Back to Journals » Therapeutics and Clinical Risk Management » Volume 16
Cardiovascular Outcomes with Sacubitril-Valsartan in Heart Failure: Emerging Clinical Data
Authors Cuthbert JJ , Pellicori P , Clark AL
Received 24 May 2020
Accepted for publication 28 June 2020
Published 4 August 2020 Volume 2020:16 Pages 715—726
DOI https://doi.org/10.2147/TCRM.S234772
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Joseph J Cuthbert,1 Pierpaolo Pellicori,2 Andrew L Clark1
1Department of Academic Cardiology, Hull York Medical School, Hull and East Yorkshire Medical Research and Teaching Centre, Castle Hill Hospital, Kingston upon Hull HU16 5JQ, UK; 2Robertson Institute of Biostatistics and Clinical Trials Unit, University of Glasgow, University Avenue, Glasgow G12 8QQ, UK
Correspondence: Joseph J Cuthbert
Department of Academic Cardiology, Hull York Medical School, Hull and East Yorkshire Medical Research and Teaching Centre, Castle Hill Hospital, Cottingham, Kingston upon Hull HU16 5JQ, UK
Tel + 44 (0)1482 461776
Fax + 44 (0)1482 461779
Email [email protected]
Abstract: One of the defining features of heart failure (HF) is neurohormonal activation. The renin-angiotensin-aldosterone-system (RAAS) and sympathetic nervous system (SNS) cause vasoconstriction and fluid retention and, in response, the secretion of natriuretic peptides (NPs) from volume and pressure-overloaded myocardium promotes vasodilation and diuresis. Inhibition of the RAAS with either angiotensin-converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB) has been the cornerstone of medical treatment for HF with a reduced ejection fraction (HFrEF) but, until recently, it was unclear how the beneficial effects of NPs may be augmented in patients with HF. Neprilysin, a metalloproteinase widely distributed throughout the body, plays a role in degrading the gross excess of circulating NPs in patients with HF. Early studies of neprilysin inhibition suggested possible physiological benefits. In 2014, the PARADIGM-HF trial found that sacubitril-valsartan, a combination of the ARB valsartan, and the neprilysin inhibitor sacubitril, was superior to enalapril in patients with HFrEF, reducing the relative risk of cardiovascular (CV) death or first hospitalisation with HF by 20%. Almost half of the patients with HF symptoms have a “preserved” ejection fraction (HFpEF); however, the PARAGON-HF study found that sacubitril-valsartan in patients with LVEF ≥ 45% had no effect on CV death or first and recurrent hospitalisations with HF compared to valsartan. Guidelines across the world have changed to include sacubitril-valsartan for patients with HFrEF yet, nearly 6 years after PARADIGM-HF, there is still uncertainty as to when and in whom sacubitril-valsartan should be started. Furthermore, there may yet be subsets of patients with HFpEF who might benefit from treatment with sacubitril-valsartan. This review will describe the mechanisms behind the outcome benefit of sacubitril-valsartan in patients with HFrEF and to consider its future role in the management of patients with HF.
Keywords: sacubitril-valsartan, heart failure, PARADIGM-HF, PARAGON-HF, natriuretic peptide, angiotensin receptor neprilysin inhibitor
Plain Language Summary
Heart failure (HF) is characterised by breathlessness that is due, in part, to activation of the renin-angiotensin-aldosterone system (RAAS) which causes widespread vasoconstriction and fluid retention. To counteract these harmful effects the heart secretes hormones called natriuretic peptides (NPs) which increase diuresis. Blockade of the RAAS has been at the centre of medical treatment of HF for the last three decades and the prognosis for patients with HF due to an impaired left ventricle (HFrEF) has vastly improved as a result. Comparatively, treatments to enhance the beneficial effects of NPs have been less successful. However, in 2014, the PARADIGM-HF trial of over 8000 patients with HFrEF found that a new drug, sacubitril-valsartan, was superior to the gold standard treatment, enalapril. Sacubitril-valsartan is a combination drug of two compounds: sacubitril, which acts increase levels of circulating NPs by preventing their enzymatic breakdown and valsartan, which acts to lessen the effects of the RAAS. Despite this success, it is still not clear as to which patients with HF sacubitril-valsartan should be given. Additionally, further trials have found that sacubitril-valsartan may not be effective for all patients with HF. This review will explore how sacubitril-valsartan might benefit some patients with HF (and not others), and how it might fit into medical practice in years to come.
Introduction
Heart failure is characterised by neurohormonal activation. Activation of the renin-angiotensin-aldosterone (RAAS) and sympathetic nervous (SNS) systems is triggered by cardiac dysfunction, whilst the natriuretic peptide (NP) system is activated by fluid retention and consequent myocardial stretch. To some extent the two systems counteract each other with the RAAS and SNS tending to cause vasoconstriction and fluid retention, and the NP system causing vasodilation and diuresis.
Patients with HF are classified into different phenotypes based on their left ventricular ejection fraction (LVEF) on imaging: LVEF <40% - HF with reduced ejection fraction (HFrEF); LVEF 40–49% - HF with mid-range ejection fraction (HFmrEF) and LVEF ≥50% - HF with preserved ejection fraction (HFpEF), which has therapeutic implications.1 However, activation of the SNS and RAAS and high levels of NPs occur in each phenotype.2
Treatment of patients with HFrEF with medications that inhibit the RAAS and SNS is one of the great success stories of modern medicine;3 the PARADIGM-HF trial of sacubitril-valsartan is one of the more recent chapters.4
Sacubitril-valsartan is a “first-in-class” angiotensin receptor neprilysin inhibitor (ARNI) combining a neprilysin inhibitor, sacubitril, with the angiotensin receptor blocker (ARB) valsartan.5 The purpose of this review is to describe and understand the mechanisms behind the outcome benefit of sacubitril-valsartan in patients with HFrEF, and to consider its future role in the management of patients with HF.
