Back to Journals » International Journal of Nanomedicine » Volume 12
Cerium oxide nanoparticles: green synthesis and biological applications
Authors Charbgoo F, Ahmad MB, Darroudi M
Received 17 October 2016
Accepted for publication 12 November 2016
Published 20 February 2017 Volume 2017:12 Pages 1401—1413
DOI https://doi.org/10.2147/IJN.S124855
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Thomas Webster
Fahimeh Charbgoo,1 Mansor Bin Ahmad,2,* Majid Darroudi3,*
1Department of Pharmaceutical Biotechnology, School of Pharmacy, Mashhad University of Medical Sciences, Mashhad, Iran; 2Department of Chemistry, Faculty of Science, Universiti Putra Malaysia, Serdang, Selangor, Malaysia; 3Nuclear Medicine Research Center, Mashhad University of Medical Sciences, Mashhad, Iran
*These authors contributed equally to this work
Abstract: CeO2 nanoparticles (NPs) have shown promising approaches as therapeutic agents in biology and medical sciences. The physicochemical properties of CeO2-NPs, such as size, agglomeration status in liquid, and surface charge, play important roles in the ultimate interactions of the NP with target cells. Recently, CeO2-NPs have been synthesized through several bio-directed methods applying natural and organic matrices as stabilizing agents in order to prepare biocompatible CeO2-NPs, thereby solving the challenges regarding safety, and providing the appropriate situation for their effective use in biomedicine. This review discusses the different green strategies for CeO2-NPs synthesis, their advantages and challenges that are to be overcome. In addition, this review focuses on recent progress in the potential application of CeO2-NPs in biological and medical fields. Exploiting biocompatible CeO2-NPs may improve outcomes profoundly with the promise of effective neurodegenerative therapy and multiple applications in nanobiotechnology.
Keywords: cerium oxide nanoparticles, green synthesis, biocompatibility, surface Ce3+, size, morphology
Introduction
CeO2 nanoparticles (NPs) have received much attention in nanotechnology due to their useful applications as catalysts, fuel cells and antioxidants in biological systems.1–5 In general, cerium can exist in two oxidation states: Ce3+ and Ce4+. Therefore, cerium dioxide can have two different oxide forms, CeO2 (Ce4+) or Ce2O3 (Ce3+), in bulk material.4,6 On the nanoscale, the cerium oxide lattice has a cubic fluorite structure, and both Ce3+ and Ce4+ can coexist on its surface. Charge deficiency due to the presence of Ce3+ is compensated by oxygen vacancy in the lattice; thus, CeO2-NPs contain intrinsic oxygen defects.7 These oxygen defects are actually sites of catalytic reactions. The concentration of oxygen defects increases with reduction in particle size.8 Therefore, CeO2-NPs have improved redox properties with respect to the bulk materials. Moreover, the presence of a mixed valance state plays an important role in scavenging reactive oxygen and nitrogen species. CeO2-NPs are found to be effective against pathologies associated with chronic oxidative stress and inflammation. Recently, CeO2-NPs have also been reported to have multienzyme, including superoxide oxidase, catalase and oxidase, and mimetic properties, and have emerged as a fascinating material in biological fields, such as in bioanalysis,9–14 biomedicine15 and drug delivery.16,17 These applications are derived from quick transition of the oxidation state between Ce3+ and Ce4+.6 The surface Ce3+:Ce4+ ratio is influenced by the microenvironment. Therefore, the microenvironment and synthesis method adopted also plays an important role in determining the biological activity and toxicity of CeO2-NPs. The CeO2-NPs have been prepared through the means of several routes and synthesis methods including solution precipitation,18 sonochemical,19 hydrothermal,20 solvothermal,21 ball milling,22 thermal decomposition,23 spray pyrolysis,24 thermal hydrolysis25 and sol–gel methods.26–28 However, applying the mentioned methods deals with several drawbacks, such as toxic solvents and reagents usage, high temperature and pressure, and the requirement of external additives as stabilizing or capping agents during the reaction. As the physiochemical properties of NPs mostly depend on the synthesis procedure, the synthesis method of NPs for biological applications is very important. The physical properties (size, surface charge, agglomeration status in liquid and coating or residual contamination of the surfactant on the surface) of NPs mainly influence interactions at the nano–bio interface.29 Moreover, the surface Ce3+:Ce4+ ratio (chemical property) also influences the biocatalysis and the biological interactions. Manipulation of the surface Ce3+:Ce4+ ratio can be achieved by controlling their synthesis method.30 However, coating the NPs with biocompatible/organic polymers increases dispersion/stability, decreases nonspecific interactions with cells and proteins, increases blood circulation time and reduces the toxicity of the NPs.31
Biomaterials possess functional groups such as –COOH, –OH and –NH2, and have the potential to stabilize and/or cap metal ions for preparation of various NPs via green chemistry methods. Recently, CeO2-NPs have been synthesized through several bio-directed methods applying natural and organic matrices as stabilizing agents in order to prepare biocompatible CeO2-NPs and solve the challenges to safely and effectively use this metal oxide for biomedicinal purposes.27,28,32 In the first part of the review, we discuss the literature on different green synthesis methods of CeO2-NPs (Table 1). Next, we discuss the effect of these CeO2-NPs on reducing their cytotoxicity in the biological environment. Finally, a brief review on the updates of the potential biological application of CeO2-NPs is presented.
  | Table 1 Green synthesis methods of CeO2-NPs |
Green approaches for CeO2-NP synthesis
Plant-mediated synthesis of CeO2-NPs
Phytosynthesis of metal and metal oxide NPs is a new emerging issue in nanoscience and technology.33 Recently, phytosynthesis of CeO2-NPs was reported using different plants, such as Gloriosa superba, Acalypha indica and even Aloe vera plant leaf extract (Figure 1).33–35 The plant extracts acted as stabilizing and capping agents in the CeO2-NPs synthesis process. Investigating biological effects of the phytosynthesized NPs, antibacterial activity of them was examined. The results showed that smaller crystal sizes with a higher surface area led to higher antibacterial activity. These reports applied bio-directed methods of CeO2-NP synthesis. However, the synthesized nanoparticles were generally so large in size that, according to literature, they were not appropriate for biomedical applications.1,36 Recently, biosynthesis of NPs using yeast and fungi has also been noted. Munusamy et al had explained rapid and extracellular synthesis of cerium oxide NPs using fungus Curvularia lunata culture media.37 The synthesized NPs had a cubic structure and exhibited antibacterial effects against different kinds of bacteria.37 It is known that CeO2-NPs cannot enter bacterial and algal cells. Noninternalized CeO2-NPs seem to show toxic effects by direct attachment of CeO2-NPs to cell walls of algae and bacteria.38–41 Several mechanisms have been suggested to demonstrate how CeO2-NPs in contact with the membrane may exert cytotoxicity. CeO2-NPs could interfere with the nutrient transport functions of the membrane,39 cause mechanical damage and membrane disruption42,43 or generate reactive oxygen species (ROS) and induce oxidative stress.38–40 The generation of ROS, most probably hydrogen peroxide, by CeO2-NPs is in agreement with observations noted by Xia et al44 and Zhao et al.45 Hydrogen peroxide is capable of freely diffusing across cell walls and membranes, inducing cell damage.
