Back to Journals » Patient Preference and Adherence » Volume 12
Changes in adherence and associated factors among patients on newly introduced prostaglandin analog and timolol fixed-combination therapy
Authors Hasebe Y, Kashiwagi K, Tsumura T , Suzuki Y, Yoshikawa K , Suzumura H , Maeda T, Takeda R, Saito H, Araie M
Received 22 March 2018
Accepted for publication 26 June 2018
Published 27 August 2018 Volume 2018:12 Pages 1567—1577
DOI https://doi.org/10.2147/PPA.S168921
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Yuka Hasebe,1 Kenji Kashiwagi,1 Toyoaki Tsumura,2 Yasuyuki Suzuki,3 Keiji Yoshikawa,4 Hirotaka Suzumura,5 Toshine Maeda,6 Ryuji Takeda,7 Hitomi Saito,8 Makoto Araie8
1Department of Ophthalmology, University of Yamanashi, Yamanashi, Japan; 2Department of Ophthalmology, Fussa Hospital, Tokyo, Japan; 3Department of Ophthalmology, Tokai University, Kanagawa, Japan; 4Yoshikawa Eye Clinic, Tokyo, Japan; 5Suzumura Eye Clinic, Tokyo, Japan; 6Maeda Eye Clinic, Tokyo, Japan; 7Faculty of Agriculture, Kinki University, Nara, Japan; 8Department of Ophthalmology, Kanto Central Hospital of the Mutual Aid Association of Public School, Tokyo, Japan
Purpose: We investigated patient adherence and factors related to a newly introduced prostaglandin analog and timolol fixed-combination eye drops (PGTFC).
Patients and methods: The Glaucoma Research on Adherence to fixed-Combination Eye drops in Japan (GRACE) study group performed a nationwide prospective questionnaire survey. Participants in this study were patients with glaucoma who were scheduled to receive any type of PGTFC for the first time. The participants answered a questionnaire on the day of PGTFC introduction and again at a return visit 4–6 weeks after PGTFC introduction. The physicians in charge were asked to complete a separate questionnaire on the day of PGTFC introduction. One of two leaflets was randomly delivered to each participant before the description of the PGTFC. One leaflet explained how to correctly instill the eye drops, and the other explained the clinical meaning of intraocular pressure reduction in addition to explaining how to correctly instill the eye drops. Nonadherence was defined as forgetting to instill the eye drops one or more times during the week before the return visit.
Results: In total, 3,597 patients (age, 68.4±12.2 years) met the study protocol requirements. PGTFC introduction significantly reduced the number of antiglaucoma eye drops from 1.93±0.78 to 1.34±0.54 (P<0.0001) and significantly improved adherence (P<0.00001). Factors significantly associated with nonadherence at the return visit included a history of nonadherence as reported by either the patient or their physician before introduction, acceptable instillation times as reported by the patient, and burdensome eye drop instillation as reported by the patient. No significant difference was observed between the two leaflets in terms of their effects on adherence.
Conclusion: PGTFC significantly improved adherence and some of the factors that were significantly associated with adherence.
Registration number: UMIN000013696
Keywords: glaucoma, fixed-combination, adherence questionnaire, Japan
Introduction
Many patients with glaucoma are required to use ocular hypotensive eye drops for long periods, even for the remainder of their life. Many new types of ocular hypotensive eye drops have been recently introduced. We previously reported that glaucoma patients instill approximately two types of ocular hypotensive eye drops on average in Japan.1 Poor adherence is recognized as one of the most important problems with medical treatment for glaucoma patients in real-world situations. According to many previous studies, several factors affect adherence, and some studies have reported that an increase in the number of eye drops used is negatively associated with adherence.2–10 Therefore, reducing the number of ocular hypotensive eye drops may improve adherence.
