Back to Journals » Infection and Drug Resistance » Volume 12
Characterization and analysis of a novel diguanylate cyclase PA0847 from Pseudomonas aeruginosa PAO1
Authors Zhang Y, Guo J, Zhang N, Yuan W, Lin Z , Huang W
Received 17 November 2018
Accepted for publication 16 February 2019
Published 21 March 2019 Volume 2019:12 Pages 655—665
DOI https://doi.org/10.2147/IDR.S194462
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Yan Zhang,1,2 Jiayi Guo,1 Ning Zhang,2 Wensu Yuan,2 Zhi Lin,2 Weidong Huang1
1Department of Biochemistry and Molecular Biology, School of Basic Medicine, Ningxia Medical University, Yinchuan 750004, People’s Republic of China; 2School of Life Sciences, Tianjin University, Tianjin 300072, People’s Republic of China
Background: As a central signaling molecule, cyclic diguanylate (c-di-GMP) is found to regulate various bacterial phenotypes, especially those involved in pathogen infection and drug resistance. Noticeably, many microbes have up to dozens of proteins that are involved in c-di-GMP metabolism. This apparent redundancy and the relevant functional specificity have become the focus of research. While a number of these proteins have been identified and investigated, the functions of PA0847, a PAS and GGDEF domain-containing protein from Pseudomonas aeruginosa PAO1, remain unclear.
Materials and methods: In the current study, microbiology, biochemistry and structural biology methods were applied to characterize the gene/protein of PA0847.
Results: We showed that PA0847 affects bacterial motility but not biofilm formation. We recorded the phenotypic influences of amino acids and compounds, and found that PA0847 is involved in response to various environmental nutrients and factors, suggesting its possible role in sensing environmental cues. Both in-vitro and in-vivo studies showed that PA0847 is an active diguanylate cyclase (DGC), whose activity depends on the neighboring PAS domain. Interestingly, PA0847 demonstrates no significant product inhibition, though the key residues of two I-sites for c-di-GMP binding are conserved in its GGDEF domain. A local structural change imposed by an adjacent tyrosine residue was identified, which indicates the structural and functional diversities of the GGDEF family proteins.
Conclusion: Our data provide evidence for understanding the signaling mechanism of the unique c-di-GMP metabolizing protein PA0847.
Keywords: Pseudomonas aeruginosa, c-di-GMP, GGDEF domain, diguanylate cyclase, structure
Introduction
Pseudomonas aeruginosa is a widespread Gram-negative bacterium that can cause a variety of acute and chronic infections. It has a complicated regulatory network of signaling molecules, which allow the bacteria to adapt to and thrive in different environments.1 As a well-established model strain, P. aeruginosa PAO1 has been fully sequenced and frequently applied for bacterial phenotypic, metabolic and physiological studies.
In recent years, signaling of the second messenger c-di-GMP (bis-(3,5)-cyclic diguanosine monophosphate) belongs to the most dynamic fields in molecular microbiology.2 C-di-GMP is involved in regulation of various bacteria physiological functions, and most noticeably, it modulates the bacteria motility and the switch between motile and sessile lifestyles. Typically, elevated intracellular level of c-di-GMP is linked to increased exopolysaccharides and biofilm formation, while decreased c-di-GMP associated with motile planktonic bacteria.3
In bacteria cells, c-di-GMP is synthesized from two molecules of GTP by diguanylate cyclases (DGCs), and degraded to pGpG by phosphodiesterases (PDEs). These enzymes contain the conserved GGDEF and EAL or HD-GYP domains.4–6 Strikingly, there is an apparent redundancy of c-di-GMP metabolic proteins encoded in bacteria genome. In P. aeruginosa PAO1, these c-di-GMP modulating proteins are highly complex, with 17 GGDEF, 5 EAL, 16 GGDEF/EAL, and 3 HD-GYP domain-containing proteins (
Noticeably, many of the c-di-GMP modulating proteins contain concatenated distinct N-terminal sensing domains, indicating that they may respond to individual environmental signals.17 Experimental data in c-di-GMP signaling is emerging dynamically. However, there are still many unresolved issues for understanding the environmental response, functional specificity and physiological importance of these c-di-GMP modulating proteins. In a recent study, a multimodal regulatory strategy, including combinations of ligand-mediated signals, protein–protein interaction and/or transcriptional regulation, was proposed in c-di-GMP networking.18 In another discussion, a “local” c-di-GMP signaling concept was suggested, where a subset of DGCs and PDEs could operate as “central interaction hubs” in a larger supermodule, with other DGCs and PDEs behaving as “lonely players” without contacting different c-di-GMP-related enzymes any more.19 These observations signify the networking of c-di-GMP metabolizing molecules far more complicated than previously thought, hence highlighting the need for investigations of individual GGDEF and EAL containing protein in details.
