Back to Journals » International Journal of Women's Health » Volume 14
Cervical Cancer in a Septate Uterus with Double Cervix and Double Vagina: A Case Report and Review of the Literature
Authors Gong Y , Xie Y , Chen L, Sui L
Received 23 November 2021
Accepted for publication 28 February 2022
Published 8 March 2022 Volume 2022:14 Pages 345—351
DOI https://doi.org/10.2147/IJWH.S350768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Yingxin Gong,1,* Yu Xie,1,* Limei Chen,1,2 Long Sui1,2
1Cervical Diseases Center, Obstetrics and Gynecology Hospital of Fudan University, Shanghai, People’s Republic of China; 2Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Long Sui; Limei Chen, Obstetrics and Gynecology Hospital of Fudan University, 419 Fangxie Road, Shanghai, 200011, People’s Republic of China, Tel +86 021-33189900, Email [email protected]; [email protected]
Abstract: Septate uterus with duplicate cervices and double vagina is a rare Müllerian duct anomaly mostly found in labor or gynecological examination. We present a case of a 40-year-old asymptomatic parous patient diagnosed with double cervix and complete vaginal septum. She was admitted to hospital due to abnormal histopathology of suspicious cervical squamous papillary carcinoma post-salpingectomy. Her genital malformation was seriously addressed due to the cervical lesion. The diagnosis of cervical cancer in the left cervix and LSIL in the right cervix was made after LEEP conization. She received laparoscopic hysterectomy with salpingectomy and partial vagina wall resection for radical resection of the lesion. We report this case to present irregular findings during colposcopy, hysterectomy, and histopathology.
Keywords: cervical cancer, duplicate cervix, double vagina, Müllerian duct anomaly, septate uterus
Introduction
Müllerian anomalies, which often refer to congenital malformations of the female reproductive tract, have a prevalence of about 5%.1 According to the 2013 ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies, the uterine abnormalities have six categories, including dysmorphic uterus, septate uterus, dysfused uterus, unilaterally formed-uterus, aplastic uterus, and unclassified malformations.2 Septate uterus is caused by resorption defect of Müllerian ducts, leading to a persistent complete or partial longitudinal uterine cavity septum. Women with Müllerian abnormalities can present with miscarriage, infertility and recurrent pelvic pain.3 In rare cases, a complete uterocervicovaginal septum was found. A retrospective study reported that 5.9% of double-chambered uterus cases were complete septate uterus with cervicovaginal septum.4 The anomaly is almost asymptomatic and only be found during other gynecological examinations or delivery.
We report a case of a cervical cancer patient with a complete uterine septum and longitudinal vaginal septum, as well as duplicate cervices, double cervical canal, and double vagina. The anomaly diagnosis was confirmed by gynecological examination, ultrasound imaging, magnetic resonance imaging (MRI), and laparoscope. The patient was treated with radical hysterectomy to eliminate the cervical neoplasm and followed up at regular intervals.
Case Report
A 40-year-old asymptomatic woman diagnosed with cervical squamous cancer was found with a complete septate uterus with double cervix and vagina. The patient came to our clinical center a week after a cervical lesion was identified during post-operative follow-up visit after right salpingectomy for an ectopic pregnancy. She had no symptoms, no abnormal vaginal bleeding or discharging. The patient had normal, regular menses with menstrual cycle of 28 days and menstruation of 3–4 days. She had moderate menstruation volume and no dysmenorrhea. The woman had one full-term labor with spontaneous vertex delivery and three abortions (two induced abortions and one rupture of tubal pregnancy). She had no family history of tumor or genital malformation.
The time of first discovery of her genital malformation was uncertain, but it was seriously addressed due to the cervical lesion. Two external cervical Ostia were visible in gynecological examination, transvaginal sonography showed “Y-shaped” endometrium converged near inner cervical ostium and cervical canals length were measured 32 mm. An round shaped intrauterine device (IUD) was detected in the right uterine cavity. The uterus measured 41mm in length, 59mm in transverse diameter, and 40 mm in anteroposterior diameter, which was relatively shorter and wider compared to normal uterus. Since MRI was planned to further visualize and classify the malformation, the patient had the IUD removed under ultrasound guidance. During the procedure, the left side cervix presented an immature appearance which was smaller than expected of a normal cervix, and the IUD was removed intact.