Fixed-combination eye drops exert a greater ocular hypotensive effect without increasing the number of eye drops used.10–16 Some previous studies have reported that the introduction of fixed-combination eye drops improves adherence.17–19 Previous studies have also reported that many factors affect adherence, and literacy regarding glaucoma may contribute to adherence. To date, no large studies in Japan have investigated the status of adherence and the factors affecting adherence among patients with glaucoma.
We established a study group, called the Glaucoma Research on Adherence to fixed-Combination Eye drops in Japan (GRACE) study, to investigate the effect of fixed-combination ocular hypotensive eye drop use on adherence using a nationwide questionnaire-based survey across Japan. This study group revealed the status of adherence and its associated factors among glaucoma patients who planned to utilize fixed-combination eye drops based on a previous paper.20 In this study, we compared the adherence before and 4–6 weeks after the introduction of prostaglandin analog and timolol fixed-combination eye drops (PGTFC) and the factors associated with adherence.
Patients and methods
This study was conducted according to the tenets of the Declaration of Helsinki. We invited all facilities belonging to the Japan Ophthalmologists Association in 2011 to participate in this study. The institutional research board (IRB) of the Ishikawa Medical Association (Kanazawa, Japan) approved the study as a main IRB for institutes that did not have their own IRB. When available, the IRB of each participating institute approved this study. The participating ophthalmologists submitted written informed consent to the council of the GRACE study group.
Subject inclusion and exclusion criteria
The subject enrollment period was from June 2011 to July 2012. All subjects satisfied the inclusion and exclusion criteria. The inclusion criteria were as follows: subjects with primary open-angle glaucoma (POAG), normal tension glaucoma (NTG), exfoliation glaucoma (XFG), or ocular hypertension (OH); subjects aged 20 years or older; subjects who had been treated with ocular hypotensive eye drops, excluding any fixed-combination eye drops; and subjects who planned to start a PGTFC by visiting an ophthalmologist due to clinical requirements. Regarding patients with different types of glaucoma, those with POAG in one eye were assigned to the POAG group, those with NTG in one eye and OH or XFG in the other eye were assigned to the NTG group, and those with XFG in one eye and OH in the other eye were assigned to the XFG group. The exclusion criteria were as follows: subjects who were unable to answer the questionnaire, those who exhibited difficulty measuring their intraocular pressure (IOP) using a Goldmann applanation tonometer, and those whose attending ophthalmologist judged the patient unsuitable for participation. The attending ophthalmologists carefully explained the protocol and the purpose of the study to their patients before obtaining written informed consent.
Study design and protocol
Details of the study design and protocol were described in a previous paper.20 Briefly, the subjects and attending ophthalmologists completed the questionnaire (Figure S1A and B) prior to the prescription of the PGTFC. The questionnaires for both the subjects and the ophthalmologists were prepared by a council of the GRACE study group. The questionnaire for the subjects included glaucoma care status, glaucoma-related visual function disability and symptoms, awareness of instillation-related difficulty, the interval between the use of different types of eye drops, the frequency of forgetting instillations during the most recent week, and the times when instillations were most likely to be forgotten. The questionnaire also included information regarding whether the subject received any assistance with instillation, the use of instillation aids, acceptable types of eye drops, acceptable frequencies of instillation, and questions confirming their general and specific knowledge about glaucoma (Figure S1A). Questions for the ophthalmologists included the type of glaucoma, the glaucoma eye drops that were prescribed before and after the induction of the PGTFC, reasons for the PGTFC induction, best-corrected visual acuity, recent IOP, mean deviation (MD) of the Humphrey visual field analyzer program central 30–2 or 24–2, and the presumed frequency of forgetting eye drop instillation during the previous week (Figure S1B). Either leaflet A or leaflet B was randomly delivered to each patient on the day of PGTFC introduction. Leaflet A explained how to correctly instill eye drops, and leaflet B explained the clinical relevance of IOP reduction in glaucoma care and included two figures demonstrating how to correctly instill the eye drops as explained in leaflet A (Figure 1A and B). The patients returned to the hospital or medical clinic at 4–6 weeks for follow-up. The patients were required to complete another questionnaire that included questions regarding the PGTFC in addition to the questions included in the first questionnaire as indicated in Figure S2.
  | Figure 1 The two leaflets: leaflet A explains how to correctly instill the eye drops (A), whereas leaflet B explains the clinical relevance of IOP reduction in glaucoma care (B), in addition to how to correctly instill the eye drops. |
The ophthalmologists were prohibited from viewing the subjects’questionnaires. The questionnaires and medical data were sent to the GRACE committee office for analysis.
