Back to Journals » Clinical Interventions in Aging » Volume 11
CHADS2 score has a better predictive value than CHA2DS2-VASc score in elderly patients with atrial fibrillation
Authors Xing Y , Ma Q , Ma X, Wang C, Zhang D , Sun Y
Received 29 January 2016
Accepted for publication 31 March 2016
Published 14 July 2016 Volume 2016:11 Pages 941—946
DOI https://doi.org/10.2147/CIA.S105360
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Yunli Xing, Qing Ma, Xiaoying Ma, Cuiying Wang, Dai Zhang, Ying Sun
Department of Geriatrics and Gerontology, Beijing Friendship Hospital, Capital Medical University, Beijing, People’s Republic of China
Aim: The study aims to compare the ability of CHA2DS2-VASc (defined as congestive heart failure, hypertension, age ≥75 years [two scores], type 2 diabetes mellitus, previous stroke, transient ischemic attack, or thromboembolism [TE] [doubled], vascular disease, age 65–74 years, and sex category) and CHADS2 (defined as congestive heart failure, hypertension, age ≥75 years, type 2 diabetes mellitus, previous stroke [doubled]) scores to predict the risk of ischemic stroke (IS) or TE among patients with nonvalvular atrial fibrillation (NVAF).
Methods: A total of 413 patients with NVAF aged ≥65 years, and not on oral anticoagulants for the previous 6 months, were enrolled in the study. The predictive value of the CHA2DS2-VASc and CHADS2 scores for IS/TE events was evaluated by the Kaplan–Meier method.
Results: During a follow-up period of 1.99±1.29 years, 104 (25.2%) patients died and 59 (14.3%) patients developed IS/TE. The CHADS2 score performed better than the CHA2DS2-VASc score in predicting IS/TE as assessed by c-indexes (0.647 vs 0.615, respectively; P<0.05). Non-CHADS2 risk factors, such as vascular disease and female sex, were not found to be predictive of IS/TE (hazard ratio 1.518, 95% CI: 0.832–2.771; hazard ratio 1.067, 95% CI: 0.599–1.899, respectively). No differences in event rates were found in patients with the CHADS2 scores of 1 and 2 (7.1% vs 7.8%). It was observed that patients with a CHADS2 score of ≥3 were most in need of anticoagulation therapy.
Conclusion: In patients with NVAF aged ≥65 years, the CHADS2 score was found to be significantly better in predicting IS/TE events when compared to the CHA2DS2-VASc score. Patients with a CHADS2 score of ≥3 were associated with high risk of IS/TE events.
Keywords: NVAF, vascular disease, sex, elderly
Background
Atrial fibrillation (AF) is a common cardiac rhythm disorder, which is responsible for substantial morbidity and mortality. The prevalence of nonvalvular atrial fibrillation (NVAF) increases with advancing age and is considered to be an important risk factor for ischemic stroke (IS) and thromboembolism (TE).1 Anticoagulation is the cornerstone for AF management. However, various studies have reported the underuse of oral anticoagulation (OAC) among elderly patients with NVAF,2–4 and the situation is more grim in the People’s Republic of China.5
Both CHADS2 (defined as congestive heart failure, hypertension, age ≥75 years, type 2 diabetes mellitus [DM], previous stroke [doubled]) and CHA2DS2-VASc (defined as congestive heart failure, hypertension, age ≥75 years [two scores], type 2 diabetes mellitus, previous stroke, transient ischemic attack [TIA], or TE [doubled], vascular disease, age 65–74 years, and sex category) scores are well-validated tools for the estimation of stroke risk in patients with AF. CHA2DS2-VASc improves the precision of identifying “low-risk” patients.6 Age is a very important factor of stroke, and it is unclear which score is better suited for use in elderly patients.7 The goal of the present study was to compare the utility of CHA2DS2-VASc and CHADS2 scores in predicting IS/TE for the patients with NVAF aged ≥65 years.