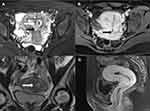
MRI showed a 5.0 cm×6.2 cm×4.8 cm uterus with flat fundus and “Y-shaped” cavity, the thickness of the right and the left endometrium were 1.7 cm and 1.5 cm with an asymmetric signal in the endometrium and cervical canal. A septum can be seen in the midline of the uterus which extended to the external cervical ostium, the distance between the endometrium of bilateral cornua uteri was 3.6 cm and its distance to the uterine fundal serosal layer was 1.0 cm. Two cervical canals and a partial septum image in the vagina can be seen. And there was no lymphovascular space invasion identified. No abnormality in the size and shape of both kidneys was observed in the MRI (Figure 1).
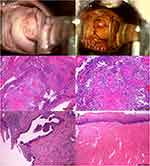
The patient received a “three-step” screening procedure for cervical cancer to clarify the condition of cervical lesions. Cytology results of Liquid-based cell testing (LCT) in both cervices were both high-grade squamous intraepithelial lesion (HSIL). Human papillomavirus (HPV) testing of both cervical smears was HPV16 positive. Colposcopy showed a double vagina, duplicate cervices, and HSIL in both cervices. Colposcopy-directed punch biopsy (CDB) and endocervical curettage (ECC) histopathology showed: 1. (LEFT ECC) cervical canal epithelial tissue; 2. (LEFT CDB) exogenous squamous papillary carcinoma located at 1–2 o’clock at the left cervix, and insufficient tissue determining invasion; 3. (RIGHT ECC) low-grade squamous intraepithelial lesion (LSIL); 4. (RIGHT CDB) LSIL in the right upper lip of the cervix (Figure 2A and B).
To define the degree and invasive extent of the lesion, the patient then received Loop Electrosurgical Excision Procedure (LEEP) conization of the left cervix. The resection began at 3 o’clock of the left cervix, part of the cervical tissue (base width radius 0.8 cm, depth 1.8 cm) was cut off, and electrocoagulation was used for hemostasis. The histopathological result showed focal invasive squamous cell carcinoma (invasive depth 2.8 mm) with a negative margin (Figure 2C and D). The diagnosis of cervical cancer stage IA1 (FIGO 2018) and stage TIA1N0M0 (AJCC 2017) in the left cervix was made.
As the patient had no fertility demands, Querleu-Morrow type A hysterectomy with salpingectomy (left) and partial vagina wall resection under laparoscopy was performed. Laparoscopy showed normal kidneys and an anteversion uterus with a size close to 4cm×5cm×4cm and regular contour. After laparoscopic examination, coagulated resection was performed along the left mesosalpinx to the left proximal uterine horn. The ovarian ligament, round ligament and part of broad ligament were clamped to the level of uterine isthmus. The bladder peritoneum was opened, both bladderpods were cut by coagulation, and the bladder was pushed down to the anterior fornix of the vagina. The left uterine arteries and veins, parametrium and the uterosacral ligament were cut by coagulation. The anterior wall of the vagina was lifted, then the uterus, cervix, and 2 cm of the vaginal wall below the external cervix were resected along the vaginal fornix. Absorbable suture was used to continuously suture the vaginal stump. Duplicate cervices and a complete vagina septum were visible after resection. The uterus specimen was 5.0cm×6.2cm×4.8cm in size with a septum extending from the uterine fundus to the cervix. The right uterine cavity measured 7 cm deep with 0.3 cm-thick endometrium and 1.5 cm-thick posterior muscular layer. The left uterine cavity was 7.5 cm deep with 0.2 cm-thick endometrium and 1.5 cm-thick posterior muscular layer.
The intraoperative diagnosis was consistent with the preoperative diagnosis and confirmed the diagnosis of the complete septate uterus as well as vagina septum. Microscopic histopathology results showed necrotic and reparative changes of the left cervix after LEEP, and no residual lesions were observed. Chronic inflammation in the right cervix was shown, the excisional margin of the vaginal wall was negative. The cervical squamous column junction was separated by the connective tissue septum, which was completely normal (Figure 2E and F). Focal adenomyosis was identified in the right myometrium while no lesion in left myometrium.
The patient’s intraoperative bleeding was 150mL and the postoperative course was uneventful. The postoperative follow-up 6 month later presented normal cytology and colposcopy result with negative HPV testing. Institutional review board/ethics committee approval of Obstetrics and Gynecology Hospital of Fudan University was obtained for this study and the patient gave her informed consent for publication of this case report.
