Back to Journals » Clinical Interventions in Aging » Volume 14
Carotid artery calcification score and its association with cognitive impairment
Authors Chu Z , Cheng L, Tong Q
Received 31 October 2018
Accepted for publication 13 December 2018
Published 18 January 2019 Volume 2019:14 Pages 167—177
DOI https://doi.org/10.2147/CIA.S192586
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Zhou Chu, Liu Cheng, Qiao Tong
Department of Vascular Surgery, The Affiliated Drum Tower Hospital of Nanjing University Medical School, Nanjing 210008, Jiangsu, China
Purpose: To retrospectively investigate the possible association between carotid artery calcification score (CS) and cognitive impairment in carotid artery stenosis (CAS) patients.
Patients and methods: Carotid artery was measured in 102 patients with cervical carotid arteries using Color Doppler ultrasound, multi-detector row spiral CT angiography and MRI scanning. Correlation analysis between CSs obtained by MD CT and cognitive scores was performed, and the correlation between CSs and vascular stenosis degree and MRI-measured plaque histological (lipid-rich necrotic nucleus [LRNC], intraplaque hemorrhage and fibrous cap surface rupture) and morphological parameters (lumen area [LA], wall area [WA], total area of blood vessels [TVA], plaque burden [PB]) was analyzed. Follow-up review analysis was conducted on 38 postoperative patients.
Results: Significant negative correlation was discovered between CS value and cognitive scores in CAS patients (R=-0.359, P<0.001), which did not exist in postoperative patients (P=0.348); CS value also showed significant correlation with WA (R=0.521, P=0.042), TVA (R=0.215, P=0.017) and PB (R=0.237, P=0.003) and had a certain predictive value for the occurrence probability of carotid plaque LRNC (P=0.029, AUC =0.780) in preoperative patients.
Conclusion: Carotid artery CSs have significant correlation with cognitive scores, which could be used as risk factor for early screening of cognitive impairment in CAS patients. The possible mechanism may be related to the calcification impact on the plaque burden.
Keywords: calcification score, carotid artery stenosis, cognitive impairment, plaque burden
Introduction
The annual incidence of ischemic stroke has raised up to 393 per 100,000 people in China, almost half of which were resulted from CAS formed by the carotid artery atherosclerotic plaque.1 In addition to increasing the risk of stroke, many lines of evidence have shown that CAS is also closely related to cognitive decline, mainly in different levels of memory, emotion, execution, space and other cognitive impairments.2,3 Kim et al4 revealed that patients with severe unilateral carotid stenosis (70%–90%) have significant cognitive impairment, with a lower memory score than the control group. Another study conducted by Mathiesen et al indicated that asymptomatic CAS patients had significantly weaker performance in attention, psychomotor speed, memory and operation ability than the normal control group.5
It is important to confirm the change of VCI as the symptom of carotid stenosis and to seek early redress of brain hypoperfusion and cerebral infarction resulting from vascular factors, which may delay the occurrence of dementia in patients.6,7 Also, seeking for early risk factors may have significant clinical implications.8
The main mechanism of CAS is the formation of carotid atherosclerotic plaque. Compared to lipid, fibrous tissue or other plaque contents, plaque calcification can be more easily found and quantified in clinical testing. The current MDCT has been able to do precision line scanning on the calcified region.9,10 In this study, we investigated the possible correlation between carotid artery CS and cognitive impairment of CAS patients, and did further research on its possible mechanism.
Material and methods
General information
This article is a retrospective study. Ranging from July 2015 to August 2018, all 165 patients were diagnosed as carotid stenosis in the program of “green channel of stroke” in our hospital. Another 40 healthy subjects were selected as normal control group.
Forty-two patients were eliminated for failing to meet the entry criteria: nervous system symptoms of incentives conflicted with carotid stenosis (cardiac thrombosis, intracranial mass, intracranial stenosis, lacunar cerebral infarction and other intracranial small vessel disease); bilateral limb dysfunction; posterior circulation symptoms and non-atherosclerosis-related vascular stenosis. To avoid the possible interference caused by contralateral CAS, 21 patients with bilateral carotid stenosis were not enrolled.