Statistical analyses
Council members (RT, YS, TT, and KK) judged whether the enrolled subjects satisfied the inclusion/exclusion criteria, and the data from subjects who fulfilled the criteria were subjected to the analysis. Logistic regression analysis was employed after adjusting the data. Because more than half of the subjects reported never missing an eye drop instillation, we classified the patients into two groups: those who reported never forgetting an instillation (good adherence) and those who missed eye drop instillations one or more times (nonadherent). The subjects who had no difficulty with instillation were categorized as “no difficulty”. The logMAR format was used to compare visual acuity. JMP 11.0 software (SAS Institute Inc., Cary, NC, USA) was used for the data analysis. The values are presented as the mean ± SD. Student’s t-tests, Wilcoxon signed-rank tests, Mann–Whitney U tests, chi-squared tests, contingency table analysis, or multivariate logistic regression analysis were employed as appropriate. P-values below 0.05 were considered significant.
Results
Participating facilities and ophthalmologists
There were 8,454 facilities that belonged to the Japan Ophthalmologists Association in 2011. Of these facilities, 1,071 facilities (12.7%) and 1,358 ophthalmologists participated in this study. The distribution of the participating facilities was evenly spread throughout Japan.
Demographics of the enrolled patients
A total of 4,430 patients were enrolled, and 833 were excluded for the following reasons: 371 patients had critical errors in their questionnaires, 256 patients violated the study protocol, 200 had a history of previously prescribed fixed-combination eye drop use, and six patients were under 20 years of age. In total, the data from 3,597 patients (81.2%), including 1,690 male and 1,907 female patients, were used in the analyses. The mean age was 68.4±12.2 years. The study included 1,852 POAG, 1,373 NTG, 14 XFG, and 228 OH patients. The total number of subjects using any type of prostaglandin analog eye drops was 3,240. Among these subjects, latanoprost was the most frequently used (54.6%) followed by travoprost (16.0%), tafluprost (13.6% subjects), and bimatoprost (8.9%), and others. The initial IOPs of the right and left eyes were 15.4±3.6 and 15.3±3.6 mmHg, respectively. Detailed demographics are presented in Table 1.
Changes in questionnaire surveys between study entry and the return visit
Table 2 compares changes in common questions between the two surveys performed at entry and the return visit. At the time of the return visit, the number of bottles of ocular hypotensive eye drops used was significantly reduced from 1.93±0.78 bottles to 1.34±0.54 bottles (P<0.0001; Wilcoxon signed-rank test). The rate of nonadherence was significantly reduced from 27.7% to 17.7% due to PGTFC introduction (P<0.0001; chi-squared test). The number of patients complaining of burdensome eye drops was also significantly reduced after the introduction of fixed-combination eye drops (P<0.0001; contingency table analysis). At entry, patients more frequently forgot to instill eye drops in the morning, followed by at noon; after PGTFC introduction, most subjects forgot to instill eye drops at noon (P<0.0001; contingency table analysis). Intervals of more than 5 minutes were most common both at entry and the visit after PGTFC introduction, and the number of patients with longer intervals was significantly increased after introduction of the fixed-combination eye drops (P<0.0001; contingency table analysis).
  | Table 2 Changes in the questionnaire survey outcomes between study entry and the return visit |
Effects of PGTFC introduction on patient attitude
Changes in patient attitude after PGTFC introduction are shown in Figure 2. A total of 46.4% or 14.0% of patients completely or partially agreed, respectively, that the PGTFC alleviated the burden of instilling eye drops (Figure 2A). A total of 46.3% of patients completely or partially agreed that PGTFC introduction contributed to a decrease in the forgetting of eye drop instillation, while 28.2% of the patients did not agree or totally disagreed with this statement (Figure 2B). However, PGTFC introduction did not influence eyedrop-induced hyperemia or a foreign body sensation (Figure 2C). In total, 42.4% or 17.4% of patients totally or partially preferred the PGTFC, respectively, while 4.7% or 6.0% of patients partially or totally disliked the PGTFC, respectively (Figure 2D).