Discussion
Congenital genital malformations in women can cause infertility as well as adverse effects on pregnancy especially for women of childbearing age. The incomplete fusion of the paired Müllerian ducts and resorption of the septum is the etiology of the uterus malformation, although the region of fusion and the direction is not well understood.5 The anomaly of the longitudinal vaginal septum, double cervix, and complete septate uterus revealed the fusion of the Müllerian duct begins in the isthmic region and moves in both caudal and cranial direction.6 This anomaly is not included in the classification of female genital anomaly, but with extended septum to the vagina comparing to complete uterus septum which extended to the outer ostium.
The vaginal septum with complete cervical-uterine septum can be asymptomatic, dyspareunia in nulligravida, abortion or preterm delivery are the main reasons for patients to seek medical advice.7 Through the literature review, we found several similar cases reported worldwide.5,6,8–11 The first case of the double uterus and double vagina was reported in 1900, Tindal et al described the detailed examination of the patient’s anomaly as septate uterus with a perforated fleshy septum to the left of the vaginal middle line and menstruation came from the right cervical canal.8 Most patients were in their twenties with symptoms as dysmenorrhea, metrorrhagia, dyspareunia, and infertility, but some of their symptoms were associated with occlusion of the menstruation. Those patients were mostly diagnosed by transvaginal 3D ultrasonography, MRI and hysteroscopy, which showed a longitudinal septum extending through the uterus, cervix, and the proximal vagina with cervical and vaginal duplication. The management of the abnormality varied greatly and required a forum to regulate and facilitate the normalization.12
Malignant lesions occurring in Müllerian anomaly create a challenge for gynecologists to manage. Cervical neoplasms occurring in patients with double uterus have been reported previously (Table 1). Most patients have symptoms of abnormal vaginal bleeding or abnormal Pap smear, and the lesion can reside in one or both cervices with different origins. Whether the malformation of duplicate cervices increases the incidence of cervical neoplasm or not is unknown as its incidence is rare. In general, the staging principle of cervical cancer in patients with double cervix is similar to normal patients except the lesion in both cervices should be described and staged separately as the lesion of each cervix may in different stage.14,19,20 Though lesions of different stages occurred in each cervix, most previously reported cases adopted radical hysterectomy as the therapy of cervical cancer and resection of the vaginal septum was optional.14,16 Only one patient with lesion of different stage on each cervix did not receive hysterectomy as it would have required vaginectomy, so bilateral cervical loop excisions were performed, and radical radiotherapy was applied to both uteri in case of cancerous progress.19 In some cases, conization can be technically difficult as the aberrant anatomical structure, and the fertility-sparing procedure in early-stage patients with fertility demands has not been reported previously.23
 |
Table 1 Summary of Previous Publication of Double Uterus with Cervical Neoplasm |
In our case, we performed hysterectomy with salpingectomy and partial vagina wall resection to fully eliminate the cervical lesion as the patient had no fertility demand. However, the cervical cancer of StageIA1 can be managed with simple hysterectomy or conization if wish for future fertility, even with double cervices. As the patient was staged TIA1N0M0, pelvic lymph node resection and sentinel lymph node mapping were unnecessary. Cervical cancer occurred in patients with duplicate cervices is rare, here we presented a novel case of cervical cancer in one of the cervical canals in a woman with a complete uterocervicovaginal septum. However, there are some limitations. The patients did not receive genetic testing thus whether genetic factor lead to the malformation and the cervical lesion was not determined. Besides, the patient’s previous delivery was not in our hospital, the detailed circumstance was unknown. The present case was not sufficient to conclude the management of cervical lesion on both cervices, and more evidence was required.
Conclusion
Cervical cancer occurred with the uterine anomaly was seldom reported with discrepant treatment. To explore the optimal therapy, especially for those with fertility demands, additional information from other case reports are needed. More evidence was required before recommendations of management of cervical cancer with uterine anomaly.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81701398) and the Science and Technology Commission of Shanghai Municipality (No. 18411963600). The authors thank the Shanghai Key Laboratory of Female Reproductive Endocrine Related Diseases.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Chan YY, Jayaprakasan K, Zamora J, et al. The prevalence of congenital uterine anomalies in unselected and high-risk populations: a systematic review. Hum Reprod Update. 2011;17(6):761–771.