Methods
Ethical approval was obtained from the Hospital Ethical Committee of Beijing Friendship Hospital. A procedure-oriented informed consent form was signed by each patient. A retrospective study was conducted by collecting patient data available at Beijing Friendship Hospital for the period between January 1, 2011, and June 30, 2013. It was possible to retrieve the data pertaining to individual patients as all data at our hospital are linked to a unique, permanent, and personal registration number, which is assigned to every patient. Patients with NVAF aged ≥65 years, and not on OAC for the previous 6 months, were enrolled in the study. Diagnosis of AF was based on electrocardiography (12-lead electrocardiography) or 24-hour Holter monitoring. Patients with valvular AF, rheumatic mitral stenosis, mechanical or bioprosthetic heart valve, and mitral valve repair and those receiving hemodialysis or on OAC were excluded from the study.
The study consisted of baseline and follow-up periods. The date of the qualifying AF diagnosis made between January 1, 2011, and June 30, 2013, was designated as the index date. Data from the baseline period, which ended on the index date, were used to obtain information about each patient’s medical history. Follow-up was performed by going through medical records available in the hospital database. Data from the follow-up period, which started from the day after the index date and ended on March 1, 2015, were used to assess the risk of IS/TE. All patients who were lost to follow-up and those who took OAC during the study period were excluded.
The primary end point was the development of IS or TE events (ie, TIA or peripheral embolism). The secondary end point was all-cause death.
IS was defined as a new, sudden focal neurological deficit resulting from a presumed cerebrovascular cause that persisted >24 hours and was not attributable to other identifiable causes, such as tumor and seizure. Events that involved symptoms that lasted <24 hours were considered as TIA. Brain imaging was sought in each case to distinguish hemorrhagic from IS. Peripheral artery embolism was defined as abrupt vascular insufficiency associated with clinical or radiographic evidence of peripheral arterial occlusion in the absence of other likely causes. Presence of vascular disease was identified from previous diagnoses, including myocardial infarction (MI), peripheral artery disease, and complex aortic plaque.
Data were expressed as mean ± SD. The analyses were performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA), except net reclassification improvement (NRI), which was analyzed using SAS9.2. Mean values and proportions of variables were compared using unpaired Student’s t-test, analysis of variance, and chi-square test. The IS/TE risk was assessed using Cox regression analysis. The cumulative incidence curve of IS/TE was plotted via the Kaplan–Meier method, with statistical significance examined using the log-rank test. We assessed the predictive accuracies of the CHADS2 and CHA2DS2-VASc scores by calculating c-indices on the basis of receiver operating characteristic (ROC) curves and NRI. Areas under the ROC curves for these two scoring systems were compared using DeLong’s test. Statistical significance was defined as a P-value of <0.05.
Results
Characteristics of patients
Baseline characteristics of the study population are listed in Table 1. The mean age of patients was 80.82±7.34 years, with 70.9% being male. The median score of CHA2DS2-VASc and CHADS2 was 4.77 and 2.95, respectively. Hypertension was the most prevalent comorbidity and was noted in 77.5% of patients. A total of 36.8% had a history of previous stroke or TIA. During the follow-up period of 1.99±1.29 years, 104 (25.2%) patients died and 59 (14.3%) patients had an IS/TE event.
On the basis of the CHADS2 score, 1.7%, 10.2%, and 86.3% of patients were classified as low risk (0 point), intermediate risk (1 point), and high risk (2–6 points), respectively.
Comparison between CHADS2 and CHA2DS2-VASc scores
Both the CHADS2 and CHA2DS2-VASc scores were the significant predictors of IS after adjusting for age and sex. Cox regression model improved from 1.286 (95% CI: 1.086–1.523) to 1.438 (95% CI: 1.187–1.743) when the CHADS2 score was used for stroke risk categorization instead of the CHA2DS2-VASc score.
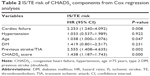
Of the components of CHADS2 and CHA2DS2-VASc scores, cardiac failure and previous stroke/TIA were strongly associated with the primary end point (hazard ratio [HR] 2.253, 95% CI: 1.240–4.092; HR 2.555, 95% CI: 1.408–4.635, respectively). Age was also found to be associated with IS/TE during follow-up. However, hypertension, DM, vascular disease, and female sex were not found to be predictive of IS/TE (Tables 2 and 3). Among patients with the vascular disease, peripheral arterial disease significantly increased the risk of stroke by 2.71-fold. Previous MI was not a significant predictor of IS/TE.
