Back to Journals » International Medical Case Reports Journal » Volume 13
Central Retinal Artery Occlusion After Nasosinal Surgery – an Insight
Authors Chowdhary S, Sawhney V , Pandya A, Sambhav K, Gupta SK
Received 25 January 2020
Accepted for publication 7 April 2020
Published 21 May 2020 Volume 2020:13 Pages 211—215
DOI https://doi.org/10.2147/IMCRJ.S247275
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Somya Chowdhary,1 Vivek Sawhney,2 Abhijit Pandya,2 Kumar Sambhav,3 Shailesh K Gupta2
1Bascom Palmer Eye Institute, University of Miami, Miami, FL, USA; 2Specialty Retina Centre, Coral Springs, FL, USA; 3Department of Ophthalmology, University of Florida, Jacksonville, FL, USA
Correspondence: Shailesh K Gupta
Specialty Retina Centre, Boca Raton, FL, USA
Tel +1561 322-3588
Email [email protected]
Objective: To describe a case of central retinal artery occlusion (CRAO) after nasosinal surgery and subject’s subsequent response to hyperbaric oxygen therapy (HBOT).
Design: Observational case report.
Results: We describe a subject with diagnosed CRAO after septoplasty, bilateral inferior turbinate reduction and balloon sinuplasty, who was given hyperbaric oxygen treatment after four days of onset of CRAO with an improvement in visual acuity and visual field.
Conclusion: Even though CRAO has been rarely reported after ENT procedures and HBOT has been previously described for the treatment, this is the case report where hyperbaric oxygen was given after four days of onset, with a possible improvement.
Keywords: central retinal artery occlusion, fundus fluorescein angiography, visual fields, electroretinogram, cherry red spot, hyperbaric oxygen therapy
Introduction
While the incidence of central retinal artery occlusion (CRAO) is 8.5 per 100,0001 of the population, incidence of CRAO after nonocular procedures has been reported to be 0.013%.2 Although there have been a few case reports discussing CRAO after ear, nose, throat (ENT) procedures, the occurrence is even rarer and several mechanisms have been proposed for such an event to occur.3,4 Documentation and a thorough ophthalmic evaluation at the initial presentation is critical in determining pathophysiology of the event. Several authors have emphasized a critical time window during which emergent management may salvage useful vision in the eye.5 There exist no clear-cut guidelines for treatment of CRAO however several treatment options have been proposed.6 The use of hyperbaric oxygen therapy (HBOT) has been evaluated by some, with successful outcomes.7–16 There have been no case reports where such a modality was tried after 72 hours of onset in a young patient with CRAO secondary to nasosinal surgery.
Herein we report a case of CRAO in an otherwise healthy young male who developed CRAO following an ENT procedure with possible improvement after HBOT treatment.
Case Report
Written informed consent has been provided by the patient to have the case details and any accompanying images published. Institutional approval was not required to publish the case details. A 25 year old healthy Caucasian male with a history of nasal obstruction and chronic sinusitis underwent septoplasty, bilateral inferior turbinate reduction and balloon sinuplasty. Immediately after waking up from general anesthesia, he noticed a painless loss of vision in the left eye. He described seeing through a small hole of vision and his vision otherwise subjectively appeared black and white. He denied any ocular pain on eye movements in either direction. An ophthalmic evaluation was done at an outside facility under emergency settings and a diagnosis of a left CRAO was established. A review of systems was uneventful. An embolic workup and cardiac evaluation were negative. A CT scan was unremarkable. CT angiogram, MRI orbit/face showed no evidence of lamina cribrosa perforation, sinus wall damage, or vascular stenosis. He was started on oral aspirin 81 mg and steroids on the day of the event after consultation with neurology along with topical brimonidine (0.2%) eye drops to reduce intraocular pressure in the left eye. CT angiogram of the neck showed a hypoplastic right vertebral artery.