2. Grimbizis GF, Gordts S, Di Spiezio Sardo A, et al. The ESHRE/ESGE consensus on the classification of female genital tract congenital anomalies. Hum Reprod. 2013;28(8):2032–2044.
3. El Saman AM, Shahin AY, Nasr A, et al. Hybrid septate uterus, coexistence of bicornuate and septate varieties: a genuine report. J Obstet Gynaecol Res. 2012;38(11):1308–1314.
4. Haddad B, Louis-Sylvestre C, Poitout P, et al. Longitudinal vaginal septum: a retrospective study of 202 cases. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):197–199.
5. Chang AS, Siegel CL, Moley KH, et al. Septate uterus with cervical duplication and longitudinal vaginal septum: a report of five new cases. Fertil Steril. 2004;81(4):1133–1136.
6. Hundley AF, Fielding JR, Hoyte L. Double cervix and vagina with septate uterus: an uncommon müllerian malformation. Obstet Gynecol. 2001;98(5 Pt 2):982–985.
7. Patton PE, Novy MJ, Lee DM, et al. The diagnosis and reproductive outcome after surgical treatment of the complete septate uterus, duplicated cervix and vaginal septum. Am J Obstet Gynecol. 2004;190(6):1669–1675.
8. Tindal D. Notes on a Case of Double Uterus (Uterus Septus) and Double Vagina. Glasgow Med J. 1900;54(3):180–183.
9. Pavone ME, King JA, Vlahos N. Septate uterus with cervical duplication and a longitudinal vaginal septum: a müllerian anomaly without a classification. Fertil Steril. 2006;85(2):
10. Wai CY, Zekam N, Sanz LE. Septate uterus with double cervix and longitudinal vaginal septum. A case report. J Reprod Med. 2001;46(6):613–617.
11. PDC M-B, Okoye EI. Uterus Septus Subtotalis Bicollis: a Rare Mullerian Duct Abnormality. Ann Clin Lab Sci. 2018;48(1):116–119.
12. Grunfeld L, Klein J, Steren C. The management of uterus septus. Fertil Steril. 2004;82(3):766.
13. Wall RL. Didelphic uterus with carcinoma in situ of both cervices. Am J Obstet Gynecol. 1958;76(4):803–806.
14. Corbett PJ, Crompton AC. Invasive carcinoma of one cervix in a uterus didelphys. Case report. Br J Obstet Gynaecol. 1982;89(2):171–172.
15. Fox S, Mones JM, Kronstadt R, et al. Bilateral and synchronous squamous cell carcinoma of the cervix in a patient with uterus didelphys. Obstet Gynecol. 1986;67(3 Suppl):76–79.
16. Tam G, Rogers M, Arnold M. Invasive carcinoma of both cervices in a patient with uterus didelphys. Aust N Z J Obstet Gynaecol. 1988;28(3):239–240.
17. Sugimori H, Hachisuga T, Nakamura S, et al. Cervical cancers in uterus didelphys. Gynecol Oncol. 1990;36(3):439–443.
18. Bakri Y, Salem H, Sadi AR, et al. Bilateral and synchronous cervical carcinoma in situ in a didelphic uterus. Int J Gynaecol Obstet. 1992;37(4):289–291.
19. Lee CD, Churn M, Haddad N, et al. Bilateral radical radiotherapy in a patient with uterus didelphys. Br J Radiol. 2000;73(869):553–556.
20. Kimball KJ, Rocconi RP, Straughn JM, et al. Unilateral cervical cancer in a patient with cervix duplex. Gynecol Oncol. 2006;103(1):346–348.
21. Loo HW, Locks SM. Squamous cell carcinoma of the cervix: report of an unusual case of bicornuate bicollis uterus treated with bilateral intracavity brachytherapy. Br J Radiol. 2010;83(991):143–146.
22. Guo LQ, Cheng AL, Bhayana D. Case of the month #169: septate uterus with cervical duplication and vaginal septum. Can Assoc Radiol J. 2011;62(3):226–228.
23. Sparic R, Dotlic J, Kovac J, et al. Management of cervical dysplasia in patient with Müllerian anomaly: diagnostic and therapeutic challenges. Eur J Gynaecol Oncol. 2017;38(3):469–472.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.