Back to Journals » Clinical Interventions in Aging » Volume 15
Cardiac Cachexia: A Well-Known but Challenging Complication of Heart Failure
Authors Krysztofiak H , Wleklik M, Migaj J , Dudek M , Uchmanowicz I , Lisiak M , Kubielas G , Straburzyńska-Migaj E, Lesiak M , Kałużna-Oleksy M
Received 25 July 2020
Accepted for publication 1 October 2020
Published 2 November 2020 Volume 2020:15 Pages 2041—2051
DOI https://doi.org/10.2147/CIA.S273967
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Walker
Helena Krysztofiak,1 Marta Wleklik,2 Jacek Migaj,1,3 Magdalena Dudek,1,3 Izabella Uchmanowicz,2 Magdalena Lisiak,2 Grzegorz Kubielas,2 Ewa Straburzyńska-Migaj,1,3 Maciej Lesiak,1,3 Marta Kałużna-Oleksy1,3
1 1st Department of Cardiology, University of Medical Sciences in Poznan, Poznan, Poland; 2Department of Clinical Nursing, Wroclaw Medical University, Wroclaw, Poland; 3Poznan University of Medical Sciences Hospital of Lord’s Transfiguration, Poznan, Poland
Correspondence:Izabella Uchmanowicz Department of Clinical Nursing, Wroclaw Medical University, Bartla 5, 51-618, Wroclaw, Poland
Tel +48 71 784 18 05
Fax +48 71 345 93 24
Email [email protected]
Abstract: Heart failure (HF) is a common complication of various cardiac diseases, and its incidence constantly increases. This is caused mainly by aging of populations and improvement in the treatment of coronary artery disease. As HF patients age, they tend to develop comorbidities, creating new problems for health-care professionals. Sarcopenia, defined as the loss of muscle mass and function, and cachexia, defined as weight loss due to an underlying illness, are muscle wasting disorders of particular relevance in the heart failure population, but they go mostly unrecognized. The coexistence of chronic HF and metabolic disorders facilitates the development of cachexia. Cachexia, in turn, significantly worsens a patient’s prognosis and quality of life. The mechanisms underlying cachexia have not been explained yet and require further research. Understanding its background is crucial in the development of treatment strategies to prevent and treat tissue wasting. There are currently no specific European guidelines or recommended therapy for cachexia treatment in HF (“cardiac cachexia”).
Keywords: cardiac cachexia, heart failure, nutritional status
Introduction
Heart failure (HF) is considered a cardiovascular disease whose incidence increases constantly. The number of hospitalizations due to HF is still rising, and it has tripled over the last three decades. Over one million hospitalizations with a primary diagnosis of HF occur each year in the U.S.1 similar to Europe.2 This is related both to the demographics, ie, aging population, and to the improved treatment of acute cardiac diseases that eventually leads to the development of chronic HF. Chronic HF is a complex syndrome accompanied by changes in various organs. Most importantly, there are changes within muscular tissue, which facilitate the development of frailty syndrome. Chronic HF is a catabolic state that may lead to cachexia when the physiological balance between anabolic and catabolic processes is impaired.3
Cachexia is a well-known problem in patients with end-stage chronic HF, and it is a risk factor of death for the patients at this stage of the disease.4 It is estimated to affect between 5% and 15% of HF patients, especially those with HF with reduced ejection fraction (HFrEF).5 However, neither left ventricular ejection fraction (LVEF) nor the New York Heart Association (NYHA) functional class is directly associated with cachexia development.6 In 34% of HF patients during a 48-month follow-up ≥6% of body weight loss was reported, while 10.5% of optimally treated outpatients with no underlying diabetes had >5% weight loss.7
Cachexia is related to malnutrition, which frequently accompanies chronic HF and has many negative consequences. There are associations between malnutrition and impaired wound healing, increased rate of postoperative complications, and mortality.8 What is important, cardiac cachexia is associated with poor outcomes and unfavorable reactions to pharmacological treatment, and poor quality of life.9 The overall prevalence of cachexia in the industrialized world increases with a greater incidence of chronic diseases. Currently, this problem affects approximately 6–12 million people. It is estimated that 1.5–2 million people die every year due to cachexia. The annual mortality due to cardiac cachexia is 20–40%.10 Although the cardiac cachexia problem is well known and well documented, it is still difficult to develop a specific therapy for its prevention and treatment due to the multifactorial myocardial injury.