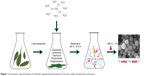  | Figure 1 Schematic representation of Gloriosa superba-based method of cerium oxide nanoparticle synthesis. |
Consequently, myco-synthesis of CeO2-NPs showed advantages including manageability, cost-effectiveness, and used techniques that were less time-consuming and required less energy,46 and therefore can be used as an economic and valuable alternative for the large-scale production of CeO2-NPs. Moreover, myco-synthesized CeO2-NPs had more stability, water dispersibility and high fluorescent properties. The fungal extracellular compounds, such as proteins (especially enzymes), and heterocyclic derivatives could act as reducing and capping agents. Other methods of plant-based CeO2-NPs synthesis were also easy, rapid and cost-effective, but the size of obtained NPs exhibited a wide distribution range, which demonstrates that the necessity of optimizing the biosynthesis methods mentioned earlier in order for application in biological systems.
Nutrient-mediated synthesis of CeO2-NPs
As mentioned, synthetic methods determine the size, charge, surface properties, solubility and morphology of NPs, therefore affecting response of CeO2-NPs in biological systems. That is why green synthesis of CeO2-NPs has received much attention recently. Several studies widely reported different nutrients and natural materials, such as egg white (EW) protein and honey for CeO2-NPs green synthesis.47,48 Kargar et al47 proposed that the two major proteins of EW, ovalbumin and lysozyme, acted as a green binders/stabilizing agents for the preparation of CeO2-NPs. The general mechanism for synthesizing CeO2-NPs in EW media includes formation of the electrostatic interaction between cerium cations (Ce3+) and oppositely charged proteins which leads to controllable growth and subsequent isotropic formation of small and stable CeO2-NPs.47,49 Some of the green methods of CeO2-NP preparation mimic the common traditional approaches in NP synthesis in a safe and eco-friendly way.48 For example, honey-based synthesis of CeO2-NPs mimics the sol–gel method. The extensive number of carbohydrates, enzymes and vitamins containing hydroxyl and amine groups in the honey matrix structure can facilitate the complexation of cerium cations (Ce3+) to an initial molecular matrix. Therefore, honey was capable of coating and stabilizing cerium species and CeO2-NPs while inhibiting their excessive aggregation or crystal growth.48 However, advancement of the EW-based method for CeO2-NP green synthesis is obvious due to nontoxic effects of CeO2-NPs at concentrations up to 800 μg/mL, compared with the safe concentration of ~25 μg/mL for honey-based CeO2-NPs. Therefore, the synthesis of CeO2-NPs in EW was found to be an excellent alternative for the preparation of CeO2-NPs, using food and bio-derived materials.
Biopolymer-mediated synthesis of CeO2-NPs
Natural polymers in the form of macromolecules can also be used as templates for bio-directed synthesis of CeO2-NPs. As the surface of the NPs could be covered by hydroxyl groups, biopolymers that intrinsically possess hydroxyl moieties are capable of stabilizing CeO2-NPs. Applying the polymers as capping/stabilizing agents, the diameter of NPs can be logically controlled.50 Kargar et al reported the green synthesis of small cerium oxide NPs, stabilized with agarose polymers via a sol–gel method.51 While heating to >90°C, the agarose powder is normally dissolved in water, and when the temperature is reduced to 35°C–40°C, semisolid gel is formed that is stable over a wide pH range of (from 3 to 9). Interpenetrating H-binding between sugar moieties resulted in production of this sol–gel network and nanochannel containing pore sizes of 200 nm. CeO2-NPs were synthesized in these nanochannels. Similarly, Darroudi et al had synthesized CeO2-NPs using starch as a capping biopolymer.27 The proposed mechanism, for starch-based synthesis of CeO2-NPs was that after dissolving starch in water, metal cations were attracted by oxygen of the OH branches. In vitro studies on Neuro2A cells demonstrated a dose-dependent toxicity with a nontoxic concentration of 175 μg/mL. Applying starch as a template for CeO2-NP synthesis by Darroudi et al27 resulted in the formation of ultrafine CeO2-NP particles that were small in size and uniform in shape. Therefore, this method seems to be more appropriate for CeO2-NP synthesis for medical purposes. Furthermore, in line with the required characteristics, this method was found to be easy, economical and green for large-scale preparation of cerium oxide in nanoscale.