Figure 1 shows the ROC curves of CHADS2 and CHA2DS2-VASc scores in predicting IS/TE. The c-indices on the basis of area under the ROC curves for the CHADS2 and CHA2DS2-VASc scores were 0.647 (95% CI: 0.599–0.693) and 0.615 (95% CI: 0.566–0.662), respectively. The difference was statistically significant in favor of the CHADS2 score (DeLong’s test, P-value =0.0498–0.05, NRI =0.237). The cut-off value of CHADS2 score was 2.5, with a specificity of 0.537 and a sensitivity of 0.780.
CHADS2 score of ≥3 identified a true high-risk cohort
The Kaplan–Meier curve of freedom from IS is shown in Figure 2. Patients with a CHADS2 score of 0 had no stroke event. Patients with a CHADS2 score of 2 had a similar event rate to those with a CHADS2 score of 1 during the follow-up period (7.1% vs 7.8%; P=0.887). Compared with a CHADS2 score of 1, patients with a CHADS2 score of 3, 4, 5, or 6 had a higher event rate (14.4%, 23.1%, 24.4%, and 10%, respectively).
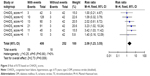
Using a CHADS2 score of 1 as the reference in the Cox regression analysis model, the HRs associated with the CHADS2 scores of 2, 3, 4, 5, and 6 were 1.09, 2.02, 3.32, 3.42, and 1.40, respectively (Figure 3). The event rates with the CHADS2 scores of 1, 2, 3, 4, 5, and 6 were 7.14%, 7.81%, 14.44%, 23.08%, 20%, and 10%, respectively. These findings indicated that a CHADS2 score of 2 had a similar event rate to a CHADS2 score of 1, and CHADS2 score ≥3 identified a cohort with a true high risk. The HR of the group with a CHADS2 score of 6 was 1.40, perhaps because of its small size.
Discussion
CHA2DS2-VASc is reported to be better than the CHADS2 score in identifying the true low-risk patients.8–12 However, for the regions and population where OAC is frequently underused, it is more important to identify the true high-risk patients. The underuse of OAC among elderly patients with NVAF has been confirmed in different settings.2–4 One of the most important reasons is that the treating physicians are not sure about which scoring system to follow to determine which patient requires the OAC the most. Therefore, it is necessary to compare the predictive value of two scores, CHA2DS2-VASc and CHADS2, in predicting IS among patients diagnosed with NVAF aged ≥65 years, and to find patients with a true high risk.
In the present study, we only included patients aged 65 years or older. This implies that every patient was added at least 1 point by the CHA2DS2-VASc system, and that both CHADS2 and CHA2DS2-VASc scores were useful parameters for predicting adverse events in patients with NVAF aged ≥65 years. However, the CHADS2 score was found to be more appropriate for patients aged ≥65 years for the categorization of stroke risk when compared with the CHA2DS2-VASc score. A CHADS2 score of ≥3 identified patients with a true high risk. Consistent with the findings of our study, Friberg et al13 found that the risk of IS in patients with a CHA2DS2-VASc score of 1 seemed to be lower than previously reported (0.1%–0.7%).