Cachexia in Heart Failure – Definition
Several definitions of cachexia due to HF were proposed. Historically, cachexia was defined as a loss of body mass (taking fluid imbalance into account) of more than 6% in the absence of any other significant illness.11 Nowadays, a multidisciplinary approach is commonly accepted. Cachexia is a multifactorial and multiple organ metabolic disorder12 that is often observed in patients affected by chronic diseases, including HF. Currently, it is primarily defined as a loss of body mass (taking fluid imbalance into account) of more than 5% in 12 months (or BMI <20 kg/m2) with accompanying conditions like decreased muscle strength, fatigue, anorexia, low fat-free mass index and abnormalities in blood biomarkers (elevated C-reactive protein and/or elevated interleukin (IL)-6, Hb <12 g/dl, or low serum albumin (<3.2 g/dL));12–15 at least three of the above must coexist. Cachexia is a generalized process involving adipose tissue, ie, energetic reserves, and other tissues, especially skeletal muscles and bones.12 Cachexia should also be understood as a nutritional syndrome because, to reverse cachexia, nutrients such as protein and calories are needed. Also, the anorectic components represent a nutritional disorder.13
Cachexia is accompanied by malnutrition, systemic nutritional deficiencies, elevated inflammatory cytokines, immune system hyperactivity, and neurohormonal alterations.16 This process results in a syndrome involving loss of body weight, fatigue, dyspnea, tachycardia, dyspepsia, and appetite loss.
Muscle wasting is an important component of cachexia. It often precedes cachexia development and can also predict poor results in HF. Interesting data shows that a handgrip test is a useful tool for detecting muscle function changes in advanced heart failure after the implantation of a ventricular assist device, which can help to better plan interventional tests in cachexia.7,17
Seeking optimal biomarkers of cachexia to facilitate diagnosis is very important. Methods of prevention and treatment are needed. Pre-albumin seems to be a biomarker of cachexia, a potential indicator of malnutrition, low-cholesterol levels, and worse prognosis in chronic HF patients.18 Appetite regulating hormones (cholecystokinin and ghrelin) can play an important role as potential biomarkers of cardiac cachexia (ie, cachexia due to heart disease). In Wang et al, plasma cholecystokinin levels and expression in the heart muscle increased in rats with cardiac cachexia and positively correlated with BNP (brain natriuretic peptide) concentrations and were significantly negatively correlated with LVEF.9 Compared to the control group, plasma-ghrelin levels in the cardiac cachexia group decreased dramatically, and the expression of ghrelin in the myocardium was also decreased, which was negatively associated with BNP levels and positively associated with the ejection fraction.9
Nutritional Status and Metabolic Processes in Heart Failure
HF is a syndrome accompanied by multiple changes. HF patients show impaired kidney function caused by a neurohormonal disorder in the renin-angiotensin-aldosterone system (RAAs). Electrolyte disorders, calcium-phosphate imbalance, lung diseases, and anemia may also be observed.19 Progression of HF involves remodeling of cardiac tissue, including metabolic changes such a slower ATP synthesis, mitochondrial dysfunction, or elevated free fatty acid concentration.20 Microelements play a crucial role in metabolic processes as co-factors. One of the most important is co-enzyme Q10 (CoQ10), a natural antioxidant for a human body that takes part in ATP synthesis. There is a high concentration of CoQ10 in the myocardium. It relieves oxidative stress and stabilizes cell membranes.21 According to research dating from 2010, decreased CoQ10 intake is associated with disease advancement as assessed using NYHA classification, decreased LVEF, and elevated NT-proBNP.22 Interestingly, it was proved more than 20 years ago, that beta-blockers and statins decrease CoQ10 concentration.23 Unfortunately, based on the available data, it is still impossible to determine whether CoQ10 has a sufficiently positive influence on biochemical and echocardiographic parameters to recommend its supplementation. The research on its influence on the state of health of HF patients is ongoing.