A total of 102 cases were enrolled: 57 cases of left carotid stenosis and 45 cases of right carotid stenosis. There were 72 males and 30 females, aged from 43 to 85 years old and 38 patients with postoperative follow-up research; patients’ residences ranged from Jiangsu, Anhui, Zhejiang, Shandong and Shanghai to other regions in China.
Protocol approval and patient consent
This study was approved by the institutional review board of the Nanjing Drum Tower Hospital. Written informed consent according to the Declaration of Helsinki was obtained from each patient involved in this study.
Inspection method
Carotid artery MDCT
Patients were scanned from the aortic arch to the white matter using GE Lightspeed VCT 64-slice spiral CT. Prospective ECG gating techniques were used, and scan parameters were as follows: 120 kV of tube voltage, 50 mA of tube current, 0.4 seconds of gantry rotation time, 0.75 mm of reconstruction thickness, 0.75 mm of slice gap, 512×512 of matrix size, 250–300 mm of the field of view. Scanning time ranged from 8 to 10 seconds and ensured that the intake scanning will be completed during one breath-hold. All image acquisition and processing was performed at GE ADW 4.3 WorkStation; the analysis software is Smart Score 3.5. Areas larger than 0.5 mm2 of higher CT value than the threshold value set as 90 HU, were identified as calcified lesion. Image window width was set to 600–1,000 H, and the window level was set to 150–400 H. Artery calcification regions from the common carotid artery to the skull of the internal carotid artery were treated together and CS value was calculated.9 Patients were divided into three groups according to CS values1: mild CS group: CS value <300;2 moderate CS group: CS value between 300 and 500;3 severe CS group: CS value >500.
Carotid artery MRI
MRI examination is produced by a 3.0 T MR scanner (General Electric Company, Boston, MA, USA). Carotid MR examinations ranging from the common carotid artery to the skull of the internal carotid artery were measured using carotid surface coils and four different contrast-weighted images were acquired: T1WI, proton-weighted imaging (PDWI), T2WI and three-dimensional time hop angiography (3D TOF MRA). Scanning parameters were as follows: T1WI: 2D fast spin echo sequence, four inversion recoveries black blood technique, repeat time (TR) =800 ms, echo time (TE) =8.8 ms. PDWI/T2WI: double echo technique, TR =3,000 ms, PD =13.1 ms for PDWI and TE =56.9 ms for T2WI. 3D TOF MRA: TR =29 ms, TE =2.1 ms, reversal angle 20°. The imaging field of view =14 cm, the imaging matrix is 256×256, the pixels in the layer are 0.55×0.55 mm2, the layer thickness is 2 mm, the number of layers is 16 and the upper and lower coverage is 32 mm. Carotid morphological measurements: LA, WA, TVA (sum of LA and WA), plaque burden = (sum of vessel outer diameter area and sum of vessel inner diameter area)/the sum of the outer diameter areas of the blood vessels. Histological indicators of carotid plaque: LRNC, IPH and FCSR. The average of the measured carotid artery parameters (the sum of the measurements of each level divided by the number of layers) is the product of this indicator for the patient. If the LRNC, IPH or FCSR occurs in any part of the carotid artery, the patient’s index is positive. Analytical images were construed by two systematically trained radiologists. Images were analyzed by software CASCADE.
Neuropsychological tests
MMSE is the most commonly used for acute neuropathological evaluation, while its detection rate for MCI is not high. MoCA has a higher sensitivity for MCI diagnosis and a wider screening for cognitive impairment groups. All the enrolled patients were assessed by trained knowledge neurologists based on MMSE and MoCA of their emotional and cognitive situation. Interval between two tests was more than 1 hour in order to avoid the same content repeated within a short time measurement, with an aim to reduce the effectiveness of learning and reduce errors. Scale of evaluation criteria were as follows1: MoCA scale was utilized based on “Beijing Union Medical College Hospital recommended version”;2 MMSE scale was utilized based on “Beijing Union Medical College Hospital Alzheimer’s Disease research group recommended version”: group with no education experience ≤19, group with primary school education experience ≤22 and group with not less than secondary education experience ≤26 represent patients with cognitive dysfunction. Patients were divided into four groups according to the assessment results1: normal cognitive function groups: MMSE + MoCA scores ≥52;2 mild cognitive impairment group: MMSE + MoCA scores between 42 and 52;3 moderate cognitive impairment group: MMSE + MoCA scores between 33 and 41;4 severe cognitive impairment group: MMSE + MoCA scores between 0 and 32.11
Carotid artery color Doppler ultrasound
All patients enrolled were examined by color Doppler ultrasound. Degree of stenosis was calculated by formulas referred to NASCET: degree of stenosis = (1 − internal carotid artery blood flow narrowest width/normal stenosis distal internal carotid artery diameter) × 100%. In accordance with the above formula, CAS degree can be divided into four levels: 1) mild stenosis: artery diameter narrowing <30%; 2) moderate stenosis: artery diameter narrowing ranges from 30% to 69%; 3) severe stenosis: artery diameter narrowing ranges from 70% to 99% and 4) complete occlusion.