Regarding unique potential of biopolymers in the development of bio-directed methods of CeO2-NP synthesis, Darroudi et al27 also used Gum tragacanth (GT) for the production of CeO2-NPs by both chemical and biological methods.28 The soluble fraction (tragacanthin or tragacanthic acid) of GT gives a sol form in distilled water, whereas the insoluble fraction (bassorin) swells to a gel form (Figure 2).52,53 While heating the sol–gel solution up to 40°C, the GT became soluble in water and the semicrystalline structures were lost. After adding the cerium nitrate to the solution, the metal cations were attracted by the oxygen of OH branches of GT polysaccharides. During the heating process, the amount of water was decreased and the nitrate decomposed to nitrogen dioxide and oxygen molecules, which were then removed from the compounds. Ce(OH)4 nuclei were converted into CeO2 nuclei via dehydration and, subsequently, highly crystallized CeO2-NPs particles grew. The required energy for the above reactions was provided by the subsequent sol–gel procedure and heat. The stabilizing effect of GT could be attributed to the steric repulsion force arising as the gum formed a layer around the cerium hydroxides and cerium oxide NPs. However, the ability of GT to stabilize CeO2-NPs might also be due to electrostatic interactions in addition to the enhancement of suspension viscosity.54,55 Although the formation of CeO2-NPs particles involved several complicated reactions,56 controlling the nucleation of initial precipitate Ce(OH)3 would mainly determine the properties of the final CeO2-NPs. Furthermore, the CeO2-NPs exhibited very low cytotoxic effects on Neuro2A cell lines, making them suitable candidates for various biological applications. Dextran was also used for CeO2-NP stabilizing and coating, as it is a biocompatible, complex and highly water-soluble polysaccharide.57 Accordingly, NPs as small as 5 nm were produced which were toxic to cancer cells at pH 6 and much less toxic to normal cells at the same pH value.57 Moreover, the importance and versatility of polyethylene glycol (PEG) for the functionalization of rare earth cerium oxide NPs were also investigated.58–60 The suggested mechanism for PEG-mediated ceria synthesis was the presence of an electrostatic driving force for the complexation.59 The branched structure of PEG is sufficient to solubilize the CeO2-NPs and create true dispersible nanopowders in aqueous solution and in certain organic solvents, providing a framework for designing a versatile hybrid metal oxide sol.58 Furthermore, chitosan-based synthesis of CeO2-NPs was also reported due to specific properties, such as good film-forming ability, biocompatibility, nontoxicity, biodegradability and antibacterial activity (Table 2).61,62
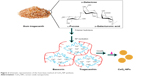  | Figure 2 Schematic representation of the Gum base method of CeO2-NP synthesis. |
  | Table 2 Advantages and challenges of different methods of CeO2-NPs green synthesis |
The toxicologic effect of green synthesized CeO2-NPs
All cerium oxide NPs contain the same core elements, however, do not display similar biological effects. There are some studies that reported prooxidant toxicity of NPs in some cases and antioxidant protective effects in others that could be attributed to different physiochemical parameters of the various NPs that were used. Method of NP synthesis, type of stabilizing agent used, and the Ce3+/Ce4+ surface ratio have been demonstrated to play major roles in producing CeO2-NPs with different physicochemical properties.63,64 The most important parameters are discussed below (Figure 3).
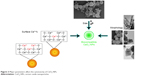  | Figure 3 Major parameters affect the cytotoxicity of CeO2-NPs. |
Particle size
Several green methods of CeO2-NPs synthesis have provided NPs as small as <10 nm. Previous results demonstrated that among different strategies reported for bio-directed synthesis of CeO2-NPs, biopolymer and nutrient-based methods provided the smallest NPs compared with plant-based processes. Reports indicated that plant-based CeO2-NP synthesis provided larger NP with antibacterial properties that exhibited high levels of cytotoxicity to bacterial cells.35,37 However, biopolymer- and nutrient-based methods have provided small NPs which show no cytotoxic effects to human cell lines at high concentrations of CeO2-NPs.27,28,47,48,51
Morphology
Morphology is another physical property that is also required to be considered for biological applications. For example, NPs in polygonal, cube or rod shapes have sharp edges and could cause mechanical damage to cells.7,65,66 Therefore, the effect of NP shape cannot be ignored for biological applications. As mentioned earlier, almost all the green methods of ceria synthesis that are mentioned herein have produced NPs with spherical morphology. However, starch-based synthesis of CeO2-NPs seems to be the most appropriate method to provide CeO2-NPs for biomedical purposes.27
Percentage of surface Ce3+
In 2015, Pulido-Reyes et al67 presented a report that differed from previous reports about CeO2-NPs synthesis. They demonstrated that neither concentration, surface charge nor size of CeO2-NPs plays any important role in their observed toxic properties. The report demonstrated that percentage of surface Ce3+ correlated with toxicity and was the main driver of CeO2-NPs toxic effects.67 They proposed that CeO2-NPs with the highest percentage of surface Ce3+ (58%) exhibited the most toxic effect, and CeO2-NPs with lower percentage of surface Ce3+ values (between 26% and 36%) were evidently nontoxic for the model organism. In fact, CeO2-NPs with lower Ce3+ and, therefore, higher Ce4+ on their surface showed catalase mimetic activity,68 which broke down H2O2 to molecular oxygen, protecting the cells against this toxic ROS. CeO2-NPs with higher Ce3+ on their surface could efficiently scavenge radicals of superoxide (superoxide dismutase [SOD] mimetic activity) and produce H2O2, which is toxic to the cells. They suggested that in a narrow range of surface Ce3+, there seemed to be a shift from SOD activity to catalase mimetic activity; however, the mechanisms and whether the observed biological effect reported at their study may also occur in other cellular systems, requires further investigation.67 However, there is no report on the effect of applying green methods of CeO2-NPs synthesis on the percentage of surface Ce3+ of NPs and this should be investigated to clearly demonstrate the effect of green synthesis of CeO2-NPs on their cytotoxicity.