HF is often accompanied by calcium-phosphate metabolic disorders associated with secondary hyperparathyroidism and vitamin D deficiency, which in turn is caused by the impairment of kidney function.21,24,25 It also seems that elevated TNF-alpha concentration due to HF is associated with lower calcitriol and vitamin D synthesis, which results in elevated renin, which may play a role in promoting cachexia.21 This is why supplementation of vitamin D in HF is growing more popular. Moreover, research shows that vitamin D supplementation decreases aldosterone concentration, which is often elevated in HF.21,24,25
It is important to keep normal iron concentration in HF patients. They often show iron deficiency (somewhat more frequent in women than in men), and inflammatory states may reduce iron absorption by 40% in these patients.26,27 One of the causes of iron deficiency in cachectic HF patients may also be loss of appetite. As a result, microcytic anemia develops, and subsequently, fatigue, dyspnea, and tachycardia in more severe cases. Anemia is a recognized cause of exacerbation of HF.26,27 It seems that intravenous iron supplementation is more effective than oral. It increases tolerance of exertion, improves the quality of life, and reduces symptoms of HF.27
New potential factors to aid HF treatment are discussed, which can be even more critical when cardiac cachexia occurs. They include thiamin (vitamin B1), creatine, amino acids, taurine, and carnitine. Supplementation of taurine is already used in the treatment of HF in Japan.21 Carnitine seems to improve cardiac metabolism, left ventricle function, and has a protective effect on myocardium during ischemia.27 It has been suggested that the addition of pentoxifylline to nutritional supplementation may reduce proinflammatory cytokine concentrations and increase anabolic processes,28 which relieves HF symptoms.
Elevation of pro-inflammatory cytokines due to HF causes chronic generalized inflammation, including the alimentary tract. This inflammation results in the thickening of intestinal walls, increased bacterial growth, and, consequently, absorption disorders.28 The newest theory attributes the malnutrition in HF patients to impaired intestinal microcirculation. It was proposed that ischemia might cause dysfunction of intestinal epithelial cells, edema, and thickening of intestinal walls, and promote collagen depositing in small intestine walls. All the above-mentioned factors have a negative influence on the absorption of nutrients and microelements. Moreover, intestinal mucosa changes allow bacterial products to pass to circulation, thus increasing inflammatory reactions. These bacterial metabolites seem to promote myocardial hyperplasia and fibrosis.29 Bacterial and functional changes may well play one of the most important roles in HF development. These changes result mainly in protein malnutrition and, consequently, muscle wasting, because muscle wasting causes elevation of serum amino acids to provide substrates for gluconeogenesis, which is much more intense in HF patients than in healthy individuals increased energy expenditure. This process may lead to the development of cardiac cachexia.29,30
Obesity Paradox
Obesity is much more prevalent in patients with HF with preserved ejection fraction (HFpEF) compared to HF with reduced ejection fraction (HFrEF), where over 80% of patients with HFpEF have BMI overweight or obesity.31
The obesity paradox is a curious phenomenon in HF. Obesity is linked to lower circulating levels of B-type natriuretic peptide, suggesting the presence of a more favorable hemodynamic profile. However, loss of body mass in obese patients improves LVEF, NYHA class, and quality of life.32,33 Paradoxically, many authors reported that a significant loss of body mass, even in overweight or obese patients, makes the prognosis worse and increases the risk of cachexia.33 Obesity measured by BMI and various other indicators have been associated with improved HF survival rates in many studies. What is more, HF patients with a BMI of over 30 kg/m2 show better survivability rates than normal-weight patients.34 The reason for this might be a high proportion of adipose tissue in obese patients. Thus, they would have more energetic reserves, which would be expected to decrease the risk of cachexia. There seems to be much ambiguous evidence in the research of the obesity paradox in HF. This phenomenon has not been thoroughly investigated yet.35
Pathological Mechanisms in the Development of Cardiac Cachexia
A general overexpression of catabolic processes is crucial in the development of cardiac cachexia. This is caused by various immunologic, metabolic, and neurohormonal changes.28,36,37 HF patients show increased energetic expenditure and a simultaneous decrease in energy intake.6 This results in compensatory proteolysis and a subsequent muscle wasting, the two processes are always associated with cachexia development.38,39
The sequence of pathological processes involved in the development of cardiac cachexia includes hypoalbuminemia. HF patients demonstrate a negative nitrogen balance, which is a product of significant proteolysis. It seems that hypoalbuminemia provokes changes in the activity of inflammatory cytokines. It causes increased secretion of TNF-alpha (tumor necrosis factor), one of the main inflammatory cytokines of the human body. TNF-alpha has a negative impact on the endothelium, which leads to lower tissue blood flow and, consequently, decreased physical fitness and absorption of nutrients.38,39 Apart from TNF-alpha, also IL-1 and IL-6 are secreted, and all of them further activation of catabolic processes.36,40 Moreover, hypoalbuminemia seems to reduce the activity of anti-inflammatory cytokines IL-10 and TGF-beta (transforming growth factor).12,41 Hence, hypoalbuminemia is associated with muscular wasting. The exact cause and sequence of the above-mentioned changes are not known.
