Back to Journals » OncoTargets and Therapy » Volume 13
Case Report of Anti-CD123 Chimeric Antigen Receptor T-Cell Therapy Followed by Radiotherapy for a Recurrence of Blastic Plasmacytoid Dendritic Cell Neoplasm After Allogeneic Hematopoietic Stem Cell Transplantation
Authors Jiang Y , Li Q, Yuan T, Jiang Y, Deng Q
Received 16 February 2020
Accepted for publication 4 April 2020
Published 22 April 2020 Volume 2020:13 Pages 3425—3430
DOI https://doi.org/10.2147/OTT.S250016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nicola Silvestris
Yi-li Jiang, Qing Li, Ting Yuan, Yan-yu Jiang, Qi Deng
Department of Hematology, Tianjin First Central Hospital, Tianjin 300192, People’s Republic of China
Correspondence: Qi Deng
Department of Hematology, Tianjin First Central Hospital, No. 24 Fukang Road, Nankai District, Tianjin, People’s Republic of China
Tel +86 13612055872
Email [email protected]
Background: Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematopoietic malignancy. There is no standard chemotherapy regimen for BPDCN, and even allogeneic hematopoietic stem cell transplantation (allo-HSCT) has not been able to extend the survival of patients with BPDCN.
Case Report: Here, we present a case of recurrence of BPDCN in a patient with new nodules in his head six months after allo-HSCT. He was enrolled in a clinical trial of anti-CD123 chimeric antigen receptor (CAR) T-cell therapy (ChiCTR1900022058). However, there were no significant changes in the nodules 28 days after anti-CD123-CAR T-cell infusion. He received radiotherapy for the nodules when the proportion of anti-CD123-CAR T-cells in the peripheral blood was 2.8% and the adverse events related to the anti-CD123-CAR T-cell therapy were resolved. The proportion of anti-CD123-CAR T-cells, the level of CD123-CAR gene desoxyribonucleic acid, and the serum levels of cytokines in the patient’s peripheral blood reached the highest peak 14 days after radiotherapy. Fortunately, the nodules disappeared gradually 28 days after radiotherapy. He achieved complete remission again from the anti-CD123-CAR T-cell therapy followed by radiotherapy. To date, he has maintained progression-free survival with complete donor chimerism for six months after the combination therapy.
Conclusion: Anti-CD123-CAR T-cell therapy followed by radiotherapy for a recurrence of blastic plasmacytoid dendritic cell neoplasm after allo-HSCT is effective.
Keywords: blastic plasmacytoid dendritic cell neoplasm, recurrence, chimeric antigen receptor, CARs, radiotherapy, immunotherapy
Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN) is a rare hematopoietic malignancy. It originates from CD4+ and CD56+, precursors of plasmacytoid dendritic cells.1 BPCDN most commonly presents with skin involvement. The second most common presentation is with bone marrow or lymph node involvement, and other systems and organs can be affected as well.2 BPDCN is characterized by rapidly progressive and aggressive biological behavior. The median overall survival (OS) is only 8.7 months.3 Even the allogeneic hematopoietic stem cell transplantation (allo-HSCT) could only extend the OS to 22.7 months.4 There is no established standard chemotherapy regimen for BPDCN. Common chemotherapies include acute myeloid leukemia (AML)-type, acute lymphoblastic leukemia (ALL)-type, or lymphoma-type chemotherapy regimens.5 The expression of CD123 on BPDCN cells is high in most patients.4 Therefore, the anti-CD123 chimeric antigen receptor (CAR) T-cell therapy may be a promising treatment option for patients with a recurrence of BPDCN. Here we retrospectively describe a successful case of treating a BPDCN recurrence six months after allo-HSCT with anti-CD123-CAR T-cell therapy followed by radiotherapy.