A CeO2-NP enters cells by energy-dependent, clathrin-mediated and caveolae-mediated endocytic pathways. Its localization in mitochondria, lysosomes and endoplasmic reticulum, as well as the cytoplasm and nucleus, were demonstrated by Singh et al.69 Considering radical scavenging properties of cerium oxide and its widespread cellular disposition, a CeO2-NP likely acts as a cellular antioxidant in multiple compartments of the cell, presenting protection against a variety of oxidant injuries.69
Biological applications of CeO2-NPs
Antibacterial effect
There are different studies that have reported antibacterial activity of CeO2-NPs and demonstrated their significant inhibition toward both gram-negative and gram-positive bacteria.34–37 It is suggested that CeO2-NPs with a particle size of over 20 nm possess antibacterial properties. Moreover, the most antibacterial effects due to the highest percentage of surface Ce3+ of NP are in agreement with Pulido-Reyes et al’s observations.67
Neurodegenerative effect
The brain and central nervous system are the most active organ systems in the body; therefore, they are particularly sensitive to oxidative stress because of high oxygen utilization, high levels of polyunsaturated fatty acid peroxidation and low levels of endogenous antioxidant systems. Increased oxidative stress and free radical production could be attributed to several neurodegenerative diseases, such as Parkinson’s disease, trauma, ischemic stroke, Alzheimer’s disease (AD) and aging.70 A beneficial therapy for neurodegenerative diseases is CeO2-NP utilization, which removes ROS or prevents their formation and affects different key points in the brain cells or central nervous tissue. Reducing ROS production, CeO2-NPs were demonstrated to affect (directly or indirectly) signal transduction pathways involved in neuronal death and neuroprotection. For example, it is reported that cerium oxide NPs could trigger neuronal survival in a human AD model through modulating the brain-derived neurotrophic factor (BDNF) pathway. BDNF is a factor involved in the signal transduction pathways of neuronal survival.71 In a similar approach, Guo et al reported that ceria NPs protect neurons against oxidative stress induced injury by modulating transforming growth factor beta (TGF-β) signaling.72 There are so many reports on the neuroprotective effect of engineered CeO2-NPs. Recently, Arya et al3 reported that CeO2-NPs promoted neurogenesis and modulated hypoxia-induced memory impairment through the AMPK–PKC–CBP signaling cascade. Using PEG-coated 3 nm CeO2-NPs, they demonstrated that NPs were efficiently localized in the brain and significantly decreased oxidative stress. Therefore, associated damage during hypoxia exposure was also reduced by applying PEG/CeO2-NPs. They also provided evidence that PEG/CeO2-NPs enhanced hippocampus neuronal survival and promoted neurogenesis.3
Regarding the reductive effect of CeO2-NPs on oxidative stress, which is known to play an important role in neurodegeneration, Fiorani et al73 had investigated the role of CeO2-NPs on microglial activation and neurodegenerative processes in light damaged retina. They demonstrated the ability of CeO2-NPs to reduce microglial activation and their migration toward the outer nuclear layer,73 raising the possibility of their use as therapeutic agents for neurodegenerative diseases.
Enzyme mimetic applications
CeO2-NPs are forms of powerful artificial oxidase enzymes capable of mimicking catalase and SOD and peroxidase-like activities (Table 3).
  | Table 3 Different types of enzyme mimicking activities of cerium oxide nanoparticles |
Oxidase-like activity of these NPs originated from surface Ce3+ atoms as the catalytic center.74 CeO2-NPs with lower Ce3+ on their surface showed catalase or peroxidase mimetic activity,68 which could break down H2O2 into water and oxygen. CeO2-NPs with higher Ce3+ on their surface could efficiently scavenge radicals of superoxide (SOD mimetic activity) and produce H2O2.
SOD mimicking activity
Comparing with natural enzymes, CeO2-NPs showed several advantages, such as high sensitivity, low cost, easy storage and catalytic stability under harsh conditions. Construction of efficient artificial enzymes, as a strong and cost-effective alternative to natural enzymes, has been an interesting subject in the field of biomimetic chemistry. In a new report on SOD-like activity of ceria, Bhushan and Gopinath75 developed a stable and biocompatible artificial enzymatic system based on CeO2-NPs that possessed high ROS scavenging activity over a period of time. They synthesized a CeO2-NP encapsulated biocompatible ceria-albumin nanoparticle (BCNP) capable of reducing intracellular ROS. The BCNPs preserved the antioxidant defense system of the cells and protected them from oxidant-mediated apoptosis.75 Importantly, the enzyme mimicking activity of CeO2-NPs remained almost constant and stable over a wide range of pH and temperature. Therefore, the as-prepared BCNPs were promising as potential candidates against ROS-induced diseases and disorders utilizing SOD-like activity of ceria.75 Moreover, the SOD ability of CeO2-NPs with sizes >5 nm and diversity in shape and a negligible Ce3+/Ce4+ ratio were also investigated by Li et al.76 So far, inherent superoxide-scavenging ability has only been found in the CeO2-NPs with sizes of <5 nm, and these bioactive CeO2-NPs showed very limited diversity with respect to shape. Li et al76 believed that without the coating of surface ligands to stabilize the oxygen vacancies, CeO2-NPs of >3 nm could not maintain a substantially higher Ce3+/Ce4+ ratio under ambient conditions when compared to their bulk counterpart.77 Therefore, even CeO2-NPs of <5 nm would lose their inherent SOD mimetic activity because of Ce3+ oxidation, and the time required to regenerate that activity would usually take days and weeks.78,79 Li et al76 proposed a strategy to significantly improve the superoxide-scavenging activity of CeO2-NPs of >5 nm. However, they activated the SOD mimetic activity of different sized CeO2-NPs within minutes by incubation with native CuZn-SOD in phosphate-buffered saline (Figure 4).76
  | Figure 4 Superoxide dismutase mimetic activity of CeO2-nanoparticles. |
Catalase mimicking activity
The first report on catalase mimicking activity of CeO2-NPs was presented by Pirmohamed et al.68 Recently, the catalytic activity of CeO2-NPs was applied in different biomedical approaches.80–82 For example, Akhtar et al have demonstrated that the catalase activity of CeO2-NPs could increase the intracellular glutathione (GSH) in cells challenged with H2O2, protecting cells from oxidative damage.80 Considering major roles of GSH in the regulation of cell growth and division, metabolism of carcinogens and protecting DNA from oxidative damage, the effect of CeO2-NPs on increasing the amount of intracellular GSH marks a revolution in medical biology. Moreover, Nicolini et al had introduced a kind of bioactive glass based on catalytic activity of CeO2-NPs, which was used for bone tissue engineering.82 The design of bioactive glasses capable of preventing oxidative stress after implantation would reduce the convalescence and decrease the amount of anti-inflammatory responses in patients. Applying biomedical properties of CeO2-NPs requires more investigation of the NPs’ fate in vivo. For example, cerium atoms of CeO2-NPs have the potential to interact with peptides, sugar and small anion molecules, such as phosphate in vitro and in vivo. Singh et al investigated the role of phosphate on stability and catalase mimetic activity of cerium oxide NPs.81,83 Given the abundance of inorganic phosphate in biological systems, they demonstrated that catalase mimetic activity of CeO2-NPs (Ce4+) is resistant to the phosphate anions, pH changes and composition of cell culture media. Thus, Singh et al provided a promising approach to more practical and attractive biomedical applications for cerium oxide NPs.