Statistical analysis
All statistical analyses were performed using SPSS for Windows (Release 19.0; SPSS, Chicago, IL, USA). Continuous data are presented as mean ± SD (X ± S). Discrete data are presented as counts and percentages. In terms of difference, the t-test was used to compare the paired measurement data, and the chi-squared test was used to compare the rate and categorical variables. In terms of relevance, Pearson correlation analysis was used. The ROC curve and AUC were calculated by logistic regression analysis. All statistics were performed with a 0.05 level of significance.
Results
Baseline characteristics
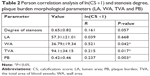
Demographic and clinical features of the patients are displayed in Table 1. The mean age was 61.5 years for CAS and 57.5 for control groups. The prevalence of hypertension and BMI was not significantly different between groups. Smoking was significantly more common in the case group. There was statistically significant difference between the two groups in CS value, carotid morphology and histological measurements. No statistically significant difference was observed between left and right CAS groups in clinical features (Specific data are listed in the Supplementary materials).
Correlation analysis between CS value and cognitive score
CS value is obviously skewed, so it is natural logarithmic transformed before analysis: ln(CS +1). ln(CS +1) and cognitive score were analyzed with Pearson correlation analysis, and the result (R=−0.359, P<0.001, Figure 1A) suggests that there is significant negative correlation between CS value and cognitive scores.
One hundred two cases enrolled were divided into four groups based on MMSE + MoCA test scores: 1) normal cognition group, n=17, 2) mild cognitive impairment group, n=44, 3) moderate cognitive impairment group, n=41, 4) severe cognitive disorders group, n=0.
One-way ANOVA of CS value with different cognitive groups indicated that CS had significant differential expression in the 3 groups (P=0.019<0.05, Figure 2A). This suggests that CS may be recognized as the risk factor for cognitive change.
Correlation analysis between CS value and CAS degree
All cases enrolled were divided into groups based on NASCET standard: 1) mild stenosis group, n=31; 2) moderate stenosis group, n=68; 3) severe stenosis group, n=24 and 4) total occlusion group, n=0.
Pearson correlation analysis was performed between ln(CS +1) and carotid stenosis. No statistically significant correlation was found between patients with stenosis (R=0.161, P=0.057, Table 2). One-way ANOVA results suggested that CS value did not have statistically significant differences among the three groups of CAS (P=0.097>0.05, Figure 2B).
The CS value was less correlated with the degree of carotid stenosis, so the mechanism of its association with cognitive changes may be independent of the degree of stenosis.
Correlation analysis between CS value and carotid plaque burden
The quantification of carotid plaque burden is currently divided into morphological parameters: LA, WA, TVA and PB; and histological parameters: LRNC, IPH and FCSR.
Correlation analysis between CS value and carotid morphological parameters
Because the carotid morphological parameters were continuous variables, the correlation between ln(CS +1) and carotid morphological parameters was analyzed by Pearson correlation analysis. The results in Table 2 showed significantly positive correlation between CS value and WA, TVA and PB (P<0.05), while no statistical correlation was observed between CS value and LA (P=0.668>0.05).
Difference analysis between CS value and carotid histological parameters
One hundred two cases enrolled in this study were divided into 3 groups based on CS values1: 1) mild, n=21; 2) moderate, n=52 and 3) severe, n=29.
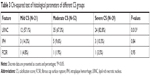
Because the carotid histology parameter is discrete data, it is chi-square tested after grouping by CS value. Table 3 shows that LRNC had significant differential expression between CAS groups with different CS values (P=0.013<0.05), while there was no such difference between IPH and FCSR (P>0.05).