Natriuretic Peptides and Neprilysin in Heart Failure
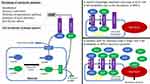
NPs – predominantly atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) – are hormones that are synthesised as pro-peptides and stored in granules within myocytes.6 In response to myocardial stretch due to increased intracardiac pressure or volume (or both), the pro-peptides are cleaved by enzymes – corin and furin for ANP and BNP, respectively – and are secreted; the biological effects are then mediated via transmembrane receptors (NPRs).7 Binding to NPR-A causes increased intracellular cyclic guanosine monophosphate (cGMP) second messenger activity which, via intracellular signalling cascades, causes increased renal sodium and water excretion, increased glomerular filtration, vasodilation, and reduced renin and aldosterone secretion (Figure 1).8
NPs are degraded via two pathways. The predominant pathway in normal physiology is via binding to a “clearance receptor”, NPR-C (the most abundant NPR), thus removing NPs from the circulation.7,8 Only once NPR-C receptors become saturated does the second pathway, hydrolysis by neprilysin, begin to play a significant role in NP clearance.9 Neprilysin is a zinc-dependant metalloproteinase distributed throughout the body and has many substrates aside from NPs including angiotensin II (ATII), bradykinin, substance P, adrenomedullin, and oxytocin among others.10 In patients with symptomatic HF who have very high levels of NPs,11 NPR-C is saturated (and may also be down-regulated due to chronic exposure to NPs),12 and thus degradation by neprilysin becomes the main pathway for NP clearance (Figure 1).13,14
From OVERTURE to PARADIGM-HF
Attempts to harness the beneficial effects of NPs in patients with cardiovascular disease has been a focus of research for over 20 years with varying degrees of success (Table 1). Despite promising Phase I and II results,15,16 trials of exogenous NPs failed to demonstrate morbidity or mortality benefit in patients admitted with HF.17,18 Trials of oral or intravenous neprilysin inhibitors were tried with the notion that blocking breakdown of NPs would result in higher circulating levels and hence a diuresis. However, because neprilysin also degrades ATII,19 neprilysin inhibitors are unlikely to benefit patients with heart failure without concurrent RAAS inhibition. Used as single agents, they yielded disappointing results during pre-clinical and early clinical stages.20–24
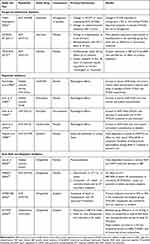 |
Table 1 Table of Studies Which Led to the Development of Combined Angiotensin Receptor Blocker and Neprilysin Inhibitor |
The IMPRESS26 and OVERTURE27 trials of omapatrilat (an oral inhibitor of both angiotensin converting enzyme (ACE) and neprilysin)25 found possible outcome benefit over ACEI in patients with HFrEF. However, development of the drug was stopped following a high incidence of angio-oedema in the omapatrilat arm of the above trials and the OCTAVE trial of omapatrilat in patients with hypertension (N~25,000).28
Sacubitril-Valsartan
Heart Failure with a Reduced Ejection Fraction
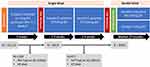
The PARADIGM-HF investigators enrolled 8442 ambulatory patients with symptomatic HFrEF (mean age 63 years, LVEF 29%, median N terminal pro-BNP (NTproBNP) 1631 pg/mL in the treatment arm). Following a run-in period during which all patients were separately titrated to target doses of enalapril and then sacubitril-valsartan, those without adverse effects to either were randomised to either sacubitril-valsartan 97/103mg twice daily (BD) or enalapril 10mg BD (Figure 2).4
Treatment with sacubitril-valsartan was associated with a 20% relative risk reduction (RRR) in the primary endpoint, a composite of cardiovascular death or first hospitalisation with heart failure compared to enalapril (Table 2).4,29 The outcome benefit was the same regardless of heart failure aetiology30 or age,31 and sacubitril-valsartan reduced the risk of recurrent admissions not just for heart failure,32 but for any cause.33
 |
Table 2 Outcomes in the PARADIGM-HF and PARAGON-HF |
A putative placebo analysis of the PARADIGM-HF data (using outcome data from the placebo arms of the SOLVD and CHARM-alternative studies as the control group)34 suggested that the number needed to treat to reduce all-cause mortality at 5 years with sacubitril-valsartan was 11, an effect size second only to beta-blockers amongst other treatments for HFrEF.35
Although there was no difference in the pre-specified outcome of new-onset diabetes mellitus (DM) between the treatment groups in PARADIGM-HF (2.9% vs 3.2%),4 sacubitril-valsartan was associated with better glycaemic control in patients with DM than enalapril.36 Similarly, while there was no difference in the pre-specified outcome of time to worsening renal function (end-stage renal failure, from >60 mL/min/1.73 m2 to <60 mL/min/1.73 m2 or a decrease in the estimated glomerular filtration rate (eGFR) of ≥50%, or by >30 mL/min/1.73 m2 from baseline), the rate of decline of eGFR was slower in patients taking sacubitril-valsartan compared to enalapril (−1.6 mL/min/1.73m2/year vs −2.0 mL/min/1.73m2/year; P<0.001), regardless of the presence of renal impairment at baseline.37
There was a greater fall in KCCQ score in the enalapril group (indicating poorer quality of life) than in the sacubitril-valsartan group,4 equivalent to the difference of 9 years of ageing.38 While this last detail is eye-catching, the difference between the groups was small (1.64 at 8 months; P=0.001) with only a weak inverse relationship between NTproBNP levels and KCCQ score.4 Whether such a small change in KCCQ score equates to any noticeable change for the patient is unknown.
