Back to Journals » International Medical Case Reports Journal » Volume 13
Challenging Clinical Cases – A Walk Through Supplemental Therapy with Intravitreal Ranibizumab Therapy Following Treatment of Diabetic Macular Edema with the 0.19 mg Fluocinolone Acetonide Implant (ILUVIEN®)
Authors Pessoa B , Melo-Beirão J , Meireles A , Menéres P
Received 15 May 2020
Accepted for publication 24 July 2020
Published 15 September 2020 Volume 2020:13 Pages 437—448
DOI https://doi.org/10.2147/IMCRJ.S262587
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Bernardete Pessoa,1,2 João Melo-Beirão,1,2 Angelina Meireles,1,2 Pedro Menéres1,2
1Ophtalmology Department, Centro Hospitalar e Universitário do Porto, Porto, Portugal; 2Unit for Multidisciplinary Research in Biomedicine, Instituto de Ciências Biomédicas Abel Salazar, Universidade do Porto, Porto, Portugal
Correspondence: Bernardete Pessoa Departamento de Oftalmologia
Hospital de Santo António, Centro Hospitalar Universitário do Porto, Porto, Portugal
Email [email protected]
Purpose: There are limited published data regarding the use of supplemental intravitreal therapies in patients with diabetic macular edema (DME) following treatment with the 0.19 mg fluocinolone acetonide (FAc; ILUVIEN®) intravitreal implant. The aim of this report was to analyze five challenging eyes that required supplemental therapies after treatment with the FAc implant.
Methods: This is a retrospective case series conducted at the Centro Hospitalar Universitário do Porto in Porto, Portugal, between 2015 and 2019. It aimed to assess the patient background, treatment history and patient outcomes in challenging clinical cases in which intravitreal injections (IVI) of ranibizumab had been given pro re nata following treatment with the FAc implant (with a minimum follow-up of 33 months). Parameters measured included best-corrected visual acuity in early treatment diabetic retinopathy scale, central macular thickness and intraocular pressure.
Patients: Five eyes (three patients) diagnosed with persistent or recurrent DME and suitable for treatment with the FAc implant according to its licensed indication in Europe.
Results: In the first 2 patients, one bilateral, DME was refractory to IVI of short-acting corticosteroids and anti-VEGF. Following FAc therapy, there was a favorable evolution and a clear regression of diabetic retinopathy (DR) severity. Supplemental treatments were adopted, but a reduced number of treatments were needed beyond three years in these cases. The third case had bilateral DME. One eye had been vitrectomized and FAc therapy led to resolution of DME within 6 months. In the contralateral eye, the control of DME was dependent on anti-VEGF supplemental treatments until a pars plana vitrectomy was performed.
Conclusion: The multifactorial nature of DME means there is a need for an individualized treatment approach to the management of DME. It also explains why some patients need a combined or a more aggressive approach to therapy in order to achieve successful outcomes for the patient.
Keywords: anti-VEGF, diabetic macular edema, fluocinolone acetonide implant, ranibizumab, supplemental therapy
Plain Language Summary
This case series describes three cases from patients with diabetic macular edema (DME) that persisted or recurred despite treatment and were subsequently treated with a 0.19 mg fluocinolone acetonide implant (ILUVIEN®). Patient eyes still proved challenging to manage and required supplemental intravitreal therapies to improve overall outcomes for these patients.
Introduction
In real-life practice, there are multiple factors contributing to suboptimal outcomes in patients with DME following anti-VEGF therapy (eg, intravitreal injections of bevacizumab [off-label], ranibizumab and aflibercept). These include the multifactorial nature of the underlying disease1,2 as well as practical elements. For example the inability of physicians to administer therapies according to treatment strategies employed in randomized controlled trials due to the excessive treatment burden (ie, monthly injections in some cases) on ophthalmologists and patients3 and missed clinical follow-up visits as a result of numerous other hospital appointments resulting in delayed or inappropriate treatment.4
The nature of intravitreal corticosteroids means they have angiostatic, anti-permeability and anti-inflammatory effects. The longer duration of effect of corticosteroids means that patients require fewer treatment injections and hospital appointments, and so they present a good treatment option in the management of diabetic macular edema (DME).4
Intravitreal corticosteroids are commonly used as second-line treatments for patients with DME.5,6 Short-acting intravitreal injections (IVI) of corticosteroids included triamcinolone acetonide (IVTA; used off-label to treat DME) and the dexamethasone implant (OZURDEX®), both of which have a duration of action ≤6 months. In contrast, the fluocinolone acetonide (FAc) intravitreal implant (ILUVIEN®) is much longer-acting with a single implant lasting for up to 36 months when injected into the vitreous cavity.
