Back to Journals » International Medical Case Reports Journal » Volume 16
Challenges in Managing Secondary Glaucoma Post-Repeat Penetrating Keratoplasty in a Developing Country
Authors Rusmayani E, Hutauruk JA, Viona V
Received 10 January 2023
Accepted for publication 16 March 2023
Published 18 March 2023 Volume 2023:16 Pages 179—185
DOI https://doi.org/10.2147/IMCRJ.S402944
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Emma Rusmayani,* Johan A Hutauruk,* Viona Viona*
Jakarta Eye Center, Jakarta, Indonesia
*These authors contributed equally to this work
Correspondence: Emma Rusmayani, Jakarta Eye Center, Terusan Arjuna Utara/1 Kedoya, West Jakarta, Jakarta, 11520, Indonesia, Email [email protected]
Purpose: To illustrate the complexity in managing secondary glaucoma post-repeat penetrating keratoplasty in a developing country.
Case Description: A patient with a history of five repeat penetrating keratoplasties (PKPs) showed good intraocular pressure (IOP) control with trabeculectomy; however, blebitis occurred as an undesirable complication. Trabeculectomy was done rather than tube implantation due to socioeconomic factors, although it’s not an ideal treatment. After the infection subsided, we performed a bleb revision with a scleral patch graft. Intraocular pressure was high in the follow-up period after the scleral patch, therefore we decided to do tube implantation. Following glaucoma tube implant surgery, the patient had good IOP control and a clear graft after six months of follow-up.
Conclusion: Secondary glaucoma post repeat PKPs is challenging in both diagnosis and management. Immediate action is imperative to control IOP, prevent glaucoma progression, and minimize corneal graft damage. In addition to medical reasons, socioeconomic factors should be considered.
Keywords: repeat penetrating keratoplasty, secondary glaucoma, blebitis, tube implant
Introduction
Secondary glaucoma post penetrating keratoplasty (PKP) is the second cause of graft failure leading to permanent visual impairment and the second cause of graft failure overall.1 Difficulties in the diagnosis and management of intraocular pressure (IOP) elevation post-PKP depict the complexity of this type of secondary glaucoma. Risk factors for secondary glaucoma post-PKP relate to anterior chamber disorganization (shallow chamber and peripheral anterior synechia [PAS] formation), long-term corticosteroid use, and inflammatory processes. Early diagnosis and prompt treatment of glaucoma may have a role in visual and graft survival outcomes.2
Case Report
A 52-year-old female with a history of repeat PKPs was referred to the glaucoma clinic due to uncontrolled IOP. The patient underwent PKP five times in the right eye from 1989 to 2019. She constantly experiences corneal rejection symptoms after several years of keratoplasty (Figure 1). The contralateral eye was diagnosed with myopic macular degeneration, and with appropriate treatment and uncomplicated cataract surgery, a best-corrected visual acuity (BCVA) of 1.00 logMAR was achieved. Therefore, treating the right eye was the immediate concern. There was no documented history of IOP elevation in the patient’s medical history prior to the fourth PKP. During that period, the IOP was adequately managed with topical antiglaucoma monotherapy, ranging from 12–14 mmHg. One week after the fifth PKP, the patient complained of pain, redness, and halos around lights. She was referred to the glaucoma service for further evaluation and management. The IOP was 42 mmHg by applanation tonometry, and the BCVA was 0.25 logMAR with a clear graft and no signs of rejection. The patient was treated with maximum topical antiglaucoma medications; however, the IOP was still 36 mmHg. We suggested tube implantation due to the uncontrolled IOP and history of repeat PKPs with a current viable graft. However, the patient was unable to attend our care due to socioeconomic factors and continued care in a tertiary clinic with no resources for glaucoma tube implantation. The patient underwent trabeculectomy with mitomycin C (MMC), and the IOP was well controlled after 22 months without antiglaucoma medications (Figures 2 and 3). In April 2022, she came to our clinic with complaints of irritation, redness, and decreased vision in the right eye. On examination, we found an incarcerated bleb with a positive Seidel test (Figures 4 and 5), 5 mmHg IOP, and BCVA hand movement. We treated this as a blebitis with broad-spectrum topical and oral antibiotics. After the infection subsided, we performed a bleb revision with scleral patch graft. One month after surgery, the IOP was 22 mmHg on maximum topical antiglaucoma medications, wound closure was good, and the corneal graft was clear (Figure 6). We decided to insert an Ahmed Glaucoma Valve (New World Medical, Inc., Rancho Cucamonga, California, USA) to lower the IOP. The tube was inserted in the posterior chamber to protect the corneal endothelium. Six months after surgery, the IOP was well controlled (11 mmHg) without antiglaucoma medications, BCVA was 0.2, and the graft remained clear (Figures 7 and 8).