PIONEER-HF and TRANSITION
PARADIGM-HF established the safety and efficacy of sacubitril-valsartan in patients with chronic heart failure, the PIONEER-HF and TRANSITION studies sought to do the same in patients who had recently been admitted with heart failure.39,40
PIONEER-HF was a head-to-head comparison of enalapril and sacubitril-valsartan initiated in hospital. Sacubitril-valsartan was associated with a greater reduction in NTproBNP between weeks 4 and 8 than enalapril (primary end-point); a significant difference was detected after 1 week of treatment.41
TRANSITION was a comparison of pre- versus post-discharge initiation of sacubitril-valsartan. There was no significant difference in the primary endpoint of the proportion of patients who reached target dose sacubitril-valsartan (97/103mg BD) by 10 weeks (45.4% vs 50.7%).42
Approximately half of the patients in PIONEER-HF and TRANSITION were ACEI or ARB naïve prior to randomisation, but this had no impact on tolerability nor the chance of achieving target dose.40,41 Neither study was powered to detect outcome benefit but sacubitril-valsartan was associated with a 42% RRR in cardiovascular death or readmission with heart failure in PIONEER-HF (P=0.007).43
Safety and Tolerability of Sacubitril-Valsartan in Patients with HFrEF
The most common adverse event with sacubitril-valsartan is symptomatic hypotension but consequent discontinuation in PARADIGM-HF was uncommon (Table 3).4,40,41,44 However, it is important to note that the run in phase of the study design means that patients eventually randomised in the trial proper were pre-selected to be tolerant of both drugs (Figure 2).4 In PARADIGM-HF, the rate of discontinuation was higher in the enalapril group than the sacubitril-valsartan group (12.3% vs 10.7%; P=0.03)4 and was lower than was seen in most other landmark studies (Table 4).4,45-49
 |
Table 3 Adverse Event Rates in PARADIGM-HF, PIONEER-HF and TRANSITION |
 |
Table 4 Rate of Discontinuation Due to an Adverse Event in Landmark Studies of Medical Therapies for Heart Failure Due to a Reduced Ejection Fraction |
Target dose sacubitril-valsartan was not particularly well tolerated: almost half (42%) of patients randomised to sacubitril-valsartan in PARADIGM-HF, all of whom started at the target dose of 97/103 mg BD, required dose reduction within the first year, and only a minority returned to target dose.50 The proportion of patients who achieved target dose by the end of the study in PIONEER-HF (55.2%) and TRANSITION (47.6%) was similarly modest.40,41 Slower up-titration from a lower starting dose of 50mg BD may enable more patients to reach target dose.51
Another substrate of neprilysin is beta amyloid protein accumulation of which is related to Alzheimer’s dementia.52 There is thus a theoretical concern that neprilysin inhibition may increase the risk of dementia.53,54, PARADIGM-HF showed no evidence of increased risk, but was, perhaps, too short to identify a trend. The PERSPECTIVE study is designed to investigate a possible link. The primary endpoint is the change in cognition from baseline to 3 years in a population of patients with HF and normal ejection fraction.55
Mechanisms of Benefit
One curiosity of the PARADIGM-HF results is the robust benefit offered by a drug that combines an ARB (a drug class with no consistent mortality benefit in HFrEF)56,57 with a medication that increases already very high levels of NPs. The mechanism of benefit is likely to be multifactorial and is not fully understood.
Reduced Cardiac Fibrosis and Reverse Remodelling
Myocardial fibrosis is central to the development of heart failure. It is driven by various peptides and hormones secreted in response to overlapping haemodynamic, neurohormonal and pro-fibrotic triggers.58 Sacubitril-valsartan reduces levels of pro-fibrotic biomarkers (aldosterone, soluble ST2, tissue inhibitor of matrix metalloproteinase, galectin-3, and N-terminal propeptide of collagen I and collagen III) to a greater extent than enalapril,59–61 and may inhibit cardiac fibroblast activity.62 Sacubitril-valsartan increases LVEF, and reduces left ventricular and atrial volumes more than either ACEI or ARB,63,64 even in patients with a normal LVEF.65
It may be that the anti-fibrotic and reverse remodelling effects are, in part, responsible for the lower risk of ventricular arrhythmia (VA) and sudden death – the most common mode of death in PARADIGM-HF – seen in patients randomised to sacubitril-valsartan. In a small study in patients with HFrEF and an implantable cardioverter-defibrillator, treatment with sacubitril-valsartan was associated with a lower burden of VA.66
Effects on Mitral Regurgitation
Functional mitral regurgitation (MR) is common amongst patients with HFrEF,67 and is associated with adverse outcome despite optimal treatment.68 The PRIME study found a greater reduction in the area and volume of the MR jet with sacubitril-valsartan compared to valsartan in 117 patients with significant functional MR (LVEF 25–49%);69 it is likely the reduction in MR severity resulted from a decrease in LV volume.70
Other Effects of Neprilysin Inhibition
Neprilysin has a greater affinity for bradykinin, adrenomedullin and substance P than it does for NPs,14,71 all of which have potentially beneficial physiological effects for patients with HF:
- Bradykinin activates B2 kinin receptors triggering release of nitric oxide (NO), prostacyclin and endothelium-derived hyperpolarising factor which cause vasodilation.72,73
- Adrenomedullin causes vasodilation, natriuresis and diuresis and decreases pulmonary capillary wedge pressure in patients with HF.74
- Substance P binds to neurokinin-1 receptors causing NO-mediated vasodilation.75 It increases myocardial perfusion and reduces hypoxic cellular damage in rat models of ischaemia-reperfusion.76
Uric acid (UA) is a product of purine metabolism by xanthine oxidase (XO) which also produces harmful reactive oxygen species; high UA levels may reflect increased oxidative stress.77 Sacubitril-valsartan was associated with reduced UA levels in PARADIGM-HF,78 which might reflect reduced action of XO. The mechanism is unknown.