HF patients also show hormonal changes. Increased catabolic processes result in lower testosterone and elevated cortisol blood concentrations. This intensifies muscle wasting and proteolysis.12 Loss of body weight provokes secretion of adiponectin, which increases lipid oxidation and tissue sensitivity to insulin. This results in lower lipid and glucose blood concentrations. Loss of body weight also increases leptin concentration. Leptin shows a similar action to adiponectin, by increased insulin sensitivity and lower lipogenesis.12,42 Nevertheless, HF patients show insulin resistance.43 The main action of leptin is an activation of the satiety center, followed by the early feeling of satiety and lower intake of nutrients.44 This may intensify cardiac cachexia.
Increased secretion of human growth hormone (hGH) with reduced IGF-1 (insulin-like growth factor) concentration may suggest that cachectic patients present tend to develop resistance to hGH.36,39 This is considered to be compensatory to ineffective action of GH on the tissue.12,39 The role of IGF-1 in HF and cardiac cachexia is still under investigation. Normally, IGF-1 plays a significant role in muscle regeneration and slows down muscular wasting.45 It stimulates protein anabolism and activates muscular satellite cells. The latter include progenitor and stem cells of muscular tissue.45,46 In a normal muscle, these cells are inactive. They are responsible for muscular fibers’ regeneration, and their activity increases after an injury (or micro-injury).46 Angiotensin II decreases the activity of these cells and TGF-beta, which may constitute another mechanism of muscular wasting in HF.38
Increased energetic expenditure in cardiac cachexia results in an imbalance between the sympathetic and parasympathetic systems. Secretion of cortisol and activation of RAAs (renin-aldosterone-angiotensin system) and natriuretic peptide system increases.38,39 Angiotensin II plays a key role in cachexia. It promotes proteolysis in the muscular tissue by increased secretion of IL-6 and glucocorticosteroids and decreased secretion of IGF-1.38 Some authors showed that the ubiquitin-dependent pathway of protein degradation plays a major role in the degradation of muscular fibril proteins in neoplastic cachexia.47 Similar processes are also likely in cardiac cachexia. Their increased activity is associated with increased oxidative stress in muscular tissue, which is provoked by nicotinamide adenine dinucleotide phosphate (NADPH) activation by angiotensin II.38,39 Angiotensin II decreases the activation of muscular satellite cells, thus disturbing regeneration of injured muscular fibers. It also increases degradation of lysosome and proteasome proteins,38 stimulates secretion of corticoid releasing hormone (CRH), and decreases secretion of neuropeptide Y (NPY) and orexin.39 Hence, it causes a feeling of satiety and decreases appetite.