The ROC curve of carotid plaque tissue was drawn by CS per 100 value to judge the predictive value of CS for carotid plaque tissue. With the increase of CS value, the probability of carotid plaque LRNC significantly increased, while IPH and FCSR did not increase significantly (P>0.05, Figure 3). This indicates that the carotid plaque burden increases at the level of both histology and morphology with the increase of CS value.
Surgical patients’ postoperative follow-up data
We performed CEA on patients who met the indications for surgery. Indications for CEA: absolute indications: patients with symptomatic CAS have been noninvasive checked of CAS ≥70% or patients have received contrast examination suggesting CAS more than 50%.
Relative indication: 1) asymptomatic carotid stenosis and noninvasive examination of stenosis ≥70% or angiography found stenosis ≥60%; 2) asymptomatic carotid stenosis and non-invasive examination of stenosis <70%, but angiography or other examinations suggest that the stenosis is in an unstable state; 3) symptomatic carotid stenosis, patients have been noninvasive checked of CAS degree between 50%–69%. At the same time, the symptomatic patients in the treatment center are expected to have a perioperative stroke incidence and mortality rate of <6%, while asymptomatic patients expected perioperative stroke incidence and mortality rate <3% and the patient’s life expectancy >5 years.12
After that, we conducted MDCT scanning and MMSE + MoCA examination on 38 patients at 24 weeks after their successful clinical operation.13
No significant correlation between cognitive scores with CS values (P=0.348>0.05, Figure 1B) was discovered. Postoperative MMSE review showed that patients had a significant improvement in the delayed memory score, but the indications in the remaining items and total scores were not obvious. Postoperative MoCA was not significantly improved in terms of computational power, verbal fluency, memory and orientation. Other achievements were significantly improved, and the total score of patients was significantly higher than that before surgery (Figure 4).
Discussion
Large population studies have shown that carotid stenosis is an independent risk factor for VCI.14,15 There are still no clear guidance or advice for clinical operation in treating cognitive impairment in asymptomatic CAS patients. Therefore, it is beneficial to confirm the change of cognitive function as a symptom of carotid stenosis, so that we could include cognitive impairment as the criteria for perioperative risk calculation and benefit from clinical operation.
Clinical diagnosis of cognitive impairment relies on examinations of cognitive function. In the present study, a combination of MMSE and MoCA was designed to detect the change in patients’ cognitive function. Consistent with the results of Watanabe et al,16 different evaluation results were observed after CEA between MMSE and MoCA. No significant difference was observed between pre- and post-MMSE scores, supporting the view that MMSE may be not suitable for detecting post-CEA cognition improvement.17 Different results between these two tests could partly be explained, according to a previous study,18 by the different content. The sub-tests of “orientation”, “registration” and “calculation” major proportions in the MMSE scale were not significantly damaged in patients with mild cognitive impairment. MoCA included not only content evaluated by MMSE but also focused on test items including “execution”, “abstraction”, “verbal fluency” and “visuo-executive”, which were improved in MoCA after CEA in the present study. Meanwhile, routine tests cannot accurately detect specific brain functional state, neither can they rule out the learning and memory effects during comparative examinations. Therefore, looking for the index factor to assess the degree of cognitive impairment in patients with CAS has positive significance for clinical benefit.
A variety of different assessment methods for artery calcification have been used for early carotid atherosclerosis research, and the conclusions about the relationship between ischemic encephalopathy and calcification were not consistent.19–25 Nandalur et al26 suggested that carotid artery calcification on CT may independently predict stroke risk. Bos et al27 have found a marginal relevance between carotid artery calcification and cognitive decline in a large community-based study with cardiovascular risk factors adjusted. CS value was chosen to be our prospective research content for the degree of carotid plaque calcification, which has been widely used as a quantitative indicator of coronary atherosclerotic plaques.25,28 Okada et al’s research found that aortic root CS value as an independent factor for predicting major adverse cardiac events in Familial Hypercholesterolemia.29 Also, recent research has found an association between metformin use and below-the-knee arterial CS value in type 2 diabetic patients.30 Our results showed that CS value has a significant correlation with the cognitive score. CS values of different cognitive status patients have a significant difference, suggesting that CS value could be independent risk factor for VCI, with possible value in the preoperative evaluation of CAS patients. Compared to MRI scanning and cognitive assessment, CS value has the advantage of operability, economic and low error.