Peroxidase mimicking activity
SOD and catalase mimetic activity of CeO2-NPs has been studied extensively; however, research regarding its peroxidase-like activity remains scant. As the newest research in this field, Tian et al exploited the peroxidase-like activity of CeO2-NPs for breast cancer cell detection using nanostructure-based enzyme-linked immunosorbent assay (ELISA).2 In the designed system, the primary antibody against a biomarker of breast cancer (CA15-3) was coated on the ELISA plate and the second antibody was directly conjugated on the surface of CeO2-NPs through electrostatic forces. In the presence of cancer cells, the primary antibody could capture the cells and the secondary antibody-conjugated CeO2-NPs would attach to them, causing oxidation of H2O2 and color change. Comparing the CeO2-NPs-based sensor with the horse radish peroxidase (HRP)-based one, the high sensitivity of CeO2-NPs-based immunoassay, with a detection limit of 0.01 ng/mL, was approximately one order of magnitude higher than the HRP system.2
Sensing applications
Different forms of biosensors were designed based on CeO2-NPs, including electrochemical, fluorometric and colorimetric sensors, which are briefly discussed here. In 2006, the catalytic activity of cerium oxide NP was exploited to develop a highly sensitive biosensor for the first time. A study has shown that synthesized electrochemical biosensors based on cerium oxide NPs were efficient tools for H2O2 detection in as low as 1 μM of water.84 Currently, interfacing H2O2 with inorganic NPs has generated a number of nanozymes showing catalase or peroxidase-like activities. Recently, Liu et al85 introduced a DNA/CeO2-NP-based fluorometric sensing system for highly sensitive detection of H2O2 (Figure 5). Liu et al probed CeO2-NPs and H2O2 interaction, applying DNA. H2O2 often causes oxidative DNA damage in the presence of redox metals; however, the ability of H2O2 to displace adsorbed DNA without cleavage was used in this study. After adding CeO2-NPs to the solution of fluorescently labeled DNA, the fluorescence was completely quenched, demonstrating the adsorption of DNA on the NPs’ surface. Interestingly, fluorescence was completely and rapidly recovered after adding H2O2. Given the sensor performance for H2O2 with a detection limit of 130 nM, Liu et al then tested the presence of glucose. H2O2 is produced in situ using glucose oxidase (GOX) and glucose. When the glucose concentration varied, a linear response was observed with a detection limit of 8.9 μM in buffer and 4.37±0.32 mM in serum.85
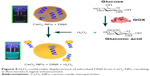  | Figure 5 H2O2 could make displacement of adsorbed DNA from CeO2-NPs, resulting in fluorescence signal enhancement. |
In other work, Sardesai et al developed a biosensor based on oxygen-rich platinum doped CeO2-NPs (Pt-ceria) and lactate oxidase for in vitro and in vivo monitoring of lactate during hypoxia.86 Integration of the oxygen-rich CeO2-NPs in the enzyme-containing layer ensured operation of the biosensor in hypoxic conditions, and provided continuous, sensitive lactate monitoring. Measurements of lactate levels in blood and tissues are important indications of the state and progress of a variety of diseases. In vitro evaluation of the biosensor demonstrated a detection limit of 100 pM and high selectivity against physiological levels of coexisting interference species, as well as a quick response time of 6 seconds. In vivo studies have been performed by placing the designed biosensor in the hippocampus of anesthetized rats. The results provided the possibility of continuous lactate monitoring under 2 hours ischemia and reperfusion.86 Moreover, all the mentioned reports have documented the ability of cerium oxide NPs to provide third-generation biosensors with high sensitivity and specificity of detection.
Angiogenesis induction
A unique property of CeO2-NPs could also induce angiogenesis in vivo. Angiogenesis is the physiological process through which new blood vessels form from pre-existing ones. In particular, CeO2-NPs trigger angiogenesis by modulating the intracellular oxygen environment and endogenously stabilizing hypoxia inducing factor 1α, which alters gene regulation. Furthermore, the high surface area, increased Ce3+/Ce4+ ratio and small size make CeO2-NPs more catalytically active toward regulating intracellular oxygen, which in turn leads to more robust induction of angiogenesis.87
Conclusion
The unique property of CeO2-NPs that makes them distinct from other antioxidants is their ability to self-regenerate their surface. Thus, one small dose can work for a long time before being cleared from the body.7 Accordingly, various kinds of CeO2-NPs have been synthesized in order to target the Achilles’ heel of any oxidative stress-associated diseases.88,89 Investigating previous literature on ceria NPs demonstrated that different synthesis methods could provide cerium oxide NPs with various catalytic and physiochemical properties that could contribute to antioxidant or prooxidant properties.29 Considering CeO2-NPs as potential therapeutic agents, it is important to pay attention to their synthesis method. Among different strategies reported for the synthesis of CeO2-NPs, green synthesis methods have shown to be promising for CeO2-NP production and in their application in biological systems. Another consideration of CeO2-NPs is that the in vitro measured properties of the NP (eg, zeta potential, size and redox activity) could change under physiological conditions.90 For example, Kumari et al has shown that the hydrodynamic diameter of CeO2-NPs increased dramatically in cell culture media due to the tendency of NPs to agglomerate in physiological conditions.91 Furthermore, adsorption of proteins in biological fluids, such as blood, could also affect the size and distribution of metal oxide NPs. Generally, smaller sized particles that are free of contamination are suitable for bio-applications. Using bio-directed methods, synthesis of small CeO2-NPs is possible. For example, as mentioned earlier, applying starch-based methods resulted in the production of CeO2-NPs as small as 6 nm. Since bio-directed methods of CeO2-NP synthesis used biocompatible stabilizers and produced nontoxic NPs, of all the different methods of CeO2-NP synthesis, green synthesis is proposed to be applied for the production of CeO2-NPs for therapeutic purposes. Moreover, green synthesis of CeO2-NPs suggests several advantages, such as cost-effectiveness, large-scale commercial production and the potential for pharmaceutical applications.
Future perspectives
CeO2-NPs were recently shown to have regenerative antioxidant activity. Therefore, low levels of CeO2-NPs can work for extended time periods. However, these NPs provided some toxicologic concerns. Currently, the green synthesis of CeO2-NPs gets more attention in order to solve the challenges regarding safety and use of this metal oxide for biomedicine, but there are still some considerations. Previous reports suggested that the protein corona provides NPs with particular biological identity which subsequently play important roles in the ultimate interactions of NPs with target cells. Therefore, physiochemical characteristics of NPs after interaction with biological fluids should be investigated in order to achieve correct interpretations of the biocompatibility of green methods of CeO2-NPs synthesis. Moreover, regarding the effect of percentage of surface Ce3+ on the properties of CeO2-NPs in biological systems, the green synthesized CeO2-NPs should be investigated from this point of view. In addition, an important consideration in clinical usage of CeO2-NPs is how cerium oxide NPs behave in biological systems. Addressing this is not a simple endeavor and requires some in vivo-based research of the effect of CeO2-NPs produced by bio-directed methods.