Sarcopenia
Cachexia is defined as weight loss due to an underlying illness, the sarcopenia is defined as the loss of muscle mass and function, and they both are muscle wasting disorders. Primary sarcopenia is age-related, whereas secondary sarcopenia is associated with various chronic diseases, including heart failure.48 Sarcopenia is a significant issue in chronic HF patients. Sarcopenia is characterized by muscle loss and decreased muscle fibers, which leads to reduced muscle strength and physical performance in symptomatic HF patients compared to healthy subjects.49 According to the European Working Group on Sarcopenia in Older People (EWGSOP) the definition of sarcopenia is both loss of muscle mass and function.50 Sarcopenia is mainly age-related,51 but it is also observed in HF patients, especially in advanced chronic HF. It may lead to reduced exercise capacity and frailty syndrome.52,53 Sarcopenia is a marker of worse prognosis in HF patients.54 Some studies show that up to 20% of HF patients may develop a significant muscular wasting.16 Another study reported that 40% of the study participants (patients with chronic HF) were at risk of or already suffered from loss of muscular strength.8,55 Sarcopenia may also develop in obese HF patients, which shows that its pathology is complex.51 Impaired myocardial relaxation in HFpEF is a reliable and independent predictor of the ability to perform aerobic exercise, and HFpEF patients simultaneously show a high incidence of sarcopenic obesity,56 which is independently associated with worse outcomes and increased mortality in chronic HF.57–60 Loss of muscle mass is associated with reduced fitness and quicker deterioration of a patient’s state. HF patients with sarcopenia require hospitalizations more often, and their risk of death is greater than those without sarcopenia.50,61 Increased catabolic stress in the skeletal muscles of chronic HF (CHF) patients results in effort intolerance, ventilatory inefficiency, chronotropic incompetence and insulin resistance, which suggests a significant influence catabolic status mechanism on the limited functional status of the patient. It has been suggested that skeletal muscle density is an independent marker of clinical outcomes.56 It has recently been shown that dilution of D3-creatine is more accurate in measuring muscle mass and more strongly associated with physical performance.48
The gold standard in diagnostics of sarcopenia is dual-energy X-ray (DXA). Also, bioelectrical impedance spectroscopy, a safer alternative, is available.16,62 The simplest method of assessing patients with sarcopenia is the measurement of muscle strength using a hand dynamometer.50 The search for effective methods of treatment is ongoing. Currently, strength training and amino-acid-based drugs are recommended. The effectiveness of other drugs, including testosterone, is currently under investigation.16 The essence of screening for sarcopenia should be emphasized. SARC-F is a simple, 5-point questionnaire that aims to identify people with high-risk complications due to sarcopenia. SARC-F contains five components: strength, assistance walking, rising from a chair, climbing stairs, and falls. SARC-F items were selected to reflect the consequences of sarcopenia. Cross-sectional SARC-F correlates with grip strength and frailty.63 According to general recommendations, sarcopenia management should start with screening, by deepening the evaluation with a dynamometric measurement or walking gait speed. If the screening test is positive, the in-depth assessment should be based on MRI, DXA. For the diagnosis of sarcopenia, physical exercise and an adequate protein supply are recommended: 1–1,5 g/kg per day.64
Clinical Interplay in Heart Failure – Cachexia, Sarcopenia, Frailty
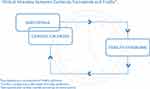
Due to a common pathophysiological pathway, patients with heart failure often suffer from a frailty syndrome with sarcopenia as a component and cardiac cachexia as the last stage. It is noteworthy that cachexic older adults are co-morbid for sarcopenia, but those who are sarcopenic are rarely co-morbid for cachexia.65 Sarcopenia in HF may ultimately progress to cardiac cachexia.56 Sarcopenia is a contributor toward the frailty syndrome, strictly physical and geriatric condition of frailty. However, frailty represents a broader age-related decline in reserve and function across multiple physiologic systems resulting in physical, cognitive, and social impairments that increase vulnerability to stressors.64,66 Frailty syndrome is an independent predictor of early disability, long-term mortality, and readmission in heart failure patients. It is important to understand that sarcopenia and cachexia may require different therapeutic approaches, even though they overlap on some points (Figure 1).