Factors associated with adherence
Table 3 shows the factors associated with adherence, as determined by multivariate logistic regression analysis. In brief, nonadherence at entry, as reported by the patients and their physicians; an acceptable frequency of eye drop instillation, as reported at entry and at the return visit; and burdensome eye drop use reported at the return visit were found to be risk factors associated with nonadherence. No significant difference was observed in adherence between patients given leaflet A and those given leaflet B.
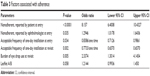  | Table 3 Factors associated with adherence |
Change in knowledge of glaucoma
All six questions were categorized into two groups. One group comprised questions 1–3, which focused on fundamental knowledge regarding eye drop use. The other group comprised questions 4–6, which focused on glaucoma-specific knowledge. The induction of PGTFC significantly improved the percentage of correct answers to questions 1, 2, 5, 6, all questions, questions in group 1, and questions in group 2, while the introduction of PGTFC significantly deteriorated the rate of correct answers to question 4 (Figure 3).
  | Figure 3 Change in the percentage of correct answers between study entry and the return visit. |
Discussion
This prospective cohort large study conducted a nationwide questionnaire-based survey regarding adherence to glaucoma eye drop use and associated factors among Japanese glaucoma patients who were newly introduced to PGTFC. A total of 3,597 patients involving more than 1,300 medical facilities participated in this study,20 which represents the largest scale study in Japan; the scale of this study was similar to or larger than that of previous studies worldwide.6,21,22 Because the patients and ophthalmologists who participated in this study were distributed evenly across Japan, we believe that the results may reflect the current Japanese status and that the sample bias of this study is limited.20
Adherence plays a key role in therapeutic effects.2 Poor adherence destabilizes IOP control in glaucoma treatment,23,24 and adherence is essential for the best outcomes of glaucoma treatment.6,25
This study revealed that introduction of PGTFC had a positive effect on adherence. PGTFC introduction reduced the number of eye drops used in this study, which may have contributed to adherence and is consistent with previous reports.26,27
The interval of eye drop instillation was significantly improved at the return visit after PGTFC introduction, and this may contribute to an increased efficacy of the eye drops. The precise reason for this is not clear, but the reduction in the number of eye drops used is possibly related to this positive effect.
This was a longitudinal study; thus, we can identify some PGTFC introduction-associated factors associated with adherence that have rarely or never been reported previously, including a history of nonadherence, an acceptable instillation frequency, and having burdensome eye drop use. These single-patient-based findings should be considered to predict adherence in addition to other factors that have previously been reported to be associated with adherence.
This study revealed that general knowledge of eye drops and glaucoma was improved after PGTFC introduction. A knowledge of glaucoma may be associated with adherence. We have reported that giving medical information to patients directly improved IOP control.28 In this study, patients randomly received one of two leaflets that were expected to result in different effects on patient knowledge. One leaflet provided fundamental information regarding correct eye drop use, and the other provided glaucoma-specific knowledge based on the presumption that improved glaucoma knowledge may improve adherence. These two leaflets may have played a role in improving the percentage of correct answers on the questionnaires, but we could not confirm that different roles were played by these two leaflets. However, this does not lead to the conclusion that glaucoma knowledge has no effect on adherence because the patients received these leaflets only once, and no further intervention influencing glaucoma knowledge was conducted in this study; thus, the provided leaflets may not have been sufficient to improve glaucoma knowledge.