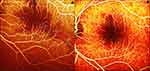
Three days later, he was seen at our facility. Clinical evaluation consisted of ocular examinations and appropriate ocular investigations. His visual acuity in the right eye was 20/20 and counting fingers at six feet in the left eye. There was relative afferent pupillary defect in the left eye. Slit lamp evaluation confirmed normal anterior segment in both eyes. Intraocular pressure (IOP) on applanation was 15 and 11 mmHg in right and left eye respectively. Fundus examination in right eye showed clear vitreous cavity, healthy appearing nerve and macula with attached retina. Fundus examination of the left eye showed attenuation of small arterioles at the posterior pole with cherry red spot (Figure 1A and B). Spectral domain optical coherence tomography (SD-OCT) evaluation showed parafoveal inner/middle retinal hyperreflectivity suggestive of inner retinal edema (Figure 2A). A fundus fluorescein angiogram (FFA) revealed delayed transits (~16 seconds) in the left eye with multiple fat emboli (Figure 3A, blue arrows) and irregular foveal hypofluorescence due to inner retinal edema in macular region. Though there was profound loss of vision still visual fields were attempted. A Humphrey visual field (HVF, 24-2 threshold test) in the left eye showed VFI of 1% with severe depression of field (Figure 4A). A full-field electroretinogram (ERG) showed increased latency with decrease in b-wave amplitude. A multicontrast visual evoked potential (VEP) showed profound reduction in amplitude and unidentifiable P100 waveform. Although the patient was more than 72 h out from the time of insult, an anterior segment tap was performed the same day to lower intraocular pressure further in an effort to dislodge the embolus. Before the procedure the patient was told that there may not be any increase in vision. The patient was advised to consider HBOT, which he agreed to. HBOT was started on the fourth day after the event. The patient was given 1.5 ATA, 100% oxygen, two 60 min sessions per day separated by four hours at an outside heathy facility. Ten treatments were administered. After 10 cycles, treatment was increased to 1.75 ATA, 100% oxygen, two 60 min sessions per day, and up to 10 treatments given. All treatment was administered via a Sechrist 3200R hyperbaric chamber (manufactured by Sechrist industries, Inc., USA). The patient underwent a total of 20 cycles of HBOT and stated there was subjective increase in vision and visual field with sequential HBOT cycles. Treatment were discontinued once subject stated that there was no improvement. It was unclear if the patient had trauma to the orbital wall or directly to the optic nerve during surgery or because fat emboli. As fat embolism is not amenable to tissue plasminogen activator therapy (tPA), he did not receive treatment with tPA.
At a subsequent evaluation done after three weeks completion of hyperbaric treatment, the patient stated subjective increase in vision and visual fields. On clinical examination his visual acuity was 20/20 in the right eye and 20/250 (through temporal island of vision) in right and left eye respectively. A fundus evaluation was similar in the right eye and in the left eye there was dull foveal reflex, with dissolution of small emboli which was noted on initial presentation. SD-OCT showed retinal thinning due to loss of inner retinal layers (Figure 2B). FFA showed normal vasculature and the emboli were not seen (Figure 3B). A 24-2 HVF showed VFI of 2% with persistent severely depressed fields, however there was small gain of temporal island of vision (noted on grey scale of HVF; Figure 4B). ERG and VEP also showed improvement. Gradually over the course of regular followup over one year the patient maintained visual acuity and field of vision.
Discussion
Central retinal artery occlusion by fat embolism has been previously reported after endoscopic sinus surgery, septoplasty and other ENT procedures3,4 as an extremely rare complication. Mechanism of CRAO occurring after ENT procedures is not fully understood. It could be due to trauma to the orbital wall or directly to the optic nerve or because of fat emboli.3,4 It is also proposed that injection of local anesthetics in combination of adrenalin injected during the ENT procedure can lead to occlusion of retinal vessels.
Inner retinal infarction occurs within 90–240 min of CRAO, retinal ganglion cells undergo infarction within 12–15 min.5 There is no generally accepted guidelines for CRAO treatment, so the treatment options may vary, also none of the current treatment options have been proven to be effective. Various modalities have been described for the treatment of CRAO such as pentoxifylline, inhalation of carbogen, sublingual isosorbide dinitrate, ocular massage with three mirror contact lens, acetazolamide, mannitol, methylprednisolone, tissue plasminogen activator, surgical modalities like anterior chamber paracentesis, neodymium:yttrium-aluminium-garnet laser, pars plana vitrectomy with removal of the embolus amongst others.6
Use of HBOT has been proposed for the treatment of the same and is thought to work by increasing oxygen delivery to the hypoxic tissues through choroidal circulation thereby preserving ischemic tissue affected by retinal artery occlusion.7,8 Various authors have given different timelines for the treatment of the same.9–12 Some authors have reported successful treatment of CRAO with this modality.12–15 For any treatment to be effective, it has to be delivered within this recommended time frame—the window of opportunity for emergent treatment after CRAO or ischemic penumbra, even though earlier administration may be associated with better outcomes. Although there has been a retrospective case series stating that HBOT is an effective modality of treatment for CRAO using fundus changes as a marker for irreversible damage and not time lapse since the event,16 most authors are in agreement that such cases should be treated within the critical time period.