The sequence architecture of PA0847 gene from P. aeruginosa PAO1, which encodes a putative GGDEF domain, suggests that it could be involved in c-di-GMP synthesis.20 However, its effects on bacterial behaviors and its enzymatic mechanisms remain elusive. In this study, we examined and identified the roles of PA0847 in the regulation of bacterial motility and biofilm formation. Further, we found that PA0847 was involved in response to a variety of environmental nutrients and factors, suggesting it could serve as a broad-range environmental sensor on the membrane of P. aeruginosa. Domain analysis of PA0847 shows a unique molecular architecture with an N-terminal periplasmic sensory domain and a C-terminal intracellular GGDEF domain. Furthermore, biochemical and structural characterization of the GGDEF domain revealed that PA0847 is an active DGC with weak product feedback inhibition. These new findings provide a basis for studying c-di-GMP signaling through a unique GGDEF domain-containing protein with environmental and clinical relevance.
Materials and methods
Bacteria phenotype analysis
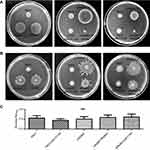
The P. aeruginosa PA0847 mutants (ΔPA0847-1/2) and wild type PAO1 strains were requested from Manoil lab. For biofilm analysis, overnight cultures were adjusted to O.D. (600 nm) 0.5, 1:100 diluted with fresh LB broth (1 ml, in Falcon 352057 tube), and incubated at 37°C, 100 rpm for the indicated periods of culture. Cells bound to the walls of the tubes were stained with 0.2% crystal violet (Sigma) for 20 min at room temperature. The tubes were then rinsed with water, air dried and photographed. For quantification, biofilms were suspended in 5 ml of 95% ethanol, and measured with a spectrophotometer at 595 nm. Each experiment was repeated at least three times.
For motility analysis, overnight cultures were adjusted to O.D. (600 nm) 0.5, 1:100 diluted with fresh LB broth, and inoculated into 100 mm petri plates. Swimming motility medium: LB medium containing 0.3% Bacto agar (BD). Swarming motility medium: 0.8% nutrient broth (OXOID) with 0.45% Bacto agar (BD), add 0.5% Glucose before use.
Complementation of PA0847 was applied by amplification of the full-length gene with its native promoter. The PCR products were cloned into the pACYC184 vector and verified by sequencing (
Amino acids and environmental factors
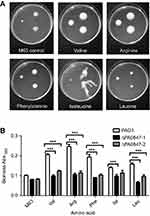
A number of amino acids and compounds were selected to be applied in the M63 medium, to study their effects on bacteria biofilm formation and motility. The medium applied is M63 supplemented with individual amino acids at the following concentrations: isoleucine (17.6 mM), leucine (25.6 mM), valine (17.6 mM), arginine (4.8 mM), and phenylalanine (8.0 mM).21 These concentrations are the same as those applied for biofilm formation analysis. The chemicals and their working concentrations are included in the
Recombinant protein expression and purification
Individual domains of PA0847 was subcloned between the BamH Ⅰ and Hind Ⅲ sites of a modified pET-32a (Novagen) vector with the S-tag and thioredoxin gene removed. The His-tagged proteins were induced for expression with optimal IPTG and purified with the Ni-NTA resin (Qiagen). The imidazole in protein solution was removed by dialysis against a Tris buffer (pH 7.6).
