubosomes into the skin after 24-hour application was less than that from the conventional cream. In spite of the reduced absorption, the cubosomal formulations still achieved higher and sustained skin retention of capsaicin as compared to the cream. This may be due to the fact that the cubosomal formulation could retard the diffusion of the drug from the skin to the systemic blood stream.
As shown in Figure 5, the release of capsaicin from F1 was faster than that from F2; meanwhile, F1 showed higher drug permeation and retention than F2, and the drug permeation was positive for the drug release.
Stability results
Either under strong light or a high temperature of 60°C for 10 days, the cubosome particles were maintained as a milk-white emulsion without obvious aggregation (Figure 8). Table 3 shows that the particle sizes of cubosomes remained stable during the 10-day stress test. Therefore, the stress test demonstrated that the cubosomes comprised a thermodynamically stable system.
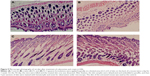
Evaluation of skin irritation
The images of skin tissues treated with cubosomes and cream formulations are shown in Figure 9. The skin structure was clear and integral without inflammatory reaction or infiltration of the inflammatory cells. Also, the skin treated with the cubosomes and cream showed very similar characteristics as that of the normal skin. These results demonstrated that both cubosomes and cream formulations had no obvious irritation to the skin.
Conclusion
The prepared cubosomes provided sustained release of capsaicin under diffusion control. Though the percutaneous absorption of capsaicin from the GMO-based cubosomes was less than that from the conventional cream, cubosomes still yielded higher and sustained skin retention of the drug than did the cream due to the retarded diffusion of the absorbed drug in the skin. Certainly, the mechanism of the retarded diffusion needs to be further investigated. The skin-targeted, sustained, and thermodynamically stable characteristics of cubosomal particles provide an interesting system for the topical delivery of capsaicin for alleviating postincision pain.
Acknowledgments
The authors gratefully acknowledge the financial support for the research (numbers: 81001643; 81173002; 81202477; 2013B021800073; 2CY13002; 2014108101049 and 201450715200682). This work was also supported in part by China Scholarship Council (numbers: 201308440229 and 201408440141). We further acknowledge Danisco Company, Denmark, for the generous gift samples of GMO.
Disclosure
The authors report no conflicts of interest in this work.
References
Huang XF, Xue JY, Jiang AQ, Zhu HL. Capsaicin and its analogues: structure-activity relationship study. Curr Med Chem. 2013;20(21):2661–2672. | ||
Sharma SK, Vij AS, Sharma M. Mechanisms and clinical uses of capsaicin. Eur J Pharmacol. 2013;720(1–3):55–62. | ||
Rollyson WD, Stover CA, Brown KC, et al. Bioavailability of capsaicin and its implications for drug delivery. J Control Release. 2014;196:96–105. | ||
Wohlrab J, Neubert RH, Heskamp ML, Michael J. Cutaneous drug delivery of capsaicin after in vitro administration of the 8% capsaicin dermal patch system. Skin Pharmacol Physiol. 2015;28(2):65–74. | ||
Sarwa KK, Mazumder B, Rudrapal M, Verma VK. Potential of capsaicin-loaded transfersomes in arthritic rats. Drug Deliv. Epub 2014 Jan 29. | ||
Kumar Sarwa K, Rudrapal M, Mazumder B. Topical ethosomal capsaicin attenuates edema and nociception in arthritic rats. Drug Deliv. Epub 2014 Feb 10. | ||
Zhu Y, Wang M, Zhang J, et al. Improved oral bioavailability of capsaicin via liposomal nanoformulation: preparation, in vitro drug release and pharmacokinetics in rats. Arch Pharm Res. 2015;38(4):512–521. | ||
Somagoni J, Boakye CH, Godugu C, et al. Nanomiemgel – a novel drug delivery system for topical application – in vitro and in vivo evaluation. PLoS One. 2014;9(12):e115952. | ||
Gupta R, Gupta M, Mangal S, Agrawal U, Vyas SP. Capsaicin-loaded vesicular systems designed for enhancing localized delivery for psoriasis therapy. Artif Cells Nanomed Biotechnol. 2014:1–10. | ||
Schoellhammer CM, Blankschtein D, Langer R. Skin permeabilization for transdermal drug delivery: recent advances and future prospects. Expert Opin Drug Deliv. 2014;11(3):393–407. | ||