We confirmed a significant correlation between CS value and cognitive impairment in non-stroke patients enrolled, which was consistent with Reis’s research results that coronary artery CS correlated with cognitive decline.31 With regard to the disappearance of correlation in postoperative patients, there was a significant reduction in CS value after surgical treatment, but CAS patient’s postoperative cognition may be in a state of “stable cognitive decline” or “mild improvement”. It is not realistic to produce cognitive changes that match CS value.
Cerebral microemboli and hypoperfusion with or without silent brain infarctions are two important mechanisms of VCI in CAS patients.24,32 The specific mechanism of CS value’s correlation with cognitive changes is still not clear. Literatures points out that there is a wide variation in the degree of calcification in the same degree of atherosclerotic lesions.33 The results of our study are similar: there is no significant correlation between CS and carotid stenosis degree, and there is no significant differential expression in patients with different degrees of stenosis. Therefore, the correlation between CS values and cognitive improvement may be independent of hypoperfusion.
Another mechanism associated with cognitive changes, cerebral microemboli, is primarily determined by the stability of atheromatous plaques,34 and also related to the total plaque burden of the arterial system.7,35 This study found that CS values were significantly positively correlated with the morphological indicators of carotid plaque burden (WA, TVA and PB), and that CS values were highly predictive of LRNC, a histological indicator of plaque stability. This suggests that CS value may be associated with total plaque burden and stability. Studies have shown that the degree of carotid calcification is related to the downstream microemboli in stroke patients.36 For the mechanism, literatures have indicated that shear stress grows larger at the edge of fibrous cap or calcified nodules protruding into the lumen of the fibrous cap in the atherosclerotic plaque; furthermore, long-tern high pressure results in poor stability.37–40
Despite the promising results, we also noted the current MDCT reconstruction measurements for assessment of CAS does not completely conform to the actual situation, which requires us to narrow the error range by ensuring enough window width in the assessment and division of CAS. Limited postoperative follow-up control, shortage of enough cognitive group division basis, and lack of experiment supporting the mechanism hypothesis all needed to be improved in future research.
Conclusion
We discovered the risk factor role of CS value in cognitive impairment of CAS patients by researching the correlation between CS value and cognitive scores. We also confirmed the significant correlation between CS value and carotid artery PB, which could be the possible mechanism of CS value’s association with cognitive impairment, but CS value shows no significant correlation with postoperative cognitive scores. The application of CS value as risk factor in clinical screening of CAS patients may have a positive effect on their clinical benefit.
Abbreviations
AUC, area under the curve; BMI, body mass index; CAS, carotid artery stenosis; CEA, carotid endarterectomy; CS, calcification score; FCSR, fibrous cap surface rupture; IPH, intraplaque hemorrhage; LA, lumen area; LRNC, lipid-rich necrotic nucleus; MCI, mild cognitive impairment; MDCT, multi-detector row spiral CT; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment; NASCET, North American Symptomatic Carotid Endarterectomy Trial Collaborators criteria; NPTs, neuropsychological tests; PB, plaque burden; ROC, receiver operating characteristic; ROI, region of interest; TVA, total area of blood vessels; VCI, vascular cognitive impairment; WA, wall area.
Disclosure
The authors report no conflicts of interest in this work.