Case Presentation
In January 2018, a 10-year-old male patient had been experiencing bilateral knee joint pain for five months. The patient received a bone marrow (BM) smear and BM biopsy when he presented with anemia. Flow cytometry showed that 68% of the abnormal cells in his BM expressed CD123, CD56, CD4, CD10, and HLA-DR. The outcomes of BM histopathological analysis and immunohistochemistry showed positive CD56, CD4, CD43, CD123, TCL1, CD303, and negative CD3, CD5, cCD79a, MPO, CD34, and CD117. The patient was diagnosed with blastic plasmacytoid dendritic cell neoplasm (BPDCN). After two cycles of VDCP chemotherapy (vincristine, liposomal doxorubicin, cyclophosphamide, and prednisone), and two cycles of VDAP (vincristine, liposomal doxorubicin, cytarabine, and prednisone), the patient achieved his first complete remission (CR). However, he did not receive allo-HSCT therapy at this time. In May 2018, one month after his first CR, the patient presented with a scalp rash that was diagnosed as recurrent BPDCN by skin biopsy. At the same time, flow cytometry showed that 12% of the abnormal cells expressed the same phenotype as the abnormal cells previously found in his BM. His disease had recurred for the first time.
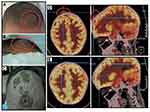
After one cycle of FAC (fludarabine, cytarabine, and cyclophosphamide) and two cycles of DOAME (dexamethasone, vincristine, cytarabine, mitoxantrone, and etoposide), the patient achieved his second CR. He subsequently received two cycles of VDMP (vincristine, dexamethasone, mitoxantrone, and pegaspargase) and two cycles of decitabine combined with CAG (aclacycin, cytarabine, and granulocyte colony-stimulating factor) followed by a haploid allo-HSCT (father to son) in January 2019. The patient developed skin grade II acute graft versus host disease (aGVHD), but maintained CR with complete donor chimerism for six months. However, in July 2019, painless nodules appeared in his head (Figure 1A). Positron emission tomography-computed tomography (PET-CT) showed that the metabolism of masses in his head increased with a standardized uptake value of 18.9 (Figure 1D). The patient was found to have 0.07% abnormal cells in his BM this time. His disease had recurred again six months after allo-HSCT.
The patient was enrolled in a clinical trial at the Department of Hematology in Tianjin First Center Hospital (Tianjin, China) and received anti-CD123-CAR T-cells expressing anti-CD123 scFv and 4-1BB-CD3ζ as well as costimulatory-activation domain therapy (ChiCTR1900022058). To manufacture CAR T, the generation and detection of anti-CD123-CAR T cells and the transduction efficiency peripheral blood mononuclear cells (PBMCs) of the patient were collected and isolated with a Ficoll density gradient centrifugation. CD3+ T cells were selected with CD3 microbeads (Miltenyi Biotec, Inc., Cambridge, MA, USA), stimulated by anti-CD3/anti-CD28 mAb-coated Human T-Expander beads (Cat. no. 11141D; Thermo Fisher Scientific, Inc., Waltham, MA, USA), and cultured in T-cell medium X-Vivo 15 (Lonza Group, Ltd., Basel, Switzerland) supplemented with 250 IU/mL interleukin-2 (IL-2; Proleukin; Novartis International AG, Basel, Switzerland). All the T cells (3x106) were transduced with a lentiviral vector encoding humanized CD123 CAR constructs (10µg; lenti-CD123-2rd-CAR) and cultured in media containing recombinant human IL-2 (30 U/mL). On the 14th day of cultivation, transduction efficiencies of anti-CD123-CAR were analyzed by flow cytometry (FCM) (BD Biosciences, San Jose, CA, USA). He received lymphodepleting chemotherapy with fludarabine (30 mg/m2/day) and cytarabine (600 mg/m2/day) from day −4 to day −2 and an infusion of anti-CD123-CAR T-cells (6 × 106 cells/kg) on day 0. The body temperature, the cytokine levels (measured by enzyme-linked immunoassay), the expansion of anti-CD123-CAR