Enzymatic activity analysis
The recombinant proteins were adjusted to 10 μM in 100 mM Tris-Cl buffer, pH 7.6, with 10 mM MgCl2, 30 nM BSA. Substrate GTP were added to the concentration of 200 μM, incubate at 37°C for 1 hr. Once the reactions ended, reaction mix were incubate in 95°C for 20 mins, centrifuged and filtered to collect the supernatants. High-performance liquid. chromatography analysis was applied with Hitachi L2000 HPLC system (Japan) using Hibar 250-4 LiChrospher 100 column (Merck) at the flow rate of 0.8 ml per minute, with mobile phase containing 95:5 methanol and 10 mM triethylammonium bicarbonate (TEAB).
Congo red staining
Congo red plates preparation: 100ml 10× stock medium containing K2HPO4 10g, (NH4)2SO4 1.75 g,Citric acid 2 g; 0.22 μm filter filtered. Dilute to 1x, adjust to pH 7.0, add MgCl2 to 1 mM, agarose 10 g/L, with Congo red 40 μg/ml.
Planktonic culture
Inoculate E. coli BL21 containing individual plasmids (GGDEF-PAS, R595A, R641A, D626A, D609A, D652A, K722A, K614A, R672A) of PA0847 into M63 medium, add 0.02% glucose, 37°C, 200 rpm overnight culture. Adjust to O.D. 600 0.5, 1:100 dilute and culture for around 3 h, add 0.05 mM IPTG and culture for 12 hrs, drop one tip and take photos to record.
Homology modeling
A three-dimensional model of PA0847 GGDEF domain (residues 576–736) was constructed in MODELLER-9 package using DGC DgcZ (PDB ID 4H54) as a structure template.22 All the structural figures were generated using UCSF Chimera.23
Results
PA0847 regulates bacterial motility without significantly affecting the biofilm formation in PAO1
To analyze the biological functions of gene of PA0847 in PAO1, we applied the PA0847 transposon mutants (ΔPA0847-1 and ΔPA0847-2,
PA0847 is involved in response to various environmental nutrients and factors
P. aeruginosa PAO1 PA0847 contains N-terminal sensing domains,20 implying its possible roles in responding to different environmental cues. Moreover, it has been reported previously that amino acids can modulate biofilm formation and motility, at least in part, by controlling the intracellular levels of c-di-GMP.21 We therefore investigated the effects of various amino acids and compounds on motility and biofilm formation for both WT PAO1 and PA0847 mutants.
As shown in Figure 2, ΔPA0847 displayed higher motility with additional amino acids of valine, arginine, phenylalanine, and isoleucine. Leucine at the concentration of 25.6 mM (Figure 2A) caused almost no difference of motility between PAO1 and ΔPA0847. Biofilm formation for ΔPA0847, compared to PAO1, generally decreased with the amino acids tested (Figure 2B). In the presence of valine, arginine, phenylalanine, isoleucine, and leucine, the differences were statistically significant. The bacterial growth was not strongly affected by these amino acids at an applied concentration (
We also examined some compounds and chemicals. As shown in
For biofilm formation, in most of the conditions tested, there were no significant differences between PAO1 and ΔPA0847. With extra sucrose, ΔPA0847 showed an increased biofilm formation. With CaCl2 and SDS added, however, the level of biofilm in ΔPA0847 was decreased significantly (
PA0847 is an active diguanylate cyclase
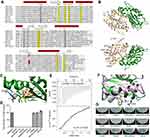
Domain analysis of PA0847 shows a unique molecular architecture among known GGDEF-containing proteins in P. aeruginosa PAO1.20 PA0847 consists of an uncharacterized N-terminal domain (NTD) of ~90 amino acid residues, a predicted periplasmic sensory domain CHASE (cyclase/histidine kinase-associated sensing extracellular) flanked by two single-pass transmembrane domains (TM), an HAMP (Histidine kinase, Adenylyl cyclase, Methyl-accepting protein, and Phosphatase) domain, a PAS (Per-Arnt-Sim) domain and a C-terminal canonical GGDEF domain (Figure 3A). To experimentally evaluate its diguanylate cyclase activity in-vitro, two recombinant proteins, GGDEF, and PAS-GGDEF dual domains, were expressed, purified and confirmed by SDS-PAGE analysis (
In addition to the in-vitro analysis, we stained both WT PAO1 and ΔPA0847 strains using Congo Red to investigate the level of c-di-GMP in vivo, as elevated c-di-GMP results in increased polysaccharides synthesis and stronger Congo Red binding. As exhibited in Figure 3E, wildtype PAO1 is more reddish than ΔPA0847. Combining both in-vitro and in-vivo results, we demonstrated that PA0847 is an active DGC.