This study has some limitations. Adherence was estimated using only questionnaires, although a large number of patients participated in the study. Adherence was difficult to accurately confirm because we did not employ electronic monitoring devices as used in previous studies.7,23,29–31 Although patients and ophthalmologists widely participated in the study across Japan, selection bias was not completely removed. We employed a longitudinal study to investigate the effect of PGTFC introduction on adherence. However, a limitation of the current results is that we did not include control patients. We employed a questionnaire survey in this study, but studies weighing bottles or checking daily logs of administration may be preferable to clarify the status of adherence.
Conclusion
This large-scale nationwide study revealed that the introduction of fixed-combination eye drops significantly improved adherence, and a history of poor adherence, burdensome instillation, and an acceptable frequency of instillation were associated with adherence. Ophthalmologists should consider these results to improve glaucoma care.
Acknowledgment
The authors deeply appreciate the cooperation of the participating subjects and ophthalmologists.
Author contributions
KK and MA conceived and designed the study. KK, YH, and RT analyzed the data and wrote the draft. TT, YS, KY, HS, TM, HS, and KK collected the data. All authors interpreted the results and critically revised the manuscript for important intellectual content.
Disclosure
Pfizer Japan Inc. financially supported this study but had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. The authors report no conflicts of interest in this work.
References
Kashiwagi K. Changes in trend of newly prescribed anti-glaucoma medications in recent nine years in a Japanese local community. Open Ophthalmol J. 2010;4:7–11. | ||
Gurwitz JH, Glynn RJ, Monane M, et al. Treatment for glaucoma: adherence by the elderly. Am J Public Health. 1993;83(5):711–716. | ||
Robin AL, Novack GD, Covert DW, Crockett RS, Marcic TS. Adherence in glaucoma: objective measurements of once-daily and adjunctive medication use. Am J Ophthalmol. 2007;144(4):533–540. | ||
Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116(6):1097–1105. | ||
Friedman DS, Quigley HA, Gelb L, et al. Using pharmacy claims data to study adherence to glaucoma medications: methodology and findings of the Glaucoma Adherence and Persistency Study (GAPS). Invest Ophthalmol Vis Sci. 2007;48(11):5052–5057. | ||
Djafari F, Lesk MR, Harasymowycz PJ, Desjardins D, Lachaine J. Determinants of adherence to glaucoma medical therapy in a long-term patient population. J Glaucoma. 2009;18(3):238–243. | ||
Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. | ||
Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009;116(12):2286–2293. | ||
Cohen Castel O, Keinan-Boker L, Geyer O, Milman U, Karkabi K. Factors associated with adherence to glaucoma pharmacotherapy in the primary care setting. Fam Pract. 2014;31(4):453–461. | ||
Takagi Y, Osaki H, Yamashita T, Kai Y. Prospective observational post-marketing study of Tafluprost 0.0015%/Timolol 0.5% combination ophthalmic solution for glaucoma and ocular hypertension: short-term efficacy and safety. Ophthalmol Ther. 2016;5(2):191–206. | ||
Denis P. Travoprost/timolol fixed combination in the management of open-angle glaucoma: a clinical review. Expert Opin Pharmacother. 2011;12(3):463–471. | ||
Pfeiffer N, Scherzer ML, Maier H, et al. Safety and efficacy of changing to the travoprost/timolol maleate fixed combination (DuoTrav) from prior mono- or adjunctive therapy. Clin Ophthalmol. 2010;4:459–466. | ||
Mandić Z, Novak-Laus K, Bojić L, et al. Safety and efficacy of monotherapy change to fixed combination (travoprost 0.004%/timolol 0.5%) in 6 months follow up period. Acta Clin Croat. 2010;49(4):411–419. | ||