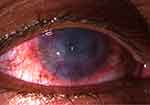 |
Figure 1 Failed graft after repeat penetrating keratoplasties. The patient complained of a significant decrease in vision, redness, grittiness, and pain. |
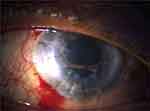 |
Figure 2 Patient one-week post-trabeculectomy with clear graft. |
 |
Figure 3 Thin, avascular bleb and no leak at one-month follow-up post-trabeculectomy with mitomycin C. |
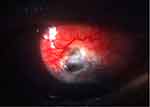 |
Figure 4 Scleral thinning 22-months post-trabeculectomy with mitomycin C. |
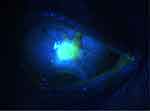 |
Figure 5 Bleb leak with positive Seidel test viewed after fluorescein staining. |
 |
Figure 6 One-week post bleb revision with scleral patch. |
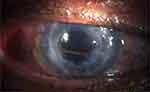 |
Figure 7 Clear graft six months after Ahmed tube implant positioned in the posterior chamber. |
 |
Figure 8 Tube positioned behind the iris with extensive peripheral anterior synechia. |
Discussion
Secondary glaucoma post-PKP is one of the most complex complications of the surgery due to difficulties in diagnosis and management.3,4 The incidence of glaucoma following PKP is relatively lower in the early postoperative period (9–31%) than in the late postoperative period (18–35%).1 The incidence of elevated IOP following PKP ranges from 5.3% to 60%.2 This wide range may be caused by inaccurate IOP measurements due to corneal irregularities.1,3,4 The limited availability of IOP measurement devices in countries with limited resources complicates the diagnosis of this type of glaucoma.
The IOP increase in the early postoperative period must be managed appropriately despite difficulties in obtaining accurate IOP measurements due to corneal surface irregularities. Late IOP spikes may occur weeks to years after PKP surgery and may result from PAS, long-term use of steroid agents, or aqueous misdirection.5 Early management of IOP elevation may affect the prognosis of graft survival and the risk of developing glaucoma. Uncontrolled elevation of IOP might lead to damage of the anterior (corneal graft) and posterior (optic nerve) structures, leading to permanent visual impairment.6 Early diagnosis and prompt treatment of this type of glaucoma play an important role in conserving graft clarity and graft survival.1 Medical management with antiglaucoma medications is the first line of treatment for glaucoma following PKP. Surgical management of secondary glaucoma post-PKP is indicated in patients with uncontrolled IOP on maximum topical antiglaucoma medications. The challenges of surgery in cases of glaucoma secondary to PKP are minimizing corneal graft complications and controlling IOP.7
Many studies have found good outcomes with trabeculectomy after PKP in terms of IOP control and graft survival.8 However, several factors contribute to poor outcomes, including limbal conjunctival scarring due to higher rates of bleb fibrosis in repeated PKP cases and anterior segment disorganization (shallow anterior chamber and PAS).9,10 Aqueous humor properties after PKP demonstrate high levels of inflammatory substances, which act as chemoattractants, promoting the fibrotic process.8 Antimetabolites, such as 5-fluorouracil (5-FU) and MMC have improved the outcomes of complex glaucoma cases by inhibiting fibroblast proliferation and promoting bleb filtering properties. As an alkylating agent, MMC inhibits the postoperative scarring response by cross-linking the DNA of conjunctival and episcleral fibroblasts and reducing their proliferation.11 MMC is a more potent agent than 5-FU, and 5-FU has shown corneal toxicity and therefore may affect corneal graft survival. Good bleb formation and IOP control has been reported in 67–80% of trabeculectomy with MMC cases, with a low rate (12–16%) of failure.12–14 Compared to conventional trabeculectomy, the use of MMC