References
Ren L, Bai L, Wu Y, et al. Prevalence of and risk factors for cognitive impairment among elderly without cardio- and cerebrovascular diseases: a population-based study in rural China. Front Aging Neurosci. 2018;10:62. | ||
Sztriha LK, Nemeth D, Sefcsik T, Vecsei L. Carotid stenosis and the cognitive function. J Neurol Sci. 2009;283(1–2):36–40. | ||
Popovic IM, Lovrencic-Huzjan A, Simundic AM, Popovic A, Seric V, Demarin V. Cognitive performance in asymptomatic patients with advanced carotid disease. Cogn Behav Neurol. 2011;24(3):145–151. | ||
Kim JE, Lee BR, Chun JE, et al. Cognitive dysfunction in 16 patients with carotid stenosis: detailed neuropsychological findings. J Clin Neurol. 2007;3(1):9–17. | ||
Mathiesen EB, Waterloo K, Joakimsen O, Bakke SJ, Jacobsen EA, Bønaa KH. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromsø study. Neurology. 2004;62(5):695–701. | ||
Silvestrini M, Viticchi G, Falsetti L, et al. The role of carotid atherosclerosis in Alzheimer’s disease progression. J Alzheimers Dis. 2011;25(4):719–726. | ||
Hansen T, Kilander L, Ahlström H, Lind L. Total atherosclerotic burden measured by magnetic resonance imaging is related to five-year decline in cognitive function. Clin Physiol Funct Imaging. 2018;38(3):373–377. | ||
Howard DP, van Lammeren GW, Rothwell PM, et al. Symptomatic carotid atherosclerotic disease: correlations between plaque composition and ipsilateral stroke risk. Stroke. 2015;46(1):182–189. | ||
Jiang JD, Li YB, Tan LL, Li SX, Liang TJ, Zhou SP. Diagnosis of carotid artery diseases using MSCTA. Chin J CT MRI. 2006;4(2):4–6. Chinese. | ||
Mujaj B, Lorza AM, van Engelen A, et al. Comparison of CT and CMR for detection and quantification of carotid artery calcification: the Rotterdam Study. J Cardiovasc Magn Reson. 2017;19(1):28. | ||
Ning C, Li H. Overview of the research and application of the Montreal Cognitive Assessment (MoCA). Chin J Nerv Ment Dis. 2009;35(10):632–634. | ||
Chen Z, Yang YG. Guidelines for the diagnosis and treatment of carotid stenosis. Chin J Vasc Surg. 2017;2(3):169–175. | ||
Wang Q, Zhou M, Zhou Y, Ji J, Raithel D, Qiao T. Effects of carotid endarterectomy on cerebral reperfusion and cognitive function in patients with high grade carotid stenosis: a perfusion weighted magnetic resonance imaging study. Eur J Vasc Endovasc Surg. 2015;50(1):5–12. | ||
Sahathevan R, Brodtmann A, Donnan GA. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7(1):61–73. | ||
Siuda J, Gorzkowska A, Opala G, Ochudło S. Vascular risk factors and intensity of cognitive dysfunction in MCI. J Neurol Sci. 2007;257(1–2):202–205. | ||
Watanabe J, Ogata T, Hamada O, et al. Improvement of cognitive function after carotid endarterectomy–a new strategy for the evaluation of cognitive function. J Stroke Cerebrovasc Dis. 2014;23(6):1332–1336. | ||
Fearn SJ, Hutchinson S, Riding G, Hill-Wilson G, Wesnes K, McCollum CN. Carotid endarterectomy improves cognitive function in patients with exhausted cerebrovascular reserve. Eur J Vasc Endovasc Surg. 2003;26(5):529–536. | ||
Roalf DR, Moberg PJ, Xie SX, Wolk DA, Moelter ST, Arnold SE. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9(5):529–537. | ||
Babiarz LS, Yousem DM, Bilker W, Wasserman BA. Middle cerebral artery infarction: relationship of cavernous carotid artery calcification. Am J Neuroradiol. 2005;26(6):1505–1511. | ||
de Weert TT, Cakir H, Rozie S, et al. Intracranial internal carotid artery calcifications: association with vascular risk factors and ischemic cerebrovascular disease. AJNR Am J Neuroradiol. 2009;30(1):177–184. | ||
Hunt KJ, Pankow JS, Offenbacher S, et al. B-mode ultrasound-detected carotid artery lesions with and without acoustic shadowing and their association with markers of inflammation and endothelial activation: the atherosclerosis risk in communities study. Atherosclerosis. 2002;162(1):145–155. | ||
Mak KF, Wong CW, Yau KW. Computed tomography evaluation of intracranial atherosclerosis in Chinese patients with transient ischemic attack or minor ischemic stroke – its distribution and association with vascular risk factors. J Stroke Cerebrovasc. 2009;18(2):158–163. | ||
Chang DH, Brinkmann S, Smith L, et al. Calcification score versus arterial stenosis grading: comparison of two CT-based methods for risk assessment of anastomotic leakage after esophagectomy and gastric pull-up. Ther Clin Risk Manag. 2018;14:721. | ||