Pre-Empting Worsening Heart Failure
During a decompensation episode, NPR-C receptors become saturated and neprilysin becomes the main pathway for NP degradation (Figure 1). A possible mechanism for benefit might thus be that, compared to a patient taking enalapril, a patient taking sacubitril-valsartan has higher levels of NPs levels at the onset of an episode of decompensation, perhaps lessening the severity of the episode.14,79 In PARADIGM-HF, patients taking sacubitril-valsartan were less likely to have worsening symptoms; to require inotropic support during admission; to be readmitted for heart failure; or to have their diuretic treatment increased as an outpatient compared to patients taking enalapril.33,80,81
Heart Failure with a Preserved Ejection Fraction
Some reports suggest that up to half of patients with HF have a normal ejection fraction on echocardiography – HFpEF,82,83 – a condition for which no treatments are known to improve outcome (other than in the specific case of underlying amyloidosis).84 The PARAMOUNT study showed that sacubitril-valsartan reduced NTproBNP and left atrial diameter and volume more than valsartan alone after 12 weeks in patients with HFpEF, although there was no significant difference between the groups after 36 weeks.64
The PARAGON-HF investigators randomised 4822 patients (mean age 72, LVEF ≥45%, median NTproBNP 904 ng/L, NYHA class II–IV, with either left ventricular hypertrophy or a dilated left atrium as evidence of cardiac dysfunction) to either sacubitril/valsartan 97/103mg BD or valsartan 160mg BD.85
The primary endpoint was a composite of first and recurrent hospitalisations for heart failure or cardiovascular death. All-cause mortality rate, change in QoL from baseline to 8 months assessed by the KCCQ, and an improvement in NYHA class from baseline to 8 months were among secondary endpoints.85
Sacubitril-valsartan had no effect on the rate of the primary composite outcome or its components (Table 2).85 Patients taking sacubitril-valsartan were more likely to have a reduction in their NYHA class (odds ratio 1.45 (95% confidence interval (CI) 1.13–1.86)), but other secondary endpoints were unaffected.85 Pre-specified sub-group analysis found possible outcome benefit for patients with LVEF 45–57% (RRR for the primary endpoint 22%), and for women (RRR 27%).85
Heart Failure with a Mid-Range Ejection Fraction
Data from PARAGON-HF suggest sacubitril-valsartan may confer benefit to patients with LVEF between 45% and 57%. The PARALLAX study of sacubitril-valsartan vs “standard therapy” for comorbidities in patients with HFpEF, and the PERSPECTIVE study described above, will include patients with an LVEF >40%. However, neither include morbidity and mortality endpoints and more work will be required.
These are muddy waters. Dividing patients into three diagnostic categories based on a poorly reproducible technique – namely, LVEF on echocardiography86 – is arbitrary at best. The same patient with the same symptoms might be described as having HFrEF on one day with one operator or HFpEF on a different day with a different operator: discrete boundaries between categories based on LVEF do not represent a biological reality.
What can be said is that there is a consistent general trend that the worse the left ventricular systolic function, the greater the likelihood of benefit from standard heart failure treatment: this is true for ACE inhibitors, beta-blockers and mineralocorticoid receptors, and now seems to be true for ARNIs as well.
Real-World Data and Future Perspectives
One of the frequent criticisms of heart failure trials is that the sample populations rarely reflect “real-world” patients. For example: the average age of a patient with heart failure in the UK is 77 years at diagnosis,87 whereas the average age of a patient in PARADIGM-HF was over 10 years younger.4 However, data from real-world populations suggest that treatment with sacubitril-valsartan is associated with significant reductions in NTproBNP,88 increases in LVEF,88 and reductions in the rate of heart failure hospitalisation compared to pre-initiation.89,90 The ARIADNE registry, which aims to describe current prescribing trends with regards to sacubitril-valsartan, is recruiting and will highlight any discrepancies.91
The role of sacubitril-valsartan is yet to be properly defined: the European Society of Cardiology (ESC) guidelines state that sacubitril-valsartan can replace “optimal” dose ACEI if patients remain symptomatic with raised NP levels,1 and the National Institute of Health and Clinical Excellence (NICE) in the UK state that sacubitril-valsartan is recommended for patients with ongoing symptoms despite “stable” dose ACEI or ARB.92 “Optimal” and “stable” are entirely different concepts and practice varies greatly as a result: estimates of the proportion of patients eligible for sacubitril-valsartan vary from 75% to 21%.93–95
In a patient not taking a RAAS inhibitor at diagnosis, it is not clear what clinical benefit titrating medications to the maximum tolerated dose then switching to sacubitril-valsartan might have over starting sacubitril-valsartan from scratch. One limiting factor might be cost: in the UK, fifty-six 10 mg enalapril tablets costs £3.98 compared to £91.56 for the same number of 97/103mg sacubitril-valsartan tablets.96,97
A significant proportion of patients in PARADIGM-HF were unable to tolerate target doses during the course of the trial despite tolerating target doses of both medications during the short run-in phase (Figure 2). Whether low-dose sacubitril-valsartan is superior to low-dose enalapril in patients who have never been able to tolerate target doses of RAAS inhibitors is unanswered by PARADIGM-HF. Approximately 4 in 5 patients with HFrEF in Europe are on sub-target doses of RAAS inhibitor.98 The proportion of patients who cannot tolerate target doses seems to be high.
Matters have been further complicated by the success of the DAPA-HF study (N=4744, average age 66, 98% NYHA II–III, median NTproBNP 1428 ng/L) which found a reduced risk of worsening heart failure or cardiovascular death with dapagliflozin, a sodium-glucose transport protein-2 inhibitor (SGLT2-I), compared to placebo (16% vs 21%; P<0.001).99 Only 10% of patients in the dapagliflozin arm were taking sacubitril/valsartan and quite how SGLT2-Is will fit into future heart failure guidelines is unclear: is the benefit conferred by dapagliflozin greater than, less than or incremental to that of sacubitril/valsartan in patients otherwise taking a beta-blocker, MRA and ACEI or ARB?