Disclosure
The authors report no conflicts of interest in this work.
References
Gagnon J, Fromm KM. Toxicity and protective effects of cerium oxide nanoparticles (Nanoceria) depending on their preparation method, particle size, cell type, and exposure route. Eur J Inorg Chem. 2015;27:4510–4517. | ||
Tian Z, Li J, Zhang Z, Gao W, Zhou X, Qu Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials. 2015;59:116–124. | ||
Arya A, Gangwar A, Singh SK, et al. Cerium oxide nanoparticles promote neurogenesis and abrogate hypoxia-induced memory impairment through AMPK–PKC–CBP signaling cascade. Int J Nanomedicine. 2016;11:1159–1173. | ||
Beaudoux X, Virot M, Chave T, Durand G, Leturcq G, Nikitenko SI. Vitamin C boosts ceria-based catalyst recycling. Green Chem. 2016;18:3656–3668. | ||
Gawande MB, Bonifacio VDB, Varma RS, et al. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst – applications in multicomponent reactions under benign conditions. Green Chem. 2013;15(5):1226–1231. | ||
Xu C, Qu X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6:e90. | ||
Das S, Dowding JM, Klump KE, McGinnis JF, Self W, Seal S. Cerium oxide nanoparticles: applications and prospects in nanomedicine. Nanomedicine (Lond). 2013;8(9):1483–1508. | ||
Deshpande S, Patil S, Kuchibhatla SV, Seal S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl Phys Lett. 2005;87(13):133113. | ||
Asati A, Santra S, Kaittanis C, Nath S, Perez JM. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew Chem Int Ed Engl. 2009;48(13):2308–2312. | ||
Asati A, Kaittanis C, Santra S, Perez JM. The pH-tunable oxidase-like activity of cerium oxide nanoparticles achieves sensitive fluorigenic detection of cancer biomarkers at neutral pH. Anal Chem. 2011;83(7):2547–2553. | ||
Li X, Sun L, Ge A, Guo Y. Enhanced chemiluminescence detection of thrombin based on cerium oxide nanoparticles. Chem Commun. 2011; 47(3):947–949. | ||
Kaittanis C, Santra S, Asati A, Perez JM. A cerium oxide nanoparticle-based device for the detection of chronic inflammation via optical and magnetic resonance imaging. Nanoscale. 2012;4(6):2117–2123. | ||
Ornatska M, Sharpe E, Andreescu D, Andreescu S. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal Chem. 2011;83(11):4273–4280. | ||
Lin Y, Xu C, Ren J, Qu X. Using thermally regenerable cerium oxide nanoparticles in biocomputing to perform label-free, resettable, and colorimetric logic operations. Angew Chem Int Ed Engl. 2012;51(50):12579–12583. | ||
Celardo I, Pedersen JZ, Traversa E, Ghibelli L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3(4):1411–1420. | ||
Li M, Shi P, Xu C, Ren J, Qu X. Cerium oxide caged metal chelator: anti-aggregation and anti-oxidation integrated H2O2-responsive controlled drug release for potential Alzheimer’s disease treatment. Chem Sci. 2013;4(6):2536–2542. | ||
Xu C, Lin Y, Wang J, et al. Nanoceria-triggered synergetic drug release based on CeO2-capped mesoporous silica host–guest interactions and switchable enzymatic activity and cellular effects of CeO2. Adv Healthc Mater. 2013;2(12):1591–1599. | ||
Chen HI, Chang HY. Synthesis of nanocrystalline cerium oxide particles by the precipitation method. Ceramics Int. 2005;31(6):795–802. | ||
Yu JC, Zhang L, Lin J. Direct sonochemical preparation of high-surface-area nanoporous ceria and ceria–zirconia solid solutions. J Colloid Interface Sci. 2003;260(1):240–243. | ||
Yan Z, Wang J, Zou R, Liu L, Zhang Z, Wang X. Hydrothermal synthesis of CeO2 nanoparticles on activated carbon with enhanced desulfurization activity. Energy Fuels. 2012;26(9):5879–5886. | ||
Chunwen S, Hong L, Huairuo Z, Zhaoxiang W, Liquan C. Controlled synthesis of CeO2 nanorods by a solvothermal method. Nanotechnology. 2005;16(9):1454. | ||
Yadav TP, Srivastava ON. Synthesis of nanocrystalline cerium oxide by high energy ball milling. Ceramics Int. 2012;38(7):5783–5789. | ||
Wang Y, Mori T, Li JG, Ikegami T. Low-temperature synthesis of praseodymium-doped ceria nanopowders. J Am Ceramic Soc. 2002; 85(12):3105–3107. | ||
Feng X, Sayle DC, Wang ZL, et al. Converting ceria polyhedral nanoparticles into single-crystal nanospheres. Science. 2006;312(5779):1504–1508. | ||
Hirano M, Fukuda Y, Iwata H, Hotta Y, Inagaki M. Preparation and spherical agglomeration of crystalline cerium(IV) oxide nanoparticles by thermal hydrolysis. J Am Ceramic Soc. 2000;83(5):1287–1289. | ||
He HW, Wu XQ, Ren W, Shi P, Yao X, Song ZT. Synthesis of crystalline cerium dioxide hydrosol by a sol–gel method. Ceramics Int. 2012;38(Suppl 1):S501–S504. | ||
Darroudi M, Sarani M, Kazemi Oskuee R, Khorsand Zak A, Hosseini HA, Gholami L. Green synthesis and evaluation of metabolic activity of starch mediated nanoceria. Ceramics Int. 2014;40(1, Part B):2041–2045. | ||
Darroudi M, Sarani M, Kazemi Oskuee R, Khorsand Zak A, Amiri MS. Nanoceria: gum mediated synthesis and in vitro viability assay. Ceramics Int. 2014;40(2):2863–2868. | ||
Dowding JM, Seal S, Self WT. Cerium oxide nanoparticles accelerate the decay of peroxynitrite (ONOO−). Drug Deliv Transl Res. 2013;3(4):375–379. | ||
Dowding JM, Das S, Kumar A, et al. Cellular interaction and toxicity depend on physicochemical properties and surface modification of redox-active nanomaterials. ACS Nano. 2013;7(6):4855–4868. | ||