Structural model of GGDEF domain and its implications in catalytic function
To understand further the catalytic mechanisms for PA0847, we analyzed the sequence and structural model of its GGDEF domain. Multiple sequence alignment showed that critical residues for DGC activity are all conserved in PA0847 GGDEF domain (Figure 4A). The overall sequence identities between PA0847 GGDEF and other GGDEF domains with known structures are close to 40%, suggesting similarities in global folding. A structural model of the dimeric PA0847 GGDEF domain in complex with two GTPs is presented in Figure 4B. PA0847 GGDEF domain displayed a conserved topology of α-β plait in GGDEF family. It is composed of a five-stranded β-sheet core surrounded by five α-helices. A close examination of the structure reveals seven critical residues (D609, K614, N617, D626, D652, E653, and K722) involved in GTP binding (Figure 4C). Single or double mutation of these residues completely disrupted GGDEF DGC activity (Figure 4D). PA0847 GGDEF also displays predicted inhibition sites, including a primary I-site (R641xxD644 motif) and a secondary I-site (R595 and R672) as shown in Figure 4B. These sites are all conserved in GGDEF-containing proteins with strong product inhibition of some DGCs (ie, PA3702 and PleD in Figure 4A).26,27 Nevertheless, the PAS-GGDEF of PA0847 only displays low binding affinity to c-di-GMP with an apparent Kd of ~59 µM (Figure 4E), indicating weak product inhibition. Mutation of the key residues in putative I-sites did not significantly increase the catalytic production of c-di-GMP (Figure 4D). Structural analysis of PA0847 GGDEF domain revealed that the first α-helix harboring the secondary I-site is shifted by approximate 15° due to bulky side-chain of Tyr700 when compared to the corresponding helix in PleD (Figure 4F). This shift in helix position may sterically hinder potential binding of c-di-GMP. A similar local structural perturbation was also observed in GGDEF domains of PA0575 and PA1120 (YfiN) which does not display product feedback inhibition.25 It is clear that both I-sites and local conformation are necessary for non-competitive inhibition.
To further verify the catalytic function of the GGDEF domain in a cellular context, we expressed the fusion proteins in Escherichia coli BL21. After 12-hr induction by IPTG in M63 medium, only the cultures of E. coli overexpressing wildtype PAS-GGDEF and mutants of R595A, R641A/R644A, and R672A maintained a similar phenotype of the clear solution with aggregated cell pellets (Figure 4G). These cells possessed lower motility and were prone to aggregate because of their higher cellular level of c-di-GMP, which were in agreement with the HPLC results. Taking all these data together, we concluded that DGC activity of PA0847 is attributed to its C-terminal GGDEF domain which did not undergo strong allosteric feedback inhibition.
Discussion
In current study, mainly through phenotypic analysis, recombinant proteins techniques, structural investigation, and both in-vitro and in-vivo biochemical studies, we demonstrated that gene PA0847 from P. aeruginosa PAO1 encodes an active DGC that was involved in c-di-GMP metabolism. PA0847 can also respond to various environmental nutrients and factors, presumably through its putative N-terminal sensory domain.