Our patient had developed CRAO following ENT procedures. Fundus pictures (Figure 1A and B) obtained initially shows a cherry red spot in the left eye (compared to the right eye it is redder) and was evident on clinical examination. SD-OCT and FFA obtained three days after initial insult confirmed the diagnosis. There were multiple emboli in retinal artery in macular area. So, it could be due to fat emboli secondary to ENT procedure resulting in CRAO. When the patient presented to our clinic, he was out of the critical time period, still considering the age of patient and visible emboli, we went ahead with paracentesis and HBOT. Though our patient did not develop any side effects with use of HBOT, it is not a risk-free procedure.17 The most common side effect is middle ear barotrauma. There are reports of paranasal sinus barotrauma, pulmonary barotrauma, pulmonary edema and others. Oxygen toxicity is rare and usually encountered as a CNS oxygen toxicity seizure. The side effects are usually self-limiting and can be avoided with adequate screening.17
The patient noted subjective increase in visual acuity and visual field after HBOT. It could be argued that it was because of the natural course of the disease. However, we consider that it was possibly due to the use of HBOT. Nevertheless, it is open for discussion. To the best of our knowledge this is the first case of improvement of vision following HBOT in an otherwise healthy young patient CRVO following ENT procedure. Although the exact mechanism of CRAO in this case cannot be pinpointed, perhaps aggressive multimodal intervention including HBOT can be beneficial in such cases. There are some limitations, we agree that assessment of visual field with microperimetry would have been a better test instead of 24-2, but that was not available and patient refused to go other facilities to get that test done. It is a case report, a larger study may help determine the true applicability of HBOT in patients presenting after 24 hours from the onset of CRAO.
In conclusion, this case report emphasizes the importance of hyperbaric oxygen in the treatment of selected cases with CRAO.
Acknowledgments
No author received financial or material support for the research and the work. No author has financial or proprietary interest related to the research.
Disclosure
The authors declare no conflicts of interest.
References
1. Rumelt S, Dorenboim Y, Rehany U. Aggressive systematic treatment for central retinal artery occlusion. Am J Ophthalmol. 1999;128(6):733–738. doi:10.1016/S0002-9394(99)00359-1
2. Berg KT, Harrison AR, Lee MS. Perioperative visual loss in ocular and nonocular surgery. Clin Ophthalmol. 2010;4:531–546.
3. Maharshak I, Hoang JK, Bhatti MT. Complications of vision loss and ophthalmoplegia during endoscopic sinus surgery. Clin Ophthalmol. 2013;7:573–580. doi:10.2147/OPTH.S40061
4. Rao GN, Rout K, Pal A. Central retinal artery occlusion and third cranial nerve palsy following nasal septoplasty. Case Rep Ophthalmol. 2012;3:321–326. doi:10.1159/000343700
5. Toblalem S, Schutz JS, Chronopoulos A. Central retinal artery occlusion- rethinking retinal survival time. BMC Ophthalmol. 2018;18:101. doi:10.1186/s12886-018-0768-4
6. Cugati S, Varma DD, Chen CS, Lee AW. Treatment options for central retinal artery occlusion. Curr Treat Options Neurol. 2013;15:63–77. doi:10.1007/s11940-012-0202-9
7. Cope A, Eggert J, O’Brien E. Retinal artery occlusion: visual outcome after treatment with hyperbaric oxygen. Diving Hyperb Med J. 2011;41:135–139.
8. Jr F B, Hagan C, Murphy-Lavoie H. Hyperbaric oxygen therapy and the eye. Undersea Hyperb Med. 2008;333–387.
9. Beiran I, Goldenberg I, Adir Y. Early hyperbaric oxygen therapy for retinal artery occlusion. Eur J Ophthalmol. 2011;11:345–350. doi:10.1177/112067210101100405
10. Hertzog LM, Meyer GM, Carsson S. Central retinal artery occlusion, treated with hyperbaric oxygen. J Hyperb Med. 1992;7:3–42.
11. Menzel-Severing J, Siekmannn U, Weinberger A, et al. Early hyperbaric oxygen treatment for nonarteritic central retinal artery obstruction. Am J Ophthalmol. 2012;153:454–459. doi:10.1016/j.ajo.2011.08.009
12. Soares A, Gomes NL, Mendonca L. The efficacy of hyperbaric oxygen therapy in the treatment of central retinal artery occlusion. BMJ Case Rep. 2017;2017:bcr2017220113. doi:10.1136/bcr-2017-220113
13. Kim SH, Cha YS, Lee Y, Kim H, Yoon IN. Successful treatment of central retinal artery occlusion using hyperbaric oxygen therapy. Clin Exp Emerg Med. 2018;5(4):278–281. doi:10.15441/ceem.17.271
14. Wu X, Chen S, Li S, Zhang J, Luan D, Zhau S. Oxygen therapy in patients with retinal artery occlusion: a meta-analysis. PLoS One. 2018;13(8):e0202154. doi:10.1371/journal.pone.0202154
15. Olson EA, Lentz K. Central retinal artery occlusion: a literature review and the rationale for hyperbaric oxygen therapy. Mo Med. 2016;113(1):53–57.
16. Hadanny A, Maliar A, Fishlev G. Reversibility of retinal ischemia due to central retinal artery occlusion by hyperbaric oxygen. Clin Ophthalmol. 2017;11:115–125. doi:10.2147/OPTH.S121307
17. Heyboer M
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.