T-cells in the peripheral blood (measured by flow cytometry), and the level of CD123-CAR gene DNA (measured by real-time quantitative polymerase chain reaction) were observed from 0 to 28 days after anti-CD123-CAR T-cell infusion. The anti-CD123-CAR T-cell therapy was well tolerated in this patient with grade 1 cytokine release syndrome (CRS). The adverse events (AEs) were manifested by fever up to 39.0°C without chills three days after infusion, accompanied by slight dizziness and weakness. These AEs related to the anti-CD123-CAR T-cell therapy were relieved eight days after infusion. The highest serum level of interleukin 6 (IL-6) was only 8.02 pg/mL on day 4. There were no significant peaks in other cytokines such as the IL-8, IL-10 and tumor necrosis factor-α (TNF-α) (Figure 2A). Similar trends were observed in the proportion of anti-CD123-CAR T-cells and the level of CD123-CAR gene DNA in the peripheral blood (Figure 2B, C). There were no significant changes in the nodules 28 days after anti-CD123-CAR T-cell infusion (Figure 1B). However, there were no abnormal cells in his BM 14 days after the anti-CD123-CAR T-cell therapy.
The levels of serum cytokines and the level of CD123-CAR gene DNA in the peripheral blood were very low (Figure 2). Meanwhile, the proportion of anti-CD123-CAR T-cells in the peripheral blood was 2.8% 28 days after anti-CD123-CAR T-cell therapy (Figure 2B). The patient received a local radiotherapy for the nodules in his head. The radiation dose was 1.8 Gy each time, 16 times in total (28.8 Gy total dose). Fourteen days after radiotherapy, his temperature reached 39.2°C without rash, diarrhea, hematuria, or other aGVHD symptoms. These AEs were relieved in the next seven days. The proportion of anti-CD123-CAR T-cells, the level of CD123-CAR gene DNA, and the serum levels of cytokines in the peripheral blood reached higher peaks 42 days after anti-CD123-CAR T-cell infusion, or 14 days after radiotherapy (Figure 2). The nodules in his head disappeared gradually 28 days after radiotherapy (Figure 1C). No abnormal metabolism throughout his body could be identified by PET-CT (Figure 1E). There were no abnormal cells in his BM. Therefore, the patient achieved CR with complete donor chimerism again from the anti-CD123-CAR T-cell therapy followed by radiotherapy in September 2019.
The patient had progression-free survival (PFS) with no aGVHD in the following 30 days. Then, the patient received two infusions of donor stem cells (CD34+ cells were 1.38×106 cells/kg and 1.79×106 cells/kg) in the following two months. He developed grade I aGVHD and recovered promptly. To date, he has maintained PFS with complete donor chimerism for six months after the anti-CD123-CAR T-cell therapy followed by radiotherapy.
Discussion
The prognosis of BPDCN is very poor. High-dose chemotherapy followed by allo-HSCT therapy has the potential to improve the OS during the patient’s first CR.6,7 Allo-HSCT therapy is more effective than autologous HSCT therapy.8 In recent years, other treatments have been used for BPDCN, especially for recurrent/refractory (R/R) patients. These new treatments and clinical trials for R/R BPDCN patients included Elzonris (tagraxofusp SL-401), which targets CD123 on tumor cells regardless of primary patients or R/R patients,9 daratumumab, which targets CD38 on tumor cells for only a few patients,10 pralatrexate, which is used for progression of BPDCN,11 venetoclax, which promotes tumor cell apoptosis,12 bortezomib, which downregulates NF-kB activity,13 and 5ʹ-azacytidine, which reduces tumor cell methylation levels.14 Anti-CD123 chimeric antigen receptor (CAR) T cells have been used in pre-clinical studies of R/R AML therapy.15 Because of the high level expression of CD123 on BPDCN cells, anti-CD123-CAR T-cell therapy could be a possible treatment for these R/R BPDCN patients.