In phenotypic assays, PA0847 mutant strains exhibited increased swimming and swarming motility phenotypes and no apparent differences in biofilm formation (Figure 1). The obvious redundancy of c-di-GMP metabolizing genes in bacteria regium has long been enigmatic. Recently, the “local signaling” theories have been proposed, indicating that these genes might form interconnected signaling networks.18,19 In agreement with this, Overhage J and colleagues had reported that expression of PA0847 could be upregulated drastically while the DGC of PA3177 was knocked out.28 Moreover, when suhB, a virulence factors regulator from PAO1, was deleted, the expression of PA0847 decreased slightly.29 Our observations combining these analyses imply that PA0847 might be involved in a complicated c-di-GMP modulating system, where more investigations for their interplays and interactions are needed.
To further analyze the catalytic activities of PA0847, we carried out both in-vitro and in-vivo assays and demonstrated that PA0847 is an active DGC (Figure 3B–E). What worth to mention is that, compared to the dual PAS-GGDEF domain, the recombinant single GGDEF domain of PA0847 exhibited only a trace amount of DGC activity. This observation implies that the DGC activity of PA0847 might be modulated and affected by the neighboring PAS domain in-vivo.
PA0847 contains the canonical product inhibition site (primary I site) of the RxxD motif, which is located upstream of the conserved GGDEF motif. It also has a secondary inhibition site which is conserved in GGDEF domains that can undergo feedback inhibition (Figure 4A and B). These two sites may form a pocket for c-di-GMP binding.30 To analyze the mechanisms involved in its enzymatic activity and the regulation, we carried out site-directed mutagenesis using alanine substitutions to generate a series of PA0847 PAS-GGDEF mutants. Unexpectedly, the DGC activity of PA0847 was not significantly affected by product inhibition (Figure 4D and G), in line with its weak binding affinity to c-di-GMP. Nevertheless, different from degenerated I-sites in PA0575 and PA1120 GGDEF domains, all the key residues for c-di-GMP binding are conserved in PA0847 GGDEF domain. Further analysis of the c-di-GMP-binding site indicated a local structural change imposed by a tyrosine residue from an adjacent β-strand when compared to other GGDEF-containing proteins with significant feedback inhibition. Therefore, the primary and secondary I-sites together with I-site local conformation are necessary conditions for product feedback inhibition. The weak product feedback inhibition found in PA0847 GGDEF domain suggests that allosteric regulation from the sensory domain could play a dominant role in the control of intracellular c-di-GMP synthesis.
Recently, there emerged a number of studies reporting that amino acids affect bacteria motility and biofilm formation, mainly through specific and functional c-di-GMP signaling networks. In B. cenocepacia, a PDE of BCAL1069 (CdpA) has been reported to regulate swimming motility by sensing the amino acids arginine and glutamate.31 For S. Typhimurium, it specifically responded to L-arginine with an increase in c-di-GMP, which required the periplasmic regulatory domain of the diguanylate cyclase (DGC) STM1987.32 In P. aeruginosa PA14, it has been reported that the two DGCs, SadC (PA4332) and RoeA (PA1107), participate in repression of swarming motility as well as boosting biofilm formation when the amino acid arginine exists.21 In a deeper analysis, it was demonstrated that sensing of the amino acid glutamate by NicD (PA4929) in P. aeruginosa results in dephosphorylation and increased cyclase activity of this DGC, glutamate thus becomes the cue to induce P. aeruginosa dispersion.33 In the current study, we probed the effects of several amino acids on PA0847. As shown in Figure 2A, with the addition of L-valine, L-arginine, L-phenylalanine and L-isoleucine, P. aeruginosa PA0847 mutant cells displayed the trend of increased motility, while their biofilm formation was also affected by L-valine, L-arginine, L-phenylalanine and L-isoleucine, and L-leucine (Figure 2B).