Jain A, Jain P, Kurmi J, et al. Novel strategies for effective transdermal drug delivery: a review. Crit Rev Ther Drug Carrier Syst. 2014;31(3):219–272. | ||
Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672. | ||
Lee KW, Nguyen TH, Hanley T, Boyd BJ. Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. Int J Pharm. 2009;365(1–2):190–199. | ||
Caffrey M. A lipid’s eye view of membrane protein crystallization in mesophases. Curr Opin Struct Biol. 2000;10(4):486–497. | ||
Shah JC, Sadhale Y, Chilukuri DM. Cubic phase gels as drug delivery systems. Adv Drug Deliv Rev. 2001;47(2–3):229–250. | ||
Pan X, Han K, Peng X, et al. Nanostructured cubosomes as advanced drug delivery system. Curr Pharm Des. 2013;19(35):6290–6297. | ||
Bender J, Ericson MB, Merclin N, et al. Lipid cubic phases for improved topical drug delivery in photodynamic therapy. J Control Release. 2005;106(3):350–360. | ||
Chang CM, Bodmeier R. Low viscosity monoglyceride-based drug delivery systems transforming into a highly viscous cubic phase. Int J Pharm. 1998;173(1–2):51–60. | ||
Wadsten-Hindrichsen P, Bender J, Unga J, Engström S. Aqueous self-assembly of phytantriol in ternary systems: effect of monoolein, distearoylphosphatidylglycerol and three water-miscible solvents. J Colloid Interface Sci. 2007;315(2):701–713. | ||
Spicer PT, Hayden KL, Lynch ML, Ofori-Boateng A, Burns JL. Novel process for producing cubic liquid crystalline nanoparticles (cubosomes). Langmuir. 2001;17(19):5748–5756. | ||
Barauskas J, Johnsson M, Joabsson F, Tiberg F. Cubic phase nanoparticles (Cubosome): principles for controlling size, structure, and stability. Langmuir. 2005;21(6):2569–2577. | ||
Salentinig S, Yaghmur A, Guillot S, Glatter O. Preparation of highly concentrated nanostructured dispersions of controlled size. J Colloid Interface Sci. 2008;326(1):211–220. | ||
Kim MK, Chung SJ, Lee MH, Cho AR, Shim CK. Targeted and sustained delivery of hydrocortisone to normal and stratum corneum-removed skin without enhanced skin absorption using a liposome gel. J Control Release. 1997;46(3):243–251. | ||
Gan L, Han S, Shen J, et al. Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. Int J Pharm. 2010;396(1–2):179–187. | ||
Morsi NM, Abdelbary GA, Ahmed MA. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. Eur J Pharm Biopharm. 2014;86(2):178–189. | ||
Yang Z, Peng X, Tan Y, et al. Optimization of the preparation process of an oral phytantriol-based amphotericin B cubosomes. J Nanomater. 2011;(2011):1–10. | ||
Peng XS, Zhou YF, Han K, Qin LZ, Wu CB. [Preparation and in vitro study on diffusion of capsaicin cubosome]. Zhongguo Zhong Yao Za Zhi. 2014;39(4):644–647. Chinese. | ||
Peng X, Yang Z, Chen M, Han K, Xiao Y, Wu C. [Preparation, characterization and content determination of cubic phase gel containing capsaicin]. Zhongguo Zhong Yao Za Zhi. 2010;35(23):3123–3126. Chinese. | ||
Liu H, Pan WS, Nie SF, Yang XG, Yan TX. [Study on in vitro release empirical method and the release mechanism of budesonide colonic localization tablet]. Yao Xue Xue Bao. 2008;43(11):1147–1151. Chinese. | ||
Peng X, Wen X, Pan X, Wang R, Chen B, Wu C. Design and in vitro evaluation of capsaicin transdermal controlled release cubic phase gels. AAPS Pharm Sci Tech. 2010;11(3):1405–1410. | ||
Rottke M, Lunter DJ, Daniels R. In vitro studies on release and skin permeation of nonivamide from novel oil-in-oil-emulsions. Eur J Pharm Biopharm. 2014;86(2):260–266. | ||
Zhang P, Gao W, Zhang L, et al. In vitro evaluation of topical microemulsion of capsaicin free of surfactant. Biol Pharm Bull. 2008;31(12):2316–2320. | ||
Lopes LB, Lopes JL, Oliveira DC, et al. Liquid crystalline phases of monoolein and water for topical delivery of cyclosporin A: characterization and study of in vitro and in vivo delivery. Eur J Pharm Biopharm. 2006;63(2):146–155. | ||
Tanaka N, Imai K, Okimoto K, et al. Development of novel sustained-release system, disintegration-controlled matrix tablet (DCMT) with solid dispersion granules of nilvadipine. J Control Release. 2005;108(2–3):386–395. | ||
Steluti R, De Rosa FS, Collett J, Tedesco AC, Bentley MV. Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. Eur J Pharm Biopharm. 2005;60(3):439–444. | ||
Kwon TK, Hong SK, Kim J-C. In vitro skin permeation of cubosomes containing triclosan. Journal of Industrial and Engineering Chemistry. 2012;18(1):563–567. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.