The mechanism of radiotherapy is to damage tumor cell DNA and to promote the release of tumor antigens locally. Increased levels of tumor antigens could promote the systemic T cell receptor (TCR) and mediate antitumor immune response.16,17 Low-dose radiation could sensitize the tumor cells to locally activated CAR-T cells in a model of pancreatic adenocarcinoma.18 In mouse glioma models, local radiotherapy combined with CAR T-cell therapy could play a synergistic role through promoting CAR T-cell migration to the tumor location and enhancing CAR T-cell activity.19 These findings suggest potential synergy between radiotherapy and CAR T-cell therapy.
In this report, the BPDCN patient relapsed after allo-HSCT. Because of the poor curative effect of anti-CD123-CAR T-cell therapy, we chose to perform radiotherapy 28 days after CAR T-cell therapy when the CAR T cells were still at 2.8% in the peripheral blood. Two weeks after his local radiotherapy, with the proportion of anti-CD123-CAR T cells reaching a higher peak, the nodules on his head disappeared gradually. This could be similar to what has been observed in mouse models. The radiotherapy releases tumor antigens, promotes anti-CD123-CAR T-cell migration to the tumor location, and enhances CAR T-cell activity. This mechanism could explain the results seen in this case.
Conclusion
Anti-CD123-CAR T-cell therapy followed by radiotherapy for a recurrence of blastic plasmacytoid dendritic cell neoplasm patient after allo-HSCT is effective. Further studies on the mechanisms of CAR-T cell therapy combined with or followed by radiotherapy are needed.
Abbreviations
BPDCN, blastic plasmacytoid dendritic cell neoplasm; allo-HSCT, allogeneic hematopoietic stem cell transplantation; AEs, adverse events; CR, complete remission; PFS, progression-free survival; CAR, chimeric antigen receptor; DNA, desoxyribonucleic acid; AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; BM, bone marrow; aGVHD, acute graft versus host disease; PET-CT, positron emission tomography-computed tomography; CRS, cytokine release syndrome; TCR, T cell receptor.
Ethics and Consent Statement
This study was approved by the Medical Ethics Committee of the Tianjin First Center Hospital (Tianjin, China) (Approval No. of Ethics Committee: 2015002X). Patient’s father signed the written informed consent for enrollment in this clinical trial (ChiCTR1900022058).
Consent for Publication
Patient’s father signed the written informed agreement to use and publish the patient’s data, case details, and images for study.
Acknowledgments
The authors acknowledge Dr. Yu-ming Li, the department chairperson of the Department of Hematology of Tianjin First Central Hospital, for his assistance in critical evaluation of the supporting literature while drafting this manuscript.
Author Contributions
Qi Deng contributed to the concept and design and interpretation of data. All authors contributed to acquisition, analysis, and interpretation of data. Yi-li Jiang drafted the article as the first author. All authors contributed equally in drafting and revising the article and gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi:10.1182/blood-2016-03-643544
2. Feuillard J, Jacob MC, Valensi F, et al. Clinical and biologic features of CD4(+)CD56(+) malignancies. Blood. 2002;99(5):1556–1563. doi:10.1182/blood.V99.5.1556
3. Pagano L, Valentini CG, Pulsoni A, et al. Blastic plasmacytoid dendritic cell neoplasm with leukemic presentation: an Italian multicenter study. Haematologica. 2013;98(2):239–246. doi:10.3324/haematol.2012.072645