Osawa K, Nakanishi R, Mcclelland RL, et al. Ischemic stroke/transient ischemic attack events and carotid artery disease in the absence of or with minimal coronary artery calcification: Results from the multi-ethnic study of atherosclerosis. Atherosclerosis. 2018;275:22–27. | ||
Wang J, Wang Y, Zhou R. Enhancement with coronary artery calcification score in detection of coronary heart disease by myocardial perfusion SPECT imaging. Chin J Nucl Med Mol Imaging. 2017;37(5):274–278. | ||
Nandalur KR, Baskurt E, Hagspiel KD, et al. Carotid artery calcification on CT may independently redict stroke risk. Am J Roentgenol. 2006;186(2):547–552. | ||
Bos D, Vernooij MW, Elias-Smale SE, et al. Atherosclerotic calcification relates to cognitive function and to brain changes on magnetic resonance imaging. Alzheimers Dement. 2012;8(5 Suppl):S104–S111. | ||
Crichton GE, Elias MF, Davey A, Alkerwi A. Cardiovascular health and cognitive function: the Maine-Syracuse Longitudinal Study. PLoS One. 2014;9(3):e89317. | ||
Okada H, Tada H, Hayashi K, et al. Aortic root calcification score as an independent factor for predicting major adverse cardiac events in familial hypercholesterolemia. J Atheroscler Thromb. 2018;25(7):634–642. | ||
Mary A, Hartemann A, Liabeuf S, et al. Association between metformin use and below-the-knee arterial calcification score in type 2 diabetic patients. Cardiovasc Diabetol. 2017;16(1):24. | ||
Reis JP, Launer LJ, Terry JG, et al. Subclinical atherosclerotic calcification and cognitive functioning in middle-aged adults: the CARDIA study. Atherosclerosis. 2013;231(1):72–77. | ||
Rodriguez-Granillo GA, Carrascosa P, Bruining N, Waksman R, Garcia-Garcia HM. Defining the non-vulnerable and vulnerable patients with computed tomography coronary angiography: evaluation of atherosclerotic plaque burden and composition. Eur Heart J-Card Img. 2016;17(5):481–491. | ||
Rumberger JA, Schwartz RS, Simons DB, Sheedy PF, Edwards WD, Fitzpatrick LA. Relation of coronary calcium determined by electron beam computed tomography and lumen narrowing determined by autopsy. Am J Cardiol. 1994;73(16):1169–1173. | ||
Boden WE, O’Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356(15):1503–1516. | ||
Ejiri K, Miyoshi T, Kohno K, et al. Protective effect of remote ischemic preconditioning on myocardial damage after percutaneous coronary intervention in stable angina patients with complex coronary lesions – subanalysis of a randomized controlled trial. Circ J. 2018;82(7):1788–1796. | ||
Wu XH, Chen XY, Fan YH, Leung TW, Wong KS. High extent of intracranial carotid artery calcification is associated with downstream microemboli in stroke patients. J Stroke Cerebrovasc Dis. 2017;26(2):442–447. | ||
Loree HM, Kamm RD, Stringfellow RG, Lee RT. Effects of fibrous cap thickness on peak circumferential stress in model atherosclerotic vessels. Circ Res. 1992;71(4):850–858. | ||
Vengrenyuk Y, Carlier S, Xanthos S, et al. A hypothesis for vulnerable plaque rupture due to stress-induced debonding around cellular microcalcifications in thin fibrous caps. Proc Natl Acad Sci U S A. 2006;103(40):14678–14683. | ||
Fukumoto Y, Hiro T, Fujii T, et al. Localized elevation of shear stress is related to coronary plaque rupture. J Am Coll Cardiol. 2008;51(6):645–650. | ||
Shah PK. Mechanisms of plaque vulnerability and rupture. J Am Coll Cardiol. 2003;41(4):S15–S22. |
Supplementary materials
A pre-experiment was executed to rule out personal error in data processing. Two qualified physicians not informed in advance were selected to process data analysis of the same ten samples, with results obtained from data paired sample test:
  | Table S1 Paired sample test results of the two physicians’ analysis |
To avoid false positive results caused by CT scan box settings, all specimens of patients with CAS underwent calcium staining (Figure S1). Stained slices of calcium were sent to IMAGE PLUS quantization process; no significant difference was found between calcification score and calcium staining.
  | Figure S1 Carotid plaque calcium staining. Stained deposition of calcium presented as black annular, and plaque rupture occurred in the “right shoulder” of fibrous cap. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.