may improve IOP control post-trabeculectomy.15 A previous study found that using MMC 0.02% applied for one minute can inhibit fibroblasts by 68%, and this effect increased to 90% with 0.04% MMC applied for five minutes. At eighteen months post-surgery, a similar success rate was seen in patients receiving MMC 0.02% for 5 minutes. The protocol used in our clinic for MMC application is 0.02% for three minutes using soaked sponges. Our patient showed good IOP control without any antiglaucoma medication after trabeculectomy with MMC for almost two years. A large MMC treatment area during surgery helps to increase the bleb area, therefore resulting in better IOP control. However, trabeculectomy with MMC results in thinner blebs. Thin avascular blebs have areas of absent conjunctival epithelium, which increases the susceptibility to infection. Serious concerns regarding the use of antimetabolites are increased risk of hypotony maculopathy, blebitis, bleb-related endophthalmitis, scleral thinning, and bleb leak.14–16 The incidence of bleb-related infection is relatively low, with a 0.55% incidence of blebitis after trabeculectomy with MMC17 and a 0.11% incidence of blebitis with leakage.18 Bleb leaks expose patients to the risk of developing endophthalmitis and should be considered a serious late postoperative complication, especially in patients with high risk of infection.14 In a previous study, the mean onset of bleb leaks was six months, but in our case, a bleb leak occurred 22 months after the surgery. Repeat PKPs also create a higher risk of fragile conjunctiva and act as an additional risk factor for scleral thinning post-trabeculectomy with MMC.16 The fragile conjunctiva and sclera lead to barrier disruption and act as locus minoris resistentiae for pathogenic bacteria to invade. Therefore, in our patient, once the infection had subsided after treatment with broad-spectrum topical antibiotics, we decided to perform a bleb revision with scleral graft to prevent further infection.
Glaucoma tube implants are effective options for control of IOP in refractory cases that have failed with maximum antiglaucoma medications and filtering surgeries.9 The rate of successful tube implantation in glaucoma secondary to PKP is as high as 95%. However, tube implantation has been correlated with shorter corneal graft survival rates, with a clear graft rate decreased from 58.5% to 25.8% in a two-year follow-up.19–21 Several reasons associated with graft failure after implantation of glaucoma drainage devices (GDDs) were tube-corneal contact (turbulent flow), trauma (micromotions), and retrograde flow of inflammatory cells into the anterior chamber.5 Tube implants have a higher success rate of 71% in IOP control and 54% graft survival in a five-year follow-up in penetrating keratoplasty cases.22 In general, tube implantation carries a higher risk of graft failure than trabeculectomy. Other studies have mentioned the benefit of implanting the GDD in the vitreous chamber to reduce the risk of graft failure.23 However, anterior vitrectomy is required prior to implantation, and this may be the reason for glaucoma surgeons’ hesitation in performing this technique. Despite placing the tube in the posterior chamber, post-PKP patients have similar risks to the anterior chamber, since usually in repeat PKP cases anterior chamber structures tend to be disorganized.5 Our patient had undergone trabeculectomy and had good IOP control. A bleb leak occurred two years post-operatively with a history of five PKP surgeries. Therefore, we decided to perform tube implantation for IOP control taking into consideration graft survival. IOP was well controlled with the Ahmed tube implant; however, because of extensive PAS, there is still a risk of tube-corneal touch, which might compromise graft survival in the long term.