Barnebey HS, Orengo-Nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140(1):1.e1–1.e8. | ||
Higginbotham EJ, Feldman R, Stiles M, Dubiner H; Fixed Combination Investigative Group. Latanoprost and timolol combination therapy vs monotherapy: one-year randomized trial. Arch Ophthalmol. 2002;120(7):915–922. | ||
Kashiwagi K. Efficacy and safety of switching to travoprost/timolol fixed-combination therapy from latanoprost monotherapy. Jpn J Ophthalmol. 2012;56(4):339–345. | ||
Herceg M, Noecker R. Travoprost/timolol fixed combination. Expert Opin Pharmacother. 2008;9(6):1059–1065. | ||
Barnebey HS, Robin AL. Adherence to fixed-combination versus unfixed travoprost 0.004%/timolol 0.5% for glaucoma or ocular hypertension: a randomized trial. Am J Ophthalmol. 2017;176:61–69. | ||
Tabet R, Stewart WC, Feldman R, Konstas AG. A review of additivity to prostaglandin analogs: fixed and unfixed combinations. Surv Ophthalmol. 2008;53(Suppl 1):S85–S92. | ||
Tsumura T, Kashiwagi K, Suzuki Y, et al. A nationwide survey of factors influencing adherence to ocular hypotensive eyedrops in Japan. Int Ophthalmol. Epub 2018 Jan 12. | ||
Park MH, Kang KD, Moon J; Korean Glaucoma Compliance Study Group. Noncompliance with glaucoma medication in Korean patients: a multicenter qualitative study. Jpn J Ophthalmol. 2013;57(1):47–56. | ||
Vandenbroeck S, de Geest S, Dobbels F, Fieuws S, Stalmans I, Zeyen T. Prevalence and correlates of self-reported nonadherence with eye drop treatment: the Belgian Compliance Study in Ophthalmology (BCSO). J Glaucoma. 2011;20(7):414–421. | ||
Cate H, Bhattacharya D, Clark A, Fordham R, Holland R, Broadway DC. Improving adherence to glaucoma medication: a randomised controlled trial of a patient-centred intervention (The Norwich Adherence Glaucoma Study). BMC Ophthalmol. 2014;14(1):32. | ||
Gray TA, Fenerty C, Harper R, et al. Individualised patient care as an adjunct to standard care for promoting adherence to ocular hypotensive therapy: an exploratory randomised controlled trial. Eye. 2012;26(3):407–417. | ||
Dreer LE, Owsley C, Campbell L, Gao L, Wood A, Girkin CA. Feasibility, patient acceptability, and preliminary efficacy of a culturally informed, health promotion program to improve glaucoma medication adherence among African Americans: “Glaucoma Management Optimism for African Americans Living with Glaucoma” (GOAL). Curr Eye Res. 2016;41(1):50–58. | ||
Loon SC, Jin J, Jin Goh M. The relationship between quality of life and adherence to medication in glaucoma patients in Singapore. J Glaucoma. 2015;24(5):e36–e42. | ||
Rosdahl JA, Swamy L, Stinnett S, Muir KW. Patient education preferences in ophthalmic care. Patient Prefer Adherence. 2014;8:565–574. | ||
Kashiwagi K, Tsukahara S. Impact of patient access to Internet health records on glaucoma medication: randomized controlled trial. J Med Internet Res. 2014;16(1):e15. | ||
Dreer LE, Girkin C, Mansberger SL. Determinants of medication adherence to topical glaucoma therapy. J Glaucoma. 2012;21(4):234–240. | ||
Hermann MM, Papaconstantinou D, Muether PS, Georgopoulos G, Diestelhorst M. Adherence with brimonidine in patients with glaucoma aware and not aware of electronic monitoring. Acta Ophthalmol. 2011;89(4):e300–e305. | ||
Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmology. 2011;118(12):2398–2402. | ||
Collaborative Normal-Tension Glaucoma Study Group. The effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. Am J Ophthalmol. 1998;126(4):498–505. |
Supplementary materials
  | Figure S1 (A) Questionnaire for patients. (B) Questionnaire for ophthalmologists. |
  | Figure S2 Questionnaire for patients when revisiting the hospital or medical clinic. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