Adschiri T, Lee YW, Goto M, Takami S. Green materials synthesis with supercritical water. Green Chem. 2011;13(6):1380–1390. | ||
Ko JW, Lee BI, Chung YJ, Park CB. Carboxymethyl cellulose-templated synthesis of hierarchically structured metal oxides. Green Chem. 2015;17(8):4167–4172. | ||
Arumugam A, Karthikeyan C, Haja Hameed AS, Gopinath K, Gowri S, Karthika V. Synthesis of cerium oxide nanoparticles using Gloriosa superba L. leaf extract and their structural, optical and antibacterial properties. Mater Sci Eng C Mater Biol Appl. 2015;49:408–415. | ||
Kannan SK, Sundrarajan M. A green approach for the synthesis of a cerium oxide nanoparticle: characterization and antibacterial activity. Int J Nanosci. 2014;13(03):1450018. | ||
Priya GS, Kanneganti A, Kumar KA, Rao KV, Bykkam S. Bio synthesis of cerium oxide nanoparticles using Aloe arbadensis Miller Gel. Int J Sci Res Publications. 2014;4(6):1–4. | ||
Kumar A, Das S, Munusamy P, et al. Behavior of nanoceria in biologically-relevant environments. Environ Sci Nano. 2014;1(6):516–532. | ||
Munusamy S, Bhakyaraj K, Vijayalakshmi L, Stephen A, Narayanan V. Synthesis and characterization of cerium oxide nanoparticles using Curvularia lunata and their antibacterial properties. Int J Innovative Res Sci Eng. 2014;2(1):318–323. | ||
Thill A, Zeyons O, Spalla O, et al. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol. 2006;40(19):6151–6156. | ||
Zeyons O, Thill A, Chauvat F, et al. Direct and indirect CeO2 nanoparticles toxicity for Escherichia coli and Synechocystis. Nanotoxicology. 2009;3(4):284–295. | ||
Rodea-Palomares I, Gonzalo S, Santiago-Morales J, et al. An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat Toxicol. 2012;122–123:133–143. | ||
Hoecke KV, Quik JTK, Mankiewicz-Boczek J, et al. Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ Sci Technol. 2009;43(12):4537–4546. | ||
Rogers NJ, Franklin NM, Apte SC, et al. Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater. Environ Chem. 2010;7(1):50–60. | ||
Rodea-Palomares I, Boltes K, Fernández-Piñas F, et al. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol Sci. 2011; 119(1):135–145. | ||
Xia T, Kovochich M, Liong M, et al. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 2008;2(10): 2121–2134. | ||
Zhao L, Peng B, Hernandez-Viezcas JA, et al. Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano. 2012; 6(11):9615–9622. | ||
Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2007;10(3):507–517. | ||
Kargar H, Ghazavi H, Darroudi M. Size-controlled and bio-directed synthesis of ceria nanopowders and their in vitro cytotoxicity effects. Ceramics Int. 2015;41(3, Part A):4123–4128. | ||
Darroudi M, Hoseini SJ, Kazemi Oskuee R, Hosseini HA, Gholami L, Gerayli S. Food-directed synthesis of cerium oxide nanoparticles and their neurotoxicity effects. Ceramics Int. 2014;40(5):7425–7430. | ||
Singh AV, Bandgar BM, Kasture M, Prasad BLV, Sastry M. Synthesis of gold, silver and their alloy nanoparticles using bovine serum albumin as foaming and stabilizing agent. J Mater Chem. 2005;15(48): 5115–5121. | ||
Darroudi M, Ahmad MB, Abdullah AH, Ibrahim NA. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int J Nanomedicine. 2011;6:569–574. | ||
Kargar H, Ghasemi F, Darroudi M. Bioorganic polymer-based synthesis of cerium oxide nanoparticles and their cell viability assays. Ceramics Int. 2015;41(1, Part B):1589–1594. | ||
Loth F. Industrial Gums: Polysaccharides and Their Derivatives. 3rd edition. Edited by Roy L. Whistler and James N. BeMiller. ISBN 0-12-746253-8. Academic Press, Inc., San Diego/New York/Boston/London/Sidney/Tokyo/Toronto 1993.642P. Acta Polymerica. 1993;44(3): 172–173. | ||
Davidson RL. Handbook of Water-Soluble Gums and Resins/Robert L. Davidson, editor in chief. New York, NY: McGraw-Hill; 1980. | ||
Remani KC, Ghosh S. Nanocrystalline ceria through homogeneous precipitation in alcohol-water mixed solvent. Trans Indian Ceramic Soc. 2009;68(4):185–188. | ||
Yokoyama A, Srinivasan KR, Fogler HS. Stabilization mechanism of colloidal suspensions by gum tragacanth: the influence of pH on stability. J Colloid Interface Sci. 1988;126(1):141–149. | ||
Khorsand Zak A, Abd Majid WH, Mahmoudian MR, Darroudi M, Yousefi R. Starch-stabilized synthesis of ZnO nanopowders at low temperature and optical properties study. Adv Powder Technol. 2013;24(3):618–624. | ||
Alpaslan E, Yazici H, Golshan NH, Ziemer KS, Webster TJ. pH-dependent activity of dextran-coated cerium oxide nanoparticles on prohibiting osteosarcoma cell proliferation. ACS Biomater Sci Eng. 2015;1(11):1096–1103. | ||
Qi L, Fresnais J, Mullera P, Theodoly O, Berretb F, Chapel P. Interfacial activity of phosphonated-polyethylene glycol functionalized cerium oxide nanoparticles. Langmuir. 2012;28(31):11448–11456. | ||
Qi L, Sehgal A, Castaing JC, et al. Redispersible hybrid nanopowders: cerium oxide nanoparticle complexes with phosphonated-PEG oligomers. ACS Nano. 2008;2(5):879–888. | ||
Satapathy S. PEG-Assisted Synthesis and Characterization of Ceria Nanoparticles. Rourkela, India: National Institute of Technology; 2011. | ||
Kaushik A, Solanki PR, Pandey MK, Ahmad S, Malhotra BD. Cerium oxide-chitosan based nanobiocomposite for food borne mycotoxin detection. Appl Phys Lett. 2009;95(17):173703. | ||