Amino acids have long been noticed to have profound effects on infection. For example, Cystic fibrosis (CF) patients are among the top victims of P aeruginosa. Strikingly, there are CF pseudomonal auxotrophy strains emerged, which are frequently associated with a few specific amino acids. These CF-adapted P. aeruginosa strains reflected bacterium-host symbiosis and coevolution during long-term chronic infection, but not nutrient availability.34 The linking of amino acids with bacteria c-di-GMP modulating genes in previous research and our current study might therefore provide an angle to deepen our understandings, mainly in bacteria physiological regulation.
Likewise, c-di-GMP signaling pathways are believed to be affected by various ions and compounds. A number of environmental signals, including CaCl2, MgCl2, CuSO4, FeCl2 or NaCl, sucrose, sodium dodecyl sulfate, and dithiothreitol, were shown to differentially regulate the expression of DGCs and PDE in Y. pestis, resulting in changed intracellular levels of c-di-GMP and biofilm formation.35 In P. aeruginosa PAO1, hypochlorite (HClO), a phagocyte-derived host defense compound, was shown to act as an activator of the DGC PA3177, whose expression leads to biofilm formation and inhibited motility.28 Very recently, in a detailed dissection in P. fluorescens Pf0-1, the authors displayed that the citrate and isocitrate are ligands for the N-terminal CACHE domain of GcbC, a DGC which is involved in the complicated LapD GcbC signaling pathway. This ligand sensing may enhance the interaction between GcbC and LapD, promote higher levels of c-di-GMP production and biofilm formation, and finally, contribute to the signaling specificity of the c-di-GMP network.36 Therefore, sensing of environmental cues not only linked to phenotypic changes but also to gene expression regulations, via the c-di-GMP signaling pathway. We also tested ions and some compounds’ effects on both wildtype PAO1 and PA0847 mutants (
It has long been noticed that c-di-GMP metabolizing proteins contain concatenated N-terminus sensory domains, such as CHASE, PAS, GAF, and HAMP.17 The molecular and structural mechanisms of individual sensory domains and their corresponding environment cues will be the focus of future research to decipher PA0847-mediated signaling in P. aeruginosa PAO1.
Conclusion
In current study, we demonstrated that as an active diguanylate cyclase from P. aeruginosa PAO1, PA0847 is involved in response to a variety of environmental nutrients and factors, suggesting its role as a broad-range environmental sensor on the membrane. We also identified a local structural change imposed by an adjacent tyrosine residue, which indicates structural and functional diversities of the GGDEF family proteins. Our work thus contributes to the understandings of c-di-GMP metabolism and signaling in bacteria.
Acknowledgments
This work was supported in part by grants from National Natural Science Foundation of China (31560042, 81260243) (to WH) and Tianjin University, Natural Science Foundation of Tianjin City (17JCYBJC24200) (to ZL).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Klockgether J, Tümmler B. Recent advances in understanding Pseudomonas aeruginosa as a pathogen. F1000Research. 2017;6:1261. doi:10.12688/f1000research.10506.1
2. Hengge R, Grundling A, Jenal U, Ryan R, Yildiz F. Bacterial signal transduction by cyclic Di-GMP and other nucleotide second messengers. J Bacteriol. 2016;198(1):15–26. doi:10.1128/JB.00331-15
3. Romling U, Galperin MY, Gomelsky M. Cyclic di-GMP: the first 25 years of a universal bacterial second messenger. Microbiol Mol Biol Rev. 2013;77(1):1–52. doi:10.1128/MMBR.00043-12
4. Ryan RP, Fouhy Y, Lucey JF, et al. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc Natl Acad Sci U S A. 2006;103(17):6712–6717. doi:10.1073/pnas.0600345103
5. Ryjenkov DA, Tarutina M, Moskvin OV, Gomelsky M. Cyclic diguanylate is a ubiquitous signaling molecule in bacteria: insights into biochemistry of the GGDEF protein domain. J Bacteriol. 2005;187(5):1792–1798. doi:10.1128/JB.187.5.1792-1798.2005
6. Schmidt AJ, Ryjenkov DA, Gomelsky M. The ubiquitous protein domain EAL is a cyclic diguanylate-specific phosphodiesterase: enzymatically active and inactive EAL domains. J Bacteriol. 2005;187(14):4774. doi:10.1128/JB.187.13.4444-4450.2005
7. Galperin MY. A census of membrane-bound and intracellular signal transduction proteins in bacteria: bacterial IQ, extroverts and introverts. BMC Microbiol. 2005;5(1):1–19. doi:10.1186/1471-2180-5-1
8. Kulasakara H, Lee V, Brencic A, et al. Analysis of Pseudomonas aeruginosa diguanylate cyclases and phosphodiesterases reveals a role for bis-(3ʹ-5ʹ)-cyclic-GMP in virulence. Proc Natl Acad Sci U S A. 2006;103(8):2839–2844. doi:10.1073/pnas.0511090103
9. Güvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Mol Microbiol. 2010;66(6):1459–1473.
10. Kazmierczak BI, Lebron MB, Murray TS. Analysis of FimX, a phosphodiesterase that governs twitching motility in Pseudomonas aeruginosa. Mol Microbiol. 2010;60(4):1026–1043. doi:10.1111/j.1365-2958.2006.05156.x
11. Merritt JH, Brothers KM, Kuchma SL, O’Toole GA. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. J Bacteriol. 2007;189(22):8154. doi:10.1128/JB.00581-07
12. Morgan R, Kohn S, Hwang SH, Hassett DJ, Sauer K. BdlA, a chemotaxis regulator essential for biofilm dispersion in Pseudomonas aeruginosa. J Bacteriol. 2006;188(21):7335–7343. doi:10.1128/JB.00599-06
13. Newell PD, Monds RD, O’Toole GA. LapD is a bis-(3ʹ,5ʹ)-cyclic dimeric GMP-binding protein that regulates surface attachment by Pseudomonas fluorescens Pf0-1. Proc Natl Acad Sci U S A. 2009;106(9):3461–3466. doi:10.1073/pnas.0808933106
14. Rossello J, Lima A, Gil M, et al. The EAL-domain protein FcsR regulates flagella, chemotaxis and type III secretion system in Pseudomonas aeruginosa by a phosphodiesterase independent mechanism. Sci Rep. 2017;7(1):10281. doi:10.1038/s41598-017-09926-3
15. Valentini M, Laventie BJ, Moscoso J, Jenal U, Filloux A. The diguanylate cyclase HsbD intersects with the HptB regulatory cascade to control Pseudomonas aeruginosa biofilm and motility. PLoS Genet. 2016;12(10):e1006473. doi:10.1371/journal.pgen.1006473
16. Ying C, Liu S, Liu C, et al. Dcsbis (PA2771) from Pseudomonas aeruginosa is a highly active diguanylate cyclase with unique activity regulation. Sci Rep. 2016;6:29499. doi:10.1038/srep29499
17. Seshasayee AS, Fraser GM, Luscombe NM. Comparative genomics of cyclic-di-GMP signalling in bacteria: post-translational regulation and catalytic activity. Nucleic Acids Res. 2010;38(18):5970–5981. doi:10.1093/nar/gkq382
18. Dahlstrom KM, Collins AJ, Doing G, et al. A multimodal strategy used by a large c-di-GMP network. J Bacteriol. 2018;200(8). doi:10.1128/JB.00703-17.
19. Sarenko O, Klauck G, Wilke FM, et al. More than enzymes that make or break cyclic Di-GMP-Local signaling in the interactome of GGDEF/EAL domain proteins of Escherichia coli. mBio. 2017;8(5). doi:10.1128/mBio.01639-17.
20. Zhulin IB, Nikolskaya AN, Galperin MY. Common extracellular sensory domains in transmembrane receptors for diverse signal transduction pathways in bacteria and archaea. J Bacteriol. 2003;185(1):285–294.