Advanced or severe glaucoma can cause disability in patients, significantly impacting their ability to work and earn income. This condition can lead to an economic burden and limit treatment options, especially for middle to low-income patients who may struggle to afford the high cost of medications or surgical procedures. The financial burden of glaucoma can make it challenging for patients to access the care they need, further exacerbating their vision loss and disability. In Indonesia, the government’s health care insurance does not cover all intraocular pressure medications or certain types of glaucoma surgeries. Moreover, doctors and centres with resources for managing complex glaucoma cases, such as secondary glaucoma post-keratoplasty, are minimal. Patients may need to travel long distances and incur additional transportation costs to see physicians and visit hospitals. These predicaments may pose challenges to patients’ adherence, compliance, and commitment to treatment.25–27 In this case, ensuring the survival of the graft and adequate management of glaucoma, which likely developed after keratoplasty, is very challenging due to the patient’s limited resources. By highlighting this case, we aim to demonstrate the difficulties Indonesian patients with limited resources encounter when attempting to access high-quality healthcare, particularly in glaucoma management. Furthermore, we hope to contribute to the development of improved health policies that can alleviate the burden on these patients and ultimately prevent cases of blindness resulting from glaucoma.
Conclusion
In conclusion, secondary glaucoma post-repeat PKP is challenging in both diagnosis and management. In a limited resources center, IOP measurements may not be available or accurate, and options for glaucoma surgical procedures may not be ideal for these cases. Although trabeculectomy provided good IOP control in this case, blebitis was an undesirable complication. Bleb leaks are referred to as “a ticking time bomb” that create a high risk of infection and sight-threatening complications.24 Furthermore, in this patient with a history of repeat PKPs, immediate action was imperative to control the IOP, prevent further glaucoma progression, and minimize corneal graft damage.
Abbreviations
BCVA, best-corrected visual acuity; IOP, intraocular pressure; 5-FU, 5 fluorouracil; MMC, mitomycin C; PAS, peripheral anterior synechiae; PKP, penetrating keratoplasty.
Ethics Approval and Informed Consent
Review and approval for the case report study were approved by the internal review ethics board of Jakarta Eye Center. The patient has been informed and given her consent to publish the data.
Funding
There is no funding support for this case report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dada T, Aggarwal A, Minudath K, et al. Post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 2008;56(4):269–277. doi:10.4103/0301-4738.41410
2. Shree N, Gandhi M, Dave A, Mathur U. Incidence and risk factors for post-penetrating keratoplasty glaucoma. Indian J Ophthalmol. 2022;70(4):1239–1245. doi:10.4103/ijo.IJO_1470_21
3. Anders LM, Gatzioufas Z, Grieshaber MC. Challenges in the complex management of post-keratoplasty glaucoma. Ther Adv Ophthalmol. 2021;13:25158414211031396.
4. Wu S, Xu J. Incidence and risk factors for post-penetrating keratoplasty glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(4):e0176261. doi:10.1371/journal.pone.0176261
5. Greenlee EC, Kwon YH. Graft failure: III. Glaucoma escalation after penetrating keratoplasty. Int Ophthalmol. 2008;28(3):191–207. doi:10.1007/s10792-008-9223-5
6. Stechschulte SU, Azar DT. Complications After Penetrating Keratoplasty. Int Ophthalmol Clin. 2000;40(1):27–43. doi:10.1097/00004397-200040010-00005
7. Ishioka M, Shimazaki J, Yamagami J, Fujishima H, Shimmura S, Tsubota K. Trabeculectomy with mitomycin C for post-keratoplasty glaucoma. Br J Ophthalmol. 2000;84(7):714–717. doi:10.1136/bjo.84.7.714
8. Gilvarry AM, Kirkness CM, Steele AD, Rice NS, Ficker LA. The management of post-keratoplasty glaucoma by trabeculectomy. Eye. 1989;3(6):713–718. doi:10.1038/eye.1989.110