The multifactorial nature of DME may be one explanation as to why some patients may require a combined treatment strategy to achieve the most efficient treatment outcomes,2,7 especially in the more challenging DME cases where supplemental therapies are required. Indeed, outcomes achieved in real-world practice show that supplemental therapy is required in around 30% of cases following the administration of the FAc implant with a mean time to additional therapy of 356.1±274.8 days.7 From a clinical perspective, the challenge is to identify patients requiring supplemental therapy.
The aim of this study was to analyze and understand the outcomes from five challenging cases where the FAc implant was administered in combination with IVI of ranibizumab.
Methods
This is a retrospective case series study performed at Centro Hospitalar Universitário do Porto (CHUP), Porto, Portugal, between 2015 and 2019.
At this center, 94 DME eyes (a total of 102 injections) have been treated with the FAc implant. Forty-six eyes of these eyes have 3 years of follow-up. In these, 25 eyes (54.3%) required supplemental intravitreal therapies and in 6 eyes (13.0%) supplementary treatment did not have any additional benefit.
Background disease, treatment history and patient outcomes were captured from five DME eyes (3 patients) with a minimum follow-up of 33 months following injection of the FAc implant. Patients were treated according to the European license and had DME that persisted or recurred despite treatment. Following treatment with the FAc implant, all patients had received IVI of ranibizumab (0.5 mg/0.05mL) as a supplemental therapy.
Data collection included patient demographics, diabetic retinopathy (DR) status and prior DME therapies.
At 1-week post-injection and then at least, every 3 months thereafter, each patient had a complete ophthalmological evaluation. This included an evaluation of best-corrected visual acuity (BCVA), assessed using the early treatment diabetic retinopathy (ETDRS) letter score; central macular thickness (CMT), assessed with spectral domain optical coherence tomography (SD-OCT Topcon 3D-OCT 1000 [used up to May 2015] or a Spectralis HRA + OCT, version 1.10.2.0; Heidelberg Engineering, Heidelberg Germany [used from June 2015]); and, intraocular pressure (IOP). Fluorescein angiography was performed on all patients at baseline and at subsequent time points as and when required.
The study was conducted according to the tenets of the Declaration of Helsinki in its latest amendment (Brazil, 2013) and was approved by the ethics committee of CHUP [2017.093 (084-DEFI/082-CES)]. All patients signed a written informed consent has been provided by the patients to have the case details and any accompanying images published.
Results
Clinical Case 1
A 68-year-old female with type 2 diabetes and diagnosed with center-involved DME in her right eye (RE) in 2012 and left eye (LE) in 2011 (please see Table 1). Comorbidities included hypertension and dyslipidemia. Both eyes were treated with macular and peripheral laser and unresponsive to prior IVI therapies (LE, 5 IVTA and 1 bevacizumab; RE, 3 IVTA and 4 bevacizumab). In November 2014, the LE underwent a pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling combined with phacoemulsification. At this time intravitreal injection of IVTA was administered with no regression of the DME. In mid-2015, changing therapy from IVTA to a FAc implant was considered based on this being a different corticosteroid and that it releases a sustained low dose of fluocinolone acetonide for up to 3 years.8 FAc implants were injected into both RE and LE.
 |
Table 1 Case 1: Interventions, Treatments and Outcomes |
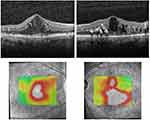
Figure 1 shows the baseline pre-FAc SD-OCT from the RE and LE.