Conclusion
Sacubitril-valsartan improves symptoms and outcome for patients with HFrEF compared with standard therapy with ACEI. The mechanism of benefit is complex and multifactorial. Different regulatory bodies have different criteria for considering sacubitril-valsartan, and there is still uncertainty as to when and in whom sacubitril-valsartan should be started. There may yet be subsets of patients with HF and LVEF >40% who might benefit from sacubitril-valsartan.
Disclosure
Professor Andrew L Clark and Joseph J Cuthbert report personal fees from Novartis, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Ponikowski P, Voors AA, Anker SD, et al. ESC scientific document group. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
2. Vergaro G, Aimo A, Prontera C, et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int J Cardiol. 2019;296:91–97. doi:10.1016/j.ijcard.2019.08.040
3. Burnett H, Earley A, Voors AA, et al. Thirty years of evidence on the efficacy of drug treatments for chronic heart failure with reduced ejection fraction: a network meta-analysis. Circ Heart Fail. 2017;10(1):e003529. doi:10.1161/CIRCHEARTFAILURE.116.003529
4. McMurray JJ, Packer M, Desai AS, et al. PARADIGM-HF investigators and committees angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004. doi:10.1056/NEJMoa1409077
5. Gu J, Noe A, Chandra P, et al. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi). J Clin Pharmacol. 2010;50(4):401–414. doi:10.1177/0091270009343932
6. Maisel AS, Duran JM, Wettersten N. Natriuretic peptides in heart failure: atrial and B-type natriuretic peptides. Heart Fail Clin. 2018;14(1):13–25. doi:10.1016/j.hfc.2017.08.002
7. Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: hormones secreted from the heart. Peptides. 2019;111:18–25. doi:10.1016/j.peptides.2018.05.012
8. Woodard GE, Rosado JA. Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol. 2008;268:59–93.
9. Okolicany J, McEnroe GA, Koh GY, Lewicki JA, Maack T. Clearance receptor and neutral endopeptidase-mediated metabolism of atrial natriuretic factor. Am J Physiol. 1992;263(3):F546–53. doi:10.1152/ajprenal.1992.263.3.F546
10. Bayes-Genis A, Barallat J, Richards AM. A test in context: neprilysin: function, inhibition, and biomarker. J Am Coll Cardiol. 2016;68(6):639–653. doi:10.1016/j.jacc.2016.04.060
11. Braunwald E. Biomarkers in heart failure. N Engl J Med. 2008;358(20):2148–2159. doi:10.1056/NEJMra0800239
12. Tsutamoto T, Kanamori T, Morigami N, Sugimoto Y, Yamaoka O, Kinoshita M. Possibility of downregulation of atrial natriuretic peptide receptor coupled to guanylate cyclase in peripheral vascular beds of patients with chronic severe heart failure. Circulation. 1993;87(1):70–75. doi:10.1161/01.CIR.87.1.70
13. Maack T. The broad homeostatic role of natriuretic peptides. Arq Bras Endocrinol Metabol. 2006;50(2):198–207. doi:10.1590/S0004-27302006000200006
14. Singh JSS, Burrell LM, Cherif M, Squire IB, Clark AL, Lang CC. Sacubitril/valsartan: beyond natriuretic peptides. Heart. 2017;103(20):1569–1577. doi:10.1136/heartjnl-2017-311295
15. Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA. 2002;287(12):1531–1540. doi:10.1001/jama.287.12.1531
16. Anker SD, Ponikowski P, Mitrovic V, Peacock WF, Filippatos G. Ularitide for the treatment of acute decompensated heart failure: from preclinical to clinical studies. Eur Heart J. 2015;36(12):715–723. doi:10.1093/eurheartj/ehu484
17. O’Connor CM, Starling RC, Hernandez AF, et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365(1):32–43. doi:10.1056/NEJMoa1100171
18. Packer M, O’Connor C, JJV M, et al. Effect of ularitide on cardiovascular mortality in acute heart failure. N Engl J Med. 2017;376(20):1956–1964. doi:10.1056/NEJMoa1601895
19. Dalzell JR, Seed A, Berry C, et al. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014;32(1):13–18. doi:10.1111/1755-5922.12053
20. Northridge DB, Jardine AG, Alabaster CT, et al. Effects of UK 69 578: a novel atriopeptidase inhibitor. Lancet. 1989;2(8663):591–593. doi:10.1016/S0140-6736(89)90714-9
21. Gros C, Souque A, Schwartz JC, et al. Protection of atrial natriuretic factor against degradation: diuretic and natriuretic responses after in vivo inhibition of enkephalinase (EC 3. 4.24.11)by acetorphan. Proc Natl Acad Sci USA. 1989;86(19):7580–7584. doi:10.1073/pnas.86.19.7580
22. Kahn JC, Patey M, Dubois-Rande JL, et al. Effect of sinorphan on plasma atrial natriuretic factor in congestive heart failure. Lancet. 1990;335(8681):118–119. doi:10.1016/0140-6736(90)90595-V
23. Good JM, Peters M, Wilkins M, Jackson N, Oakley CM, Cleland JG. Renal response to candoxatrilat in patients with heart failure. J Am Coll Cardiol. 1995;25(6):1273–1281. doi:10.1016/0735-1097(94)00561-4
24. Cleland JGF, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. Lancet. 1998;351(9116):1657–1658. doi:10.1016/S0140-6736(05)77712-6