Hassannejad H, Nouri A. Synthesis and evaluation of self-healing cerium-doped chitosan nanocomposite coatings on AA5083-H321. Int J Electrochem Sci. 2016;11:2106–2118. | ||
Karakoti A, Singh S, Dowding JM, Seal S, Self WT. Redox-active radical scavenging nanomaterials. Chem Soc Rev. 2010;39(11):4422–4432. | ||
Alili L, Sack M, von Montfort C, et al. Downregulation of tumor growth and invasion by redox-active nanoparticles. Antioxid Redox Signal. 2013;19(8):765–778. | ||
Dahle J, Arai Y. Environmental geochemistry of cerium: applications and toxicology of cerium oxide nanoparticles. Int J Environ Res Public Health. 2015;12(2):1253–1278. | ||
Prabaharan DMDM, Sadaiyandi K, Mahendran M, Sagadevan S. Structural, optical, morphological and dielectric properties of cerium oxide nanoparticles. Mater Res. 2016;19(2):478–482. | ||
Pulido-Reyes G, Rodea-Palomares I, Das S, et al. Untangling the biological effects of cerium oxide nanoparticles: the role of surface valence states. Sci Rep. 2015;5:15613. | ||
Pirmohamed T, Dowding JM, Singh S, et al. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem Commun. 2010;46(16):2736–2738. | ||
Singh S, Kumar A, Karakoti A, Seal S, Self WT. Unveiling the mechanism of uptake and sub-cellular distribution of cerium oxide nanoparticles. Mol Biosyst. 2010;6(10):1813–1820. | ||
Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7(1):65–74. | ||
D’Angelo B, Santucci S, Benedetti E, et al. Cerium oxide nanoparticles trigger neuronal survival in a human Alzheimer disease model by modulating BDNF pathway. Curr Nanosci. 2009;5(2):p167. | ||
Guo C, Smith R, Gant TW, Leonard MO. Cerium dioxide nanoparticles protect against oxidative stress induced injury through modulation of TGF-β signalling. Toxicol Res. 2015;4(2):464–475. | ||
Fiorani L, Passacantando M, Santucci S, Di Marco S, Bisti S, Maccarone R. Cerium oxide nanoparticles reduce microglial activation and neurodegenerative events in light damaged retina. PLoS One. 2015;10(10):e0140387. | ||
Juarez R, Corma A, Garcia H. Gold nanoparticles promote the catalytic activity of ceria for the transalkylation of propylene carbonate to dimethyl carbonate. Green Chem. 2009;11(7):949–952. | ||
Bhushan B, Gopinath P. Antioxidant nanozyme: a facile synthesis and evaluation of the reactive oxygen species scavenging potential of nanoceria encapsulated albumin nanoparticles. J Mater Chem B. 2015; 3(24):4843–4852. | ||
Li Y, He X, Yin JJ, et al. Acquired superoxide-scavenging ability of ceria nanoparticles. Angew Chem Int Ed Engl. 2015;54(6):1832–1835. | ||
Zhang D, Wen X, Shi L, Yan T, Zhang J. Enhanced capacitive deionization of graphene/mesoporous carbon composites. Nanoscale. 2012;4(17):5440–5446. | ||
Heckert EG, Karakoti AS, Seal S, Self WT. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29(18):2705–2709. | ||
Karakoti AS, Singh S, Kumar A, et al. PEGylated nanoceria as radical scavenger with tunable redox chemistry. J Am Chem Soc. 2009;131(40):14144–14145. | ||
Akhtar MJ, Ahamed M, Alhadlaq HA, Khan MA, Alrokayan SA. Glutathione replenishing potential of CeO2 nanoparticles in human breast and fibrosarcoma cells. J Colloid Interface Sci. 2015;453:21–27. | ||
Singh R, Singh S. Role of phosphate on stability and catalase mimetic activity of cerium oxide nanoparticles. Colloids Surf B Biointerfaces. 2015;132:78–84. | ||
Nicolini V, Gambuzzi E, Malavasi G, et al. Evidence of catalase mimetic activity in Ce3+/Ce4+ doped bioactive glasses. J Phys Chem B. 2015;119(10):4009–4019. | ||
Singh S, Dosani T, Karakoti AS, Kumar A, Seal S, Self WT. A phosphate-dependent shift in redox state of cerium oxide nanoparticles and its effects on catalytic properties. Biomaterials. 2011;32(28):6745–6753. | ||
Patil SD. Fundamental Aspects of Regenerative Cerium Oxide Nanoparticles and Their Applications in Nanobiotechnology [dissertation]. Florida, USA: Department of Mechanical, Materials and Aerospace Engineering University of Central Florida; 2006. | ||
Liu B, Sun Z, Huang PJJ, Liu J. Hydrogen peroxide displacing DNA from nanoceria: mechanism and detection of glucose in serum. J Am Chem Soc. 2015;137(3):1290–1295. | ||
Sardesai NP, Ganesana M, Karimi A, Leiter JC, Andreescu S. Platinum-doped ceria based biosensor for in vitro and in vivo monitoring of lactate during hypoxia. Anal Chem. 2015;87(5):2996–3003. | ||
Das S, Singh S, Dowding JM, et al. The induction of angiogenesis by cerium oxide nanoparticles through the modulation of oxygen in intracellular environments. Biomaterials. 2012;33(31):7746–7755. | ||
Estevez AY, Erlichman JS. Cerium oxide nanoparticles for the treatment of neurological oxidative stress diseases. Oxidative Stress: Diagnostics, Prevention, and Therapy. Vol 1083: New York: American Chemical Society; 2011:255–288. | ||
Andreescu S, Hepel M. Oxidative Stress: Diagnostics, Prevention, and Therapy. Vol. 1083. ACS Symposium Series. New York: American Chemical Society; 2011. | ||
Estevez AY, Erlichman JS. The potential of cerium oxide nanoparticles (nanoceria) for neurodegenerative disease therapy. Nanomedicine (Lond). 2014;9(10):1437–1440. | ||
Kumari M, Singh SP, Chinde S, Rahman MF, Mahboob M, Grover P. Toxicity study of cerium oxide nanoparticles in human neuroblastoma cells. Int J Toxicol. 2014;33(2):86–97. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