Supplemental Treatments Following FAc Therapy in the RE (51 Months of Follow-Up)
At 4, 6, 7 and 8 months post-injection of FAc, IVI of ranibizumab (combined with cataract surgery at month 8) were performed due to persistent DME. No other therapies were required from month 8 to 39 (Figure 2). At month 39, four IVI of ranibizumab were given to manage the recurrence of mild focal DME and no further injections were then required from month 48.
Supplemental Therapies Following FAc Therapy in the LE, Vitrectomized (50 Months of Follow-Up)
The LE was managed in a similar fashion to the RE with the first IVI of ranibizumab given at month 4 (Figure 2). A total of 3 IVI of ranibizumab were required each year, with the last 3 monthly IVI of ranibizumab been given at month 39, with no further treatments up to month 50.
After FAc implant, through more than 48 months of follow-up, 9 and 12 IVI of ranibizumab IVI were considered necessary to control refractory DME in RE and in LE, respectively (Figure 2), with an improved DR status at the end of follow-up (see Figure 3). There were no visible hard exudates (HE) and an almost normal macular anatomy was observed, although some temporal macular atrophy with external photoreceptor disruption was noticed in the LE.
At baseline, CMT was 621 μm in the RE and 590 μm in the LE and improved to 298 and 265 μm at year 4 post-FAc therapy. BCVA also improved in RE (increasing from 50 letters at baseline to 85 letters at last observation) and LE (increased from 70 to 77 letters). Both eyes received timolol 1-week post-IVTA, due to an elevation in IOP above 21 mmHg, and following treatment remained at ~18 mmHg, which was maintained throughout the follow-up period. Glycemic control also remained stable with glycated haemoglobin (HbA1c) remaining between 7.9% and 8% throughout.
Clinical Case 2
A 60-year-old female with type 2 diabetes and DME described for the first time in the RE in 2011 against a background of severe non-proliferative DR (Figure 4; Table 2). Macular LASER and pan-retinal photocoagulation (PRP) were performed in both eyes in 2011 and in 2012. Comorbidities included hypertension and dyslipidemia. In 2013, after referral to our department, several IV therapies were performed to the RE (4 bevacizumab, 1 IVTA, 3 bevacizumab, 7 ranibizumab, 2 aflibercept and 1 dexamethasone implant, by this order). DME was unresponsive to all these IV therapies. Prior to FAc therapy, administered in June 2016, IVTA evoked changes in IOP were being managed with timolol, brinzolamide and brimonidine. Subsequent therapy with the dexamethasone implant led to a further rise in IOP within one week and surgery (cyclophotocoagulation guided with transillumination) was performed. At this point, IOP was ~16 mmHg and managed without medication. One week after the administration of the FAc, timolol and brimonidine eye drops were required to manage IOP (at ~16 mmHg).
 |
Table 2 Case 2: Interventions, Treatments and Outcomes |
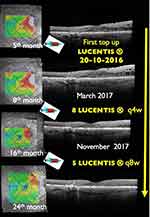 |
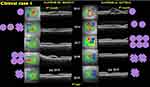
Figure 5 Clinical case 2: SD-OCT images showing the evolution while top-up treatment approach was undergone. A total of 14 IVI of ranibizumab were added between months 5 and 24. |
Supplemental Treatments Following FAc Therapy (39 Months of Follow-Up)
Cataract surgery was performed at day 40 with no worsening of DME. Due to persistence of DME, IVI of ranibizumab was added at months 4, 7, 9 and monthly thereafter up to month 20 with two more injections given at months 23 and 24 (Figure 5). A total of 6 and 10 IVI of ranibizumab were added in year 1 and year 2, respectively. From month 24, the macula was dry and no supplemental therapies were required. The reduction in edema was accompanied by an improvement in DR status with no HE and a nearly normal central macular anatomy (Figure 6).
CMT and BCVA were 557 μm and 40 letters at baseline, respectively, and improved to 251 μm and 72 letters by month 39 post-FAc injection. At baseline the patient had poor glycemic control (HbA1c=8.5%) and progressively improved to 7.7% at the end of follow-up period.