In the present study, cardiac failure, age, and history of previous stroke were found to be the independent predictors of IS/TE. Although vascular disease and female sex were not associated with IS/TE risk, both are the additional “non-CHADS2” risk factors that are incorporated into the CHA2DS2-VASc score as per 2012 European Society of Cardiology guidelines.14
Several studies have been conducted to assess the impact of atherosclerotic vascular disease on stroke in patients with AF. Peripheral arterial disease significantly increased the risk of stroke in all observational studies with the reported risk ranging from 1.3-fold to 2.5-fold.15,16 Complex aortic plaque in the descending aorta has also been reported as a significant risk factor.17–19 However, there is no conclusive evidence that previous MI is a predictor of IS.20 In our study, vascular disease included previous MI and peripheral arterial disease. We found peripheral arterial disease to significantly increase the risk of stroke by 2.71-fold. Previous MI was not a significant predictor of IS/TE, which is consistent with the findings of Lin et al.21
Though female sex is another “non-CHADS2” risk factor and has been reported to be associated with IS/TE in patients with AF,22,23 the said association is considered as controversial. Various studies have reported that female sex is associated with an increased risk of stroke in only those patients with AF aged ≥75 years, whereas female patients aged <65 years without other risk factors do not require anticoagulation therapy.24,25 Moreover, most of the clinical trials supporting female sex as a risk factor are from the western countries. However, studies conducted in the eastern countries have not reported similar results.26 It has been reported that female sex increases the risk for their comorbidities, such as heart and renal failures.22 In our study, which enrolled patients aged ≥65 years, there was no significant difference in the rate of hypertension, previous stroke/TIA, DM, and CHF in females when compared with males (73.4% vs 75.5%, 39.1% vs 37.7%, 37.5% vs 34.3%, and 23% vs 22.5%, respectively). In addition, the rate of IS/TE in females was not found to be significantly different from males (13.4% vs 14.6%; P>0.05). Therefore, in-line with other studies, the findings of our study indicate that female sex need not be considered when deciding on the antithrombotic therapy.27
In our study, the cut-off value for a very high risk of stroke when using the CHADS2 score was 3, which was determined by ROC curve analysis. In fact, the event rates during the follow-up period among patients with the CHADS2 scores of 1 and 2 were almost the same (7%), thus indicating intermediate risk in the CHADS2 score of 1. Both the CHADS2 scores of 1 and 2 need OAC.
It is important to note the limitations of our study. Being a retrospective analysis, follow-up was performed by assessing medical records available in the hospital database only, hence some clinically relevant events may have been missed. The study had a limited number of patients, especially in the group of CHADS2 scores 0 and 9. The HR associated with a CHADS2 score of 6 (relative risk [RR] =1.4 [95% CI {0.16–12.09}]) is considerably lower than that with a CHADS2 score of RR =5 (3.42, 95% CI [1.03–12.42]). for the size of sample. We will enlarge the sample in the future.
Conclusion
For patients with NVAF aged 65 years or older, both vascular disease and female sex were not the predictors of IS/TE risk. The use of the CHADS2 score significantly improves the classification of patients with AF at high risk of stroke compared with the CHA2DS2-VASc score. Thus, future large-scale studies involving multiple centers are needed to further corroborate our findings.
Acknowledgments
This study was supported by Beijing Health Care Research Fund (J 09-04), Basic and Clinical Research Cooperation Fund, and Capital Medical University (13JL57).
Disclosure
The authors report no conflicts of interest in this work.
References
Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27(10):1760–1764. | ||
Marcucci M, Iorio A, Nobili A, et al. Factors affecting adherence to guidelines for antithrombotic therapy in elderly patients with atrial fibrillation admitted to internal medicine wards. Eur J Intern Med. 2010;21(6):516–523. | ||
Tulner LR, Van Campen JP, Kuper IM, et al. Reasons for undertreatment with oral anticoagulants in frail geriatric outpatients with atrial fibrillation: a prospective, descriptive study. Drugs Aging. 2010;27(1):39–50. | ||