21. Bernier SP, Ha DG, Khan W. Modulation of Pseudomonas aeruginosa surface-associated group behaviors by individual amino acids through c-di-GMP signaling. Res Microbiol. 2011;162(7):680–688. doi:10.1016/j.resmic.2011.04.014
22. Webb B, Sali A. Protein structure modeling with Modeller. Methods Mol Biol. 2017;1654:39–54. doi:10.1007/978-1-4939-7231-9_4
23. Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25(13):1605–1612. doi:10.1002/jcc.20084
24. Jacobs MA, Alwood A, Thaipisuttikul I, et al. Comprehensive transposon mutant library of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2003;100(24):14339–14344. doi:10.1073/pnas.2036282100
25. Giardina G, Paiardini A, Fernicola S, et al. Investigating the allosteric regulation of YfiN from Pseudomonas aeruginosa: clues from the structure of the catalytic domain. PLoS One. 2013;8(11):e81324. doi:10.1371/journal.pone.0081324
26. Wassmann P, Chan C, Paul R, et al. Structure of BeF3- -modified response regulator PleD: implications for diguanylate cyclase activation, catalysis, and feedback inhibition. Structure. 2007;15(8):915–927. doi:10.1016/j.str.2007.06.016
27. De N, Navarro MV, Raghavan RV, Sondermann H. Determinants for the activation and autoinhibition of the diguanylate cyclase response regulator WspR. J Mol Biol. 2009;393(3):619–633. doi:10.1016/j.jmb.2009.08.030
28. Strempel N, Nusser M, Neidig A, Brennerweiss G, Overhage J. The oxidative stress agent hypochlorite stimulates c-di-GMP synthesis and biofilm formation in Pseudomonas aeruginosa. Front Microbiol. 2017;8:2311. doi:10.3389/fmicb.2017.02311
29. Li K, Yang G, Debru AB, et al. SuhB regulates the motile-sessile switch in Pseudomonas aeruginosa through the Gac/Rsm pathway and c-di-GMP signaling. Front Microbiol. 2017;8:1045. doi: 10.3389/fmicb.2017.01045
30. Römling U, Liang ZX, Dow JM. Progress in understanding the molecular basis underlying functional diversification of cyclic dinucleotide turnover proteins. J Bacteriol. 2017;199(5):
31. Kumar B, Sorensen JL, Cardona ST. A c-di-GMP-modulating protein regulates swimming motility of burkholderia cenocepacia in response to arginine and glutamate. Front Cell Infect Microbiol. 2018;8. doi:10.3389/fcimb.2018.00056
32. Mills E, Petersen E, Kulasekara BR, Miller SI. A direct screen for c-di-GMP modulators reveals a Salmonella Typhimurium periplasmic ʟ-arginine-sensing pathway. Sci Signal. 2015;8(380):ra57. doi:10.1126/scisignal.aaa1796
33. Basu RA, Sauer K. Diguanylate cyclase NicD-based signalling mechanism of nutrient-induced dispersion by Pseudomonas aeruginosa. Mol Microbiol. 2014;94(4):771. doi:10.1111/mmi.12802
34. Qin X. Chronic pulmonary pseudomonal infection in patients with cystic fibrosis: a model for early phase symbiotic evolution. Crit Rev Microbiol. 2016;42(1):144–157. doi:10.3109/1040841X.2014.907235
35. Ren GX, Fan S, Guo XP, Chen S, Sun YC. Differential regulation of c-di-GMP metabolic enzymes by environmental signals modulates biofilm formation in Yersinia pestis. Front Microbiol. 2016;7(8412):821. doi:10.3389/fmicb.2016.00821
36. Giacalone D, Smith TJ, Collins A, Sondermann H, Koziol LJ, O’Toole G. Ligand-mediated biofilm formation via enhanced physical interaction between a diguanylate cyclase and its receptor. mBio. 2018;9(4). doi:10.1128/mBio.01254-18
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.