Clinical Case 3
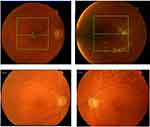
Fifty-six-year-old male, type 2 diabetic, diagnosed with bilateral DME in July 2014. The RE had severe non-proliferative DR and the LE had proliferative DR (please see Table 3). Both eyes were pseudophakic. Comorbidities included hypertension, dyslipidemia, stroke (in 2012) and acute myocardial infarction (2005). Both eyes were treated with macular (in 2014) and PRP (March 2015). The RE underwent PPV with ILM peeling in September 2014 due to an epiretinal membrane (ERM). RE had been treated with 1 IVTA in 2014 and with 2 IVTA in 2015 and LE with 1 IVTA in 2014 and another in 2015 with incomplete responses observed. Figure 7 shows the RE and LE status observed pre-FAc.
 |
Table 3 Case 3: Interventions, Treatments and Outcomes |
Supplemental Treatments Following FAc Therapy in RE (33 Months of Follow-Up)
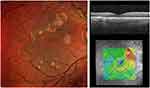
The FAc implant was given in December 2016 and DME was controlled without additional therapy, although some mild recurrences were observed. At month 33, the macula was dry with a CMT of 256 μm, decreasing from a baseline of 763 μm. BCVA increased from 40 to 77 letters and IOP was stable (~12 mmHg) with no medication required (Figure 8).
Supplemental Therapies Following FAc Therapy in LE (33 Months of Follow-Up)
FAc therapy was started in January 2017 and supplemental IVI of ranibizumab every 1.5 months were needed to control DME between May 2017 and December 2017. A fluorescein angiography in September 2017 showed no evidence of new vessels or ischemia and up to April 2018 no supplemental IVI of ranibizumab were required. Severe recurrence of DME led to further supplemental IVI of ranibizumab every 1.5 months, which were ineffective. In May 2019, a PPV was conducted with concomitant confluence of PRP (see Figure 8, top panel). By month 33 (ie, 3 months after PPV) the macula was dry (CMT decreased to 251 μm from 631 μm at baseline), BCVA improved (to 73 letters from 50 letters) and IOP remained stable (~10 mmHg) without medication. During this period, glycemic control was quite varied and at the end of the follow-up period HbA1c had decreased to 6.1% from 11.6% at baseline.
Discussion
The FAc implant is licensed for the treatment of DME that is insufficiently responsive to available therapies. The current review of challenging DME case series shows that DME can be effectively managed over the longer-term with the FAc implant when combined with supplemental treatments. A better knowledge of the patient’s clinical background (patient characteristics and demographics, as well as the clinical condition of the patient) and results regarding supplemental treatments after the administration of the FAc implant is important to the treating physician as it provides insights into the timing of these therapies and the decision process involved. These are all important considerations that are not well documented in the literature but essential for making informed decisions about supplemental therapy.
Analyzing our first two clinical cases, both with more than 36 months of follow-up, it may seem controversial to have given additional therapies, particularly in case 1. Nevertheless, these cases were refractory to short-acting IVI of corticosteroid and anti-VEGF. With the treatment strategy adopted, we obtained a clear regression of DR severity, with a complete disappearance of the HE, a dry macula and treatment interval free from supplemental therapies lasting 3 (RE) and 11 (LE) months in case 1, and 15 months in case 2. This is particularly relevant in case 1, with four years of follow-up post-FAc implantation. In the LE of case 1 it can be also inferred that PPV with ILM peeling may be the explanation for the temporal macular atrophy and the reduced gain in visual acuity.9 It is unlikely that differences between LE and RE are explained by changes in the clearance of FAc from the vitreous of the eye as past studies report similar outcomes in vitrectomised and non-vitrectomised eyes.4,10
In the third clinical bilateral case treated with the FAc implant, the different DR and vitreous status may be the explanation for the different response to treatment. In the RE, after the FAc implant, DME was effectively controlled without any additional treatment as opposed to LE with DME that was controlled with additional IVI of ranibizumab. At baseline, the RE was vitrectomized with PRP and a non-PDR status whereas the LE was not vitrectomized and received incomplete PRP and had PDR. After FAc implantation, despite supplemental PRP and the disappearance of the retina neovascularization according to the fluorescein angiography repeated 9 months post-injection of the FAc implant, the response of LE to treatment was not so favorable comparable with the RE. Nevertheless, after vitrectomy and confluence of PRP, at 33 months after the FAc implant was administered, a complete resolution of DME was achieved after 3 months. Probably VEGF levels were in fact increased in LE. That would explain the response to top-up ranibizumab and the absence of neovascularization at the time of the angiography (window effect during anti-VEGF treatment). Vitrectomy could also represent a way for the removal of angiogenic, inflammatory and tractional factors, and possibly with PRP confluence contributing to enhanced DME control.