25. Liao WC, Vesterqvist O, Delaney C, et al. Pharmacokinetics and pharmacodynamics of the vasopeptidase inhibitor, omapatrilat in healthy subjects. Br J Clin Pharmacol. 2003;56(4):395–406. doi:10.1046/j.1365-2125.2003.01888.x
26. Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet. 2000;356(9230):615–620. doi:10.1016/S0140-6736(00)02602-7
27. Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the omapatrilat versus enalapril randomized trial of utility in reducing events (OVERTURE). Circulation. 2002;106(8):920–926. doi:10.1161/01.CIR.0000029801.86489.50
28. Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the omapatrilat cardiovascular treatment vs. enalapril (OCTAVE) trial. Am J Hypertens. 2004;17(2):103–111. doi:10.1016/j.amjhyper.2003.09.014
29. Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J. 2015;36(30):1990–1997. doi:10.1093/eurheartj/ehv186
30. Balmforth C, Simpson J, Shen L, et al. Outcomes and effect of treatment according to etiology in HFrEF: an analysis of PARADIGM-HF. JACC Heart Fail. 2019;7(6):457–465. doi:10.1016/j.jchf.2019.02.015
31. Jhund PS, Fu M, Bayram E, et al. PARADIGM-HF investigators and committees. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J. 2015;36(38):2576–2584. doi:10.1093/eurheartj/ehv330
32. Mogensen UM, Gong J, Jhund PS, et al. Effect of sacubitril/valsartan on recurrent events in the prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF). Eur J Heart Fail. 2018;20(4):760–768. doi:10.1002/ejhf.1139
33. Desai AS, Claggett BL, Packer M, et al. Influence of sacubitril/valsartan (LCZ696) on 30-day readmission after heart failure hospitalization. J Am Coll Cardiol. 2016;68(3):241–248. doi:10.1016/j.jacc.2016.04.047
34. McMurray J, Packer M, Desai A, et al. PARADIGM-HF committees and investigators. A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J. 2015;36(7):434–439. doi:10.1093/eurheartj/ehu455
35. Srivastava PK, Claggett BL, Solomon SD, et al. Estimated 5-year number needed to treat to prevent cardiovascular death or heart failure hospitalization with angiotensin receptor-neprilysin inhibition vs standard therapy for patients with heart failure with reduced ejection fraction: an analysis of data from the PARADIGM-HF trial. JAMA Cardiol. 2018;3(12):1226–1231. doi:10.1001/jamacardio.2018.3957
36. Seferovic JP, Claggett B, Seidelmann SB, et al. Effect of sacubitril/valsartan versus enalapril on glycaemic control in patients with heart failure and diabetes: a post-hoc analysis from the PARADIGM-HF trial. Lancet Diabetes Endocrinol. 2017;5(5):333–340. doi:10.1016/S2213-8587(17)30087-6
37. Damman K, Gori M, Claggett B, et al. Renal effects and associated outcomes during angiotensin-neprilysin inhibition in heart failure. JACC Heart Fail. 2018;6(6):489–498. doi:10.1016/j.jchf.2018.02.004
38. Chandra A, Lewis EF, Claggett BL, et al. Effects of sacubitril/valsartan on physical and social activity limitations in patients with heart failure: a secondary analysis of the PARADIGM-HF trial. JAMA Cardiol. 2018;3(6):498–505. doi:10.1001/jamacardio.2018.0398
39. Velazquez EJ, Morrow DA, DeVore AD, et al. Rationale and design of the comParIson of sacubitril/valsartaN versus enalapril on effect on nt-pRo-bnp in patients stabilized from an acute heart failure episode (PIONEER-HF) trial. Am Heart J. 2018;198:145–151. doi:10.1016/j.ahj.2018.01.004
40. Pascual-Figal D, Wachter R, Senni M, et al. Rationale and design of TRANSITION: a randomized trial of pre-discharge vs. post-discharge initiation of sacubitril/valsartan. ESC Heart Fail. 2018;5(2):327–336. doi:10.1002/ehf2.12246
41. Velazquez EJ, Morrow DA, DeVore AD, et al. PIONEER-HF investigators. Angiotensin-neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539–548. doi:10.1056/NEJMoa1812851
42. Wachter R, Senni M, Belohlavek J, et al. Initiation of sacubitril/valsartan in haemodynamically stabilised heart failure patients in hospital or early after discharge: primary results of the randomised TRANSITION study. Eur J Heart Fail. 2019;21(8):998–1007. doi:10.1002/ejhf.1498
43. Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139(19):2285–2288. doi:10.1161/CIRCULATIONAHA.118.039331
44. Vardeny O, Claggett B, Kachadourian J, et al. Incidence, predictors, and outcomes associated with hypotensive episodes among heart failure patients receiving sacubitril/valsartan or enalapril: the paradigm-hf trial (prospective comparison of angiotensin receptor neprilysin inhibitor with angiotensin-converting enzyme inhibitor to determine impact on global mortality and morbidity in heart failure). Circ Heart Fail. 2018;11(4):e004745.
45. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the cooperative north scandinavian enalapril survival study (CONSENSUS). N Engl J Med. 1987;316(23):1429–1435. doi:10.1056/NEJM198706043162301
46. Investigators SOLVD, Yusuf S, Pitt B, Davis CE, Hood WB, Cohn JN. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325(5):293–302.