Scowcroft AC, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: an analysis using the General Practice Research Database (GPRD) 2000–2009. Heart. 2013;99(2):127–132. | ||
Zhou Z, Hu D. An epidemiological study on the prevalence of atrial fibrillation in the Chinese population of mainland China. J Epidemiol. 2008;18(5):209–216. | ||
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. | ||
Zhu WG, Xiong QM, Hong K. Meta-analysis of CHADS2 versus CHA2DS2-VASc for predicting stroke and thromboembolism in atrial fibrillation patients independent of anticoagulation. Tex Heart Inst J. 2015;42(1):6–15. | ||
Olesen JB, Lip GY, Hansen ML, et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. BMJ. 2011;342:d124. | ||
Potpara TS, Polovina MM, Licina MM, Marinkovic JM, Prostran MS, Lip GY. Reliable identification of “truly low” thromboembolic risk in patients initially diagnosed with “lone” atrial fibrillation: the Belgrade atrial fibrillation study. Circ Arrhythm Electrophysiol. 2012;5(2):319–326. | ||
Chao TF, Liu CJ, Chen SJ, et al. Atrial fibrillation and the risk of ischemic stroke: does it still matter in patients with a CHA2DS2-VASc score of 0 or 1? Stroke. 2012;43(10):2551–2555. | ||
Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731–2738. | ||
Chao TF, Lin YJ, Tsao HM, et al. CHADS(2) and CHA(2)DS(2)-VASc scores in the prediction of clinical outcomes in patients with atrial fibrillation after catheter ablation. J Am Coll Cardiol. 2011;58(23):2380–2385. | ||
Friberg L, Skeppholm M, Terent A. Benefit of anticoagulation unlikely in patients with atrial fibrillation and a CHA2DS2-VASc score of 1. J Am Coll Cardiol. 2015;65(3):225–232. | ||
Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC guidelines for the management of atrial fibrillation: an update of the 2010 ESC guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. | ||
Goto S, Bhatt DL, Rother J, et al. Prevalence, clinical profile, and cardiovascular outcomes of atrial fibrillation patients with atherothrombosis. Am Heart J. 2008;156(5):855–863,863.e2. | ||
Conway DS, Lip GY. Comparison of outcomes of patients with symptomatic peripheral artery disease with and without atrial fibrillation (the West Birmingham Atrial Fibrillation Project). Am J Cardiol. 2004;93(11):1422–1425, A10. | ||
Blackshear JL, Pearce LA, Hart RG, et al. Aortic plaque in atrial fibrillation: prevalence, predictors, and thromboembolic implications. Stroke. 1999;30(4):834–840. | ||
Manning WJ, Douglas PS. Transesophageal echocardiography and atrial fibrillation: added value or expensive toy? Ann Intern Med. 1998;128(8):685–687. | ||
Zabalgoitia M, Halperin JL, Pearce LA, Blackshear JL, Asinger RW, Hart RG. Transesophageal echocardiographic correlates of clinical risk of thromboembolism in nonvalvular atrial fibrillation. Stroke Prevention in Atrial Fibrillation III Investigators. J Am Coll Cardiol. 1998;31(7):1622–1626. | ||
Anandasundaram B, Lane DA, Apostolakis S, Lip GY. The impact of atherosclerotic vascular disease in predicting a stroke, thromboembolism and mortality in atrial fibrillation patients: a systematic review. J Thromb Haemost. 2013;11(5):975–987. | ||
Lin LY, Lee CH, Yu CC, et al. Risk factors and incidence of ischemic stroke in Taiwanese with nonvalvular atrial fibrillation – a nation wide database analysis. Atherosclerosis. 2011;217(1):292–295. | ||
Dagres N, Nieuwlaat R, Vardas PE, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro Heart Survey on atrial fibrillation. J Am Coll Cardiol. 2007;49(5):572–577. | ||
Fang MC, Singer DE, Chang Y, et al. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the AnTicoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2005;112(12):1687–1691. | ||
Mikkelsen AP, Lindhardsen J, Lip GY, Gislason GH, Torp-Pedersen C, Olesen JB. Female sex as a risk factor for stroke in atrial fibrillation: a nationwide cohort study. J Thromb Haemost. 2012;10(9):1745–1751. | ||
Wagstaff AJ, Overvad TF, Lip GY, Lane DA. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM. 2014;107(12):955–967. | ||
Tomita H, Okumura K, Inoue H, et al. Validation of risk scoring system excluding female sex from CHA2DS2-VASc in Japanese patients with nonvalvular atrial fibrillation-subanalysis of the J-RHYTHM registry. Circ J. 2015;79(8):1719–1726. | ||
Verma A, Cairns JA, Mitchell LB, et al. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30(10):1114–1130. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.