4. Garnache-Ottou F, Feuillard J, Ferrand C, et al. Extended diagnostic criteria for plasmacytoid dendritic cell leukaemia. Br J Haematol. 2009;145(5):624–636. doi:10.1111/j.1365-2141.2009.07679.x
5. Pagano L, Valentini CG, Grammatico S, Pulsoni A. Blastic plasmacytoid dendritic cell neoplasm: diagnostic criteria and therapeutical approaches. Br J Haematol. 2016;174(2):188–202. doi:10.1111/bjh.14146
6. Aoki T, Suzuki R, Kuwatsuka Y, et al. Long-term survival following autologous and allogeneic stem cell transplantation for blastic plasmacytoid dendritic cell neoplasm. Blood. 2015;125(23):3559–3562. doi:10.1182/blood-2015-01-621268
7. Dietrich S, Andrulis M, Hegenbart U, et al. Blastic plasmacytoid dendritic cell neoplasia (BPDC) in elderly patients: results of a treatment algorithm employing allogeneic stem cell transplantation with moderately reduced conditioning intensity. Biol Blood Marrow Transplant. 2011;17(8):1250–1254. doi:10.1016/j.bbmt.2010.12.706
8. Kharfan-Dabaja MA, Al Malki MM, Deotare U, et al. Haematopoietic cell transplantation for blastic plasmacytoid dendritic cell neoplasm: a North American multicentre collaborative study. Br J Haematol. 2017;179(5):781–789. doi:10.1111/bjh.14954
9. Pemmaraju N, Lane AA, Sweet KL, et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2019;380(17):1628–1637. doi:10.1056/NEJMoa1815105
10. Iversen KF, Holdgaard PC, Preiss B, Nyvold CG, Plesner T. Daratumumab for treatment of blastic plasmacytoid dendritic cell neoplasm. A single-case report. Haematologica. 2019;104(9):e432–e433. doi:10.3324/haematol.2018.214635
11. Sato S, Tanaka E, Tamai Y. Blastic plasmacytoid dendritic cell neoplasm with response to pralatrexate. Ann Hematol. 2019;98(3):801–803. doi:10.1007/s00277-019-03611-3
12. Agha ME, Monaghan SA, Swerdlow SH. Venetoclax in a patient with a blastic plasmacytoid dendritic-cell neoplasm. N Engl J Med. 2018;379(15):1479–1481. doi:10.1056/NEJMc1808354
13. Philippe L, Ceroi A, Bole-Richard E, et al. Bortezomib as a new therapeutic approach for blastic plasmacytoid dendritic cell neoplasm. Haematologica. 2017;102(11):1861–1868. doi:10.3324/haematol.2017.169326
14. Khwaja R, Daly A, Wong M, et al. Azacitidine in the treatment of blastic plasmacytoid dendritic cell neoplasm: a report of 3 cases. Leuk Lymphoma. 2016;57(11):2720–2722. doi:10.3109/10428194.2016.1160084
15. Arcangeli S, Rotiroti MC, Bardelli M, et al. Balance of anti-CD123 chimeric antigen receptor binding affinity and density for the targeting of acute myeloid leukemia. Mol Ther. 2017;25(8):1933–1945. doi:10.1016/j.ymthe.2017.04.017
16. Sridharan V, Margalit DN, Lynch SA, et al. Definitive chemoradiation alters the immunologic landscape and immune checkpoints in head and neck cancer. Br J Cancer. 2016;115(2):252–260. doi:10.1038/bjc.2016.166
17. Tang C, Wang X, Soh H, et al. Combining radiation and immunotherapy: a new systemic therapy for solid tumors? Cancer Immunol Res. 2014;2(9):831–838. doi:10.1158/2326-6066.CIR-14-0069
18. DeSelm C, Palomba ML, Yahalom J, et al. Low-dose radiation conditioning enables CAR T cells to mitigate antigen escape. Mol Ther. 2018;26(11):2542–2552. doi:10.1016/j.ymthe.2018.09.008
19. Weiss T, Weller M, Guckenberger M, Sentman CL, Roth P. NKG2D-based CAR T cells and radiotherapy exert synergistic efficacy in glioblastoma. Cancer Res. 2018;78(4):1031–1043. doi:10.1158/0008-5472.CAN-17-1788
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.