47. Packer M, Fowler MB, Roecker EB, et al., Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) Study Group. Effect of carvedilol on the morbidity of patients with severe chronic heart failure: results of the carvedilol prospective randomized cumulative survival (COPERNICUS) study. Circulation. 2002;106(17):2194–2199. doi:10.1161/01.CIR.0000035653.72855.BF
48. Pitt B, Zannad F, Remme WJ, et al. Wittes J for the randomized aldactone evaluation study investigators. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341(10):709–717. doi:10.1056/NEJM199909023411001
49. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364(1):11–21. doi:10.1056/NEJMoa1009492
50. Vardeny O, Claggett B, Packer M, et al.. Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) Investigators. Efficacy of sacubitril/valsartan vs. enalapril at lower than target doses in heart failure with reduced ejection fraction: the PARADIGM-HF trial. Eur J Heart Fail. 2016;18(10):1228–1234. doi:10.1002/ejhf.580
51. Senni M, McMurray JJ, Wachter R, et al. Initiating sacubitril/valsartan (LCZ696) in heart failure: results of TITRATION, a double-blind, randomized comparison of two uptitration regimens. Eur J Heart Fail. 2016;18(9):1193–1202. doi:10.1002/ejhf.548
52. Bateman RJ, Munsell LY, Morris JC, Swarm R, Yarasheski KE, Holtzman DM. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12(7):856–861. doi:10.1038/nm1438
53. Meilandt WJ, Cisse M, Ho K, et al. Neprilysin overexpression inhibits plaque formation but fails to reduce pathogenic Abeta oligomers and associated cognitive deficits in human amyloid precursor protein transgenic mice. version 2. J Neurosci. 2009;29(7):1977–1986. doi:10.1523/JNEUROSCI.2984-08.2009
54. Langenickel TH, Tsubouchi C, Ayalasomayajula S, et al. The effect of LCZ696 (sacubitril/valsartan) on amyloid-β concentrations in cerebrospinal fluid in healthy subjects. Br J Clin Pharmacol. 2016;81(5):878–890. doi:10.1111/bcp.12861
55. The United States National Library of Medicine. Clinicaltrials.gov. Efficacy and safety of LCZ696 compared to valsartan on cognitive function in patients with chronic heart failure and preserved ejection fraction (PERSPECTIVE). Available from: https://clinicaltrials.gov/ct2/show/NCT02884206.
56. Granger CB, McMurray JJ, Yusuf S, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function intolerant to angiotensin-converting-enzyme inhibitors: the CHARM-alternative trial. Lancet. 2003;362(9386):772–776. doi:10.1016/S0140-6736(03)14284-5
57. McMurray JJ, Ostergren J, Swedberg K, et al. CHARM investigators and committees. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-added trial. Lancet. 2003;362(9386):713–767. doi:10.1016/S0140-6736(03)14283-3
58. González A, Schelbert EB, Díez J, Butler J. Myocardial interstitial fibrosis in heart failure: biological and translational perspectives. J Am Coll Cardiol. 2018(71):1696–1706.
59. O’Meara E, Prescott MF, Claggett B, et al. Independent prognostic value of serum soluble ST2 measurements in patients with heart failure and a reduced ejection fraction in the PARADIGM-HF trial (prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure). Circ Heart Fail. 2018;11(5):e004446.
60. Zile MR, O’Meara E, Claggett B, et al. Effects of sacubitril/valsartan on biomarkers of extracellular matrix regulation in patients with HFrEF. J Am Coll Cardiol. 2019;73(7):795–806. doi:10.1016/j.jacc.2018.11.042
61. Morrow DA, Velazquez EJ, DeVore AD, et al. Cardiovascular biomarkers in patients with acute decompensated heart failure randomized to sacubitril-valsartan or enalapril in the PIONEER-HF trial. Eur Heart J. 2019;40(40):3345–3352. doi:10.1093/eurheartj/ehz240
62. Burke RM, Lighthouse JK, Mickelsen DM, Small EM. Sacubitril/valsartan decreases cardiac fibrosis in left ventricle pressure overload by restoring PKG signaling in cardiac fibroblasts. Circ Heart Fail. 2019;12(4):e005565. doi:10.1161/CIRCHEARTFAILURE.118.005565
63. Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8(13):e012272. doi:10.1161/JAHA.119.012272
64. Januzzi JL
65. Solomon SD, Zile M, Pieske B, et al. Prospective comparison of ARNI with ARB on management of heart failUre with preserved ejectioN fracTion (PARAMOUNT) investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a Phase 2 double-blind randomised controlled trial. Lancet. 2012;380(9851):1387–1395. doi:10.1016/S0140-6736(12)61227-6
66. Martens P, Nuyens D, Rivero-Ayerza M, et al. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol. 2019;108(10):1074–1082. doi:10.1007/s00392-019-01440-y
67. Trichon BH, Felker GM, Shaw LK, Cabell CH, O’Connor CM. Relation of frequency and severity of mitral regurgitation to survival among patients with left ventricular systolic dysfunction and heart failure. Am J Cardiol. 2003;91(5):538–543. doi:10.1016/S0002-9149(02)03301-5
68. Asgar AW, Mack MJ, Stone GW. Secondary mitral regurgitation in heart failure: pathophysiology, prognosis, and therapeutic considerations. J Am Coll Cardiol. 2015;65(12):1231–1248. doi:10.1016/j.jacc.2015.02.009
69. Kang DH, Park SJ, Shin SH, et al. Angiotensin receptor neprilysin inhibitor for functional mitral regurgitation. Circulation. 2019;139(11):1354–1365. doi:10.1161/CIRCULATIONAHA.118.037077
70. Mullens W, Martens P. Sacubitril/valsartan to reduce secondary mitral regurgitation. Circulation. 2019;139(11):1366–1370. doi:10.1161/CIRCULATIONAHA.118.038135
71. Hubers SA, Brown NJ. Combined angiotensin receptor antagonism and neprilysin inhibition. Circulation. 2016;133(11):1115–1124. doi:10.1161/CIRCULATIONAHA.115.018622