In the third patient, the RE is an example, which illustrates that DME relapses during the 36 months after the FAc implant and shows it could be controlled without any additional treatment. A mild DME recurrence appeared in the second year after administration of the FAc implant and resolved in a period of 5 to 6 months.
Conclusion
This analysis intends to stress the importance of an individualized approach for DME, which is a multifactorial disease that is not fully understood, where many local and systemic factors (demonstrated by similar behavior in bilateral cases) may influence the course, presentation and the response to treatment. The challenging cases presented here show that DME can still be effectively managed over the long term with the FAc implant combined with supplemental treatments as and when required.
Disclosure
None of the authors has any conflicting interests to disclose for this work. B. Pessoa had full access to all the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
References
1. Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96:1501–1510. doi:10.1016/S0161-6420(89)32699-6
2. Chakravarthy U, Yang Y, Lotery A. Clinical evidence of the multifactorial nature of diabetic macular edema. Retina. 2018;38:343–351. doi:10.1097/IAE.0000000000001555
3. Amoaku WMK, Saker S, Stewart EA. A review of therapies for diabetic macular oedema and rationale for combination therapy. Eye. 2015;29:1115–1130. doi:10.1038/eye.2015.110
4. La Mantia A, Hawrami A, Laviers H, Patra A, Zambarakji H. Treatment of refractory diabetic macular edema with a fluocinolone acetonide implant in vitrectomized and non-vitrectomized eyes. Int J Ophthalmol. 2018;11(12):1951–1956. doi:10.18240/ijo.2018.12.13
5. Ciulla TA, Bracha P, Pollack J, Williams DF. Real-world outcomes of anti–vascular endothelial growth factor therapy in diabetic macular edema in the United States. Ophthalmol Retina. 2018;2(12):1179–1187. doi:10.1016/j.oret.2018.06.004
6. Elman MJ, Aiello LP, Beck RW, et al.; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–1077. doi:10.1016/j.ophtha.2010.02.031
7. Chakravarthy U, Taylor SR, Koch FHJ, Castro de Sousa JP, Bailey C, ILUVIEN Registry Safety Study (IRISS) Investigators Group. Changes in intraocular pressure after intravitreal fluocinolone acetonide (ILUVIEN): real-world experience in three European countries. Br J Ophthalmol. 2019;103(8):1072–1077. doi:10.1136/bjophthalmol-2018-312284
8. Yang Y, Bailey C, Loewenstein A, Massin P. Intravitreal corticosteroids in diabetic macular edema: pharmacokinetic considerations. Retina. 2015;35(12):2440–2449. doi:10.1097/IAE.0000000000000726
9. Zur D, Iglicki M, Loewenstein A. The role of steroids in the management of diabetic macular edema. Ophthalmic Res. 2019;62(4):231–236. doi:10.1159/000499540
10. Pessoa B, Coelho J, Correia N, Ferreira N, Beirao M, Meireles A. Fluocinolone acetonide intravitreal implant 190 µg (ILUVIEN®) in vitrectomized versus nonvitrectomized eyes for the treatment of chronic diabetic macular edema. Ophthalmic Res. 2018;59(2):68–75. doi:10.1159/000484091
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.