9. Ayyala RS. Penetrating keratoplasty and glaucoma. Surv Ophthalmol. 2000;45(2):91–105. doi:10.1016/S0039-6257(00)00141-7
10. Kirkness CM, Steele AD, Ficker LA, Rice NS. Coexistent corneal disease and glaucoma managed by either drainage surgery and subsequent keratoplasty or combined drainage surgery and penetrating keratoplasty. Br J Ophthalmol. 1992;76(3):146–152. doi:10.1136/bjo.76.3.146
11. Lin LT, Chen JT, Lu DW, et al. Antifibrotic role of low-dose mitomycin-c-induced cellular senescence in trabeculectomy models. PLoS One. 2020;15(6):e0234706. doi:10.1371/journal.pone.0234706
12. Figueiredo RS, Araujo SV, Cohen EJ, Rapuano CJ, Katz LJ, Wilson RP. Management of coexisting corneal disease and glaucoma by combined penetrating keratoplasty and trabeculectomy with mitomycin-C. Ophthalmic Surg Lasers. 1996;27(11):903–909. doi:10.3928/1542-8877-19961101-03
13. Ayyala RS, Pieroth L, Vinals AF, et al. Comparison of mitomycin C trabeculectomy, glaucoma drainage device implantation, and laser neodymium: YAGcyclophotocoagulation in the management of intractable glaucoma after penetrating keratoplasty. Ophthalmology. 1998;105(8):1550–1556. doi:10.1016/S0161-6420(98)98046-0
14. Ayyala RS, Bellows AR, Thomas JV, Hutchinson BT. Bleb infections: clinically different courses of “blebitis” and endophthalmitis. Ophthalmic Surg Lasers. 1997;28(6):452–460. doi:10.3928/1542-8877-19970601-04
15. Bindlish R, Condon GP, Schlosser JD, D’Antonio J, Lauer KB, Lehrer R. Efficacy and safety of mitomycin-C in primary trabeculectomy: five-year follow-up. Ophthalmology. 2002;109(7):1336–1341. doi:10.1016/S0161-6420(02)01069-2
16. Belyea DA, Dan JA, Stamper RL, Lieberman MF, Spencer WH. Late onset of sequential multifocal bleb leaks after glaucoma filtration surgery with 5-fluorouracil and mitomycin C. Am J Ophthalmol. 1997;124(1):40–45. doi:10.1016/S0002-9394(14)71642-3
17. Vaziri K, Kishor K, Schwartz SG, et al. Incidence of bleb-associated endophthalmitis in the United States. Clin Ophthalmol Auckl NZ. 2015;9:317–322.
18. Alwitry A, King AJ. Surveillance of late-onset bleb leak, blebitis and bleb-related endophthalmitis — a UK incidence study. Graefes Arch Clin Exp Ophthalmol. 2012;250(8):1231–1236. doi:10.1007/s00417-011-1920-5
19. Almousa R, Nanavaty MA, Daya SM, Lake DB. Intraocular pressure control and corneal graft survival after implantation of Ahmed valve device in high-risk penetrating keratoplasty. Cornea. 2013;32(8):1099–1104. doi:10.1097/ICO.0b013e31828d2a17
20. Kwon YH, Taylor JM, Hong S, et al. Long-term results of eyes with penetrating keratoplasty and glaucoma drainage tube implant. Ophthalmology. 2001;108(2):272–278. doi:10.1016/S0161-6420(00)00496-6
21. Arroyave CP, Scott IU, Fantes FE, Feuer WJ, Murray TG. Corneal graft survival and intraocular pressure control after penetrating keratoplasty and glaucoma drainage device implantation. Ophthalmology. 2001;108(11):1978–1985. doi:10.1016/S0161-6420(01)00803-X
22. Sugar A, Tanner JP, Dontchev M, et al. Recipient risk factors for graft failure in the cornea donor study. Ophthalmology. 2009;116(6):1023–1028. doi:10.1016/j.ophtha.2008.12.050
23. Sidoti PA, Mosny AY, Ritterband DC, Seedor JA. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108(6):1050–1058. doi:10.1016/S0161-6420(01)00583-8
24. American Academy of Ophthalmology. A ticking time bomb: how to fix a leaking bleb. Available from: https://www.aao.org/eyenet/article/how-to-fix-A-leaking-bleb.
25. Delgado MF, Abdelrahman AM, Terahi M, et al. Management of glaucoma in developing countries: challenges and opportunities for improvement. ClinicoEcon Outcomes Res. 2019;11:591–604. doi:10.2147/CEOR.S218277
26. Butt NH, Ayub MH, Ali MH. Challenges in the management of glaucoma in developing countries. Taiwan J Ophthalmol. 2016;6(3):119–122. doi:10.1016/j.tjo.2016.01.004
27. Leite MT, Sakata LM, Medeiros FA. Managing glaucoma in developing countries. Arq Bras Oftalmol. 2011;74:83–84. doi:10.1590/S0004-27492011000200001
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