72. Pelc LR, Gross GJ, Warltier DC. Mechanism of coronary vasodilation produced by bradykinin. Circulation. 1991;83(6):2048–2056. doi:10.1161/01.CIR.83.6.2048
73. Kon V, Fogo A, Ichikawa I, Hellings SE, Bills T. Bradykinin causes selective efferent arteriolar dilation during angiotensin I converting enzyme inhibition. Kidney Int. 1993;44(3):545–550. doi:10.1038/ki.1993.279
74. Nagaya N, Satoh T, Nishikimi T, et al. Hemodynamic, renal, and hormonal effects of adrenomedullin infusion in patients with congestive heart failure. Circulation. 2000;101(5):498–503. doi:10.1161/01.CIR.101.5.498
75. Mione MC, Ralevic V, Burnstock G. Peptides and vasomotor mechanisms. Pharmacol Ther. 1990;46(3):429–468. doi:10.1016/0163-7258(90)90027-Y
76. Dehlin HM, Levick SP. Substance P in heart failure: the good and the bad. Int J Cardiol. 2014;170(3):270–277. doi:10.1016/j.ijcard.2013.11.010
77. Bergamini C, Cicoira M, Rossi A, Vassanelli C. Oxidative stress and hyperuricaemia: pathophysiology, clinical relevance, and therapeutic implications in chronic heart failure. Eur J Heart Fail. 2009;11(5):444–452. doi:10.1093/eurjhf/hfp042
78. Mogensen UM, Køber L, Jhund PS, et al. PARADIGM-HF investigators and committees. Sacubitril/valsartan reduces serum uric acid concentration, an independent predictor of adverse outcomes in PARADIGM-HF. Eur J Heart Fail. 2018;20(3):514–522. doi:10.1002/ejhf.1056
79. Myhre PL, Vaduganathan M, Claggett B, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019;73(11):1264–1272. doi:10.1016/j.jacc.2019.01.018
80. Packer M, McMurray JJ, Desai AS, et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation. 2015;131(1):54–61. doi:10.1161/CIRCULATIONAHA.114.013748
81. Vardeny O, Claggett B, Kachadourian J, et al. Reduced loop diuretic use in patients taking sacubitril/valsartan compared with enalapril: the PARADIGM-HF trial. Eur J Heart Fail. 2019;21(3):337–341. doi:10.1002/ejhf.1402
82. Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF registry. J Am Cardiol. 2007;50(8):768–777. doi:10.1016/j.jacc.2007.04.064
83. Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269. doi:10.1056/NEJMoa051530
84. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016(375):1868–1877.
85. Solomon SD, McMurray JJ, Anand IS, et al. PARAGON-HF investigators and committees. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med. 2019;381(17):1609–1620. doi:10.1056/NEJMoa1908655
86. Kaufmann BA, Min SY, Goetschalckx K, et al. How reliable are left ventricular ejection fraction cut offs assessed by echocardiography for clinical decision making in patients with heart failure? Int J Cardiovasc Imaging. 2013;29(3):581–588. doi:10.1007/s10554-012-0122-5
87. Conrad N, Judge A, Tran J, et al. Temporal trends and patterns in heart failure incidence: a population-based study of 4 million individuals. Lancet. 2018;391(10120):572–580. doi:10.1016/S0140-6736(17)32520-5
88. Pharithi RB, Ferre-Vallverdu M, Maisel AS, et al. Sacubitril-valsartan in a routine community population: attention to volume status critical to achieving target dose. ESC Heart Fail. 2020;7(1):158–166. doi:10.1002/ehf2.12547
89. Moliner-Abós C, Rivas-Lasarte M, Pamies Besora J, et al. Sacubitril/valsartan in real-life practice: experience in patients with advanced heart failure and systematic review. Cardiovasc Drugs Ther. 2019;33(3):307–314. doi:10.1007/s10557-019-06858-0
90. Martens P, Lambeets S, Lau CW, Dupont M, Mullens W. Impact of sacubitril/valsartan on heart failure admissions: insights from real-world patient prescriptions [published correction appears in acta cardiol. 2019 Aug;74(4):367]. Acta Cardiol. 2019;74(2):115–122. doi:10.1080/00015385.2018.1473825
91. Zeymer U, Clark AL, Barrios V, et al. Management of heart failure with reduced ejection fraction in Europe: design of the ARIADNE registry. ESC Heart Fail. 2020;7(2):727–736. doi:10.1002/ehf2.12569
92. National Institute for Health and Clinical Excellence QS103. Chronic heart failure in adults. London: NICE; 2018. Available from: https://www.nice.org.uk/guidance/qs9/chapter/List-of-quality-statements.
93. Simpson J, Benson L, Jhund PS, Dahlström U, McMurray JJ, Lund LH. Real world eligibility for sacubitril/valsartan in unselected heart failure patients: data from the swedish heart failure registry. Cardiovasc Drugs Ther. 2019;33(3):315–322. doi:10.1007/s10557-019-06873-1
94. Pellicori P, Urbinati A, Shah P, et al. What proportion of patients with chronic heart failure are eligible for sacubitril-valsartan? Eur J Heart Fail. 2017;19(6):768–778. doi:10.1002/ejhf.788
95. Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril-valsartan in a real-world heart failure population: a community-based single-centre study. ESC Heart Fail. 2018;5(2):337–343. doi:10.1002/ehf2.12251
96. The British National Formulary. Sacubitril with valsartan. Available from: https://bnf.nice.org.uk/medicinal-forms/sacubitril-with-valsartan.html.
97. The British National Formulary. Enalapril maleate. [cited May 13, 2020]. Available from: https://bnf.nice.org.uk/medicinal-forms/enalapril-maleate.html.
98. Maggioni AP, Anker SD, Dahlström U, et al. Heart failure association of the ESC. Are hospitalized or ambulatory patients with heart failure treated in accordance with European Society of Cardiology guidelines? Evidence from 12 440 patients of the ESC heart failure long‐term registry. Eur J Heart Fail. 2013;15:1173–1184. doi:10.1093/eurjhf/hft134
99. McMurray JJ, Solomon SD, Inzucchi SE, et al. DAPA-HF trial committees and investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381(21):1995–2008. doi:10.1056/NEJMoa1911303
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.