Clinical Significance of Cachexia in Heart Failure Patients and Treatment Options
There are still no European guidelines for dealing with cardiac cachexia. In the Recommendation for managing cardiac cachexia and Sarcopenia by Heart Failure Society of America Scientific Statements Committee, the most important points relate to:
- perform annual screening tests for unintentional weight loss due to the strong relationship between cachexia and adverse clinical outcomes;
- define cachexia as unintentional edema-free weight loss >7.5% from 6 to 12 months or BMI <20 kg/m2 for documented sarcopenia, cachexia or abnormal biochemical indicators;
- refer patients meeting the above criteria to an in-depth nutritional assessment;
- sarcopenia diagnosis based on screening using SARC-F (≤4 points), walking speed test (≤ to 0.8 m/s), grip strength <27 kg for men or <16 kg for women;
- protein supply in patients with identified cachexia or in the cachexia risk group at an amount ≥1.1 g/kg daily;
- special evaluation of patients with cachexia or malnutrition referred to cardiac surgery or patients with terminal status for enteral or parenteral nutritional support.66,67
Potential therapeutic methods target different cardiac cachexia pathogenesis mechanisms, including neurohormonal abnormalities, immune activation and inflammation, metabolic, hormonal imbalance, and gastrointestinal disorders.68 In patients with cardiac cachexia, long-term use of beta-blockers resulted in increased weight gain, decreased plasma noradrenaline levels, and partial reversal of cachexia. Growth hormone is an anabolic hormone that participates in protein synthesis and is associated with muscle mass gain. In studies of patients with non-ischemic HF, ghrelin administration within three weeks increased food intake, GH, left ventricular mass, LVEF, muscle strength, and lean muscle mass. While multifactorial effects of ghrelin show potential for the treatment of myocardial cachexia, further, well-controlled studies are needed.68
Currently, both prevention and treatment of cachexia bases on non-pharmacological methods. Regular physical activity combined with an appropriate diet and nutritional supplementation seems the most important. Promising results of fat-rich and protein-rich diets have been reported.69 Some authors argue that carnitine shows a positive effect on skeletal muscles. This however has never been demonstrated for the cardiac muscle.70 The most effective type of physical activity for HF patients is aerobic training. It has been proved to reduce the negative remodeling of the heart and to improve ventricular function and tolerance of exertion.7,71 Aerobic training reduces oxidative stress and catabolic processes within muscles. What is more, it promotes protein synthesis. Out of pharmacological treatment, intravenous iron usage in anemia27 and ACEi/ARB (angiotensin converting enzyme inhibitor/angiotensin receptor blocker)72 shows some promise in cachectic patients though the efficacy of such a treatment has not been proven yet. It has been suggested that both ACEi and ARB may also have some influence on muscle wasting.11,38,72 The analysis of the COPERNICUS trial has shown that carvedilol has the potential to stop and partially reverse muscle wasting in cachectic patients with severe chronic HF (LVEF<25% and dyspnea at rest or during minimal activity).73 The effects of other supplements, including essential amino acids, omega-3 polyunsaturated fatty acids, or pharmacological agents like immunomodulators, anabolic hormones, appetite stimulants, and other new drugs, are currently under investigation.74 Table 1 summarizes the results of randomized controlled trials on supplementation in HF patients.
 |
Table 1 The Summary of Randomized Controlled Trials on Suplementatiion in HF Patients |
Currently, two management options are under serious consideration: anti-TNF-alpha therapy and appetite stimulants.12 A research of thalidomide to treat cardiac cachexia is ongoing because of its potential to reduce the secretion of TNF-alpha and to relieve the symptoms of wasting.7,12 Studies of megestrol and testosterone in therapy to improve appetite are ongoing.7 However, according to many authors, targeting ghrelin for the treatment of anorexia and cachexia shows the greatest promise. Ghrelin is an adipokine that induces hunger by stimulation of the hypothalamic feeding center. Normally, it is synthesized in the stomach while fasting. It decreases secretion of TNF-alpha, IL-1, and IL-6, and it increases the secretion of IL-10. Moreover, ghrelin has been shown to improve the function of the left ventricle and tolerance of exertion and promote mass muscle gain.7,37 Also, it should be emphasized that the method for bioimpedance analysis can provide valuable measurements of body composition in the context of cachexia, sarcopenia, and frailty.75
It was also shown that such predictors as brain natriuretic peptide (BNP), estimated plasma volume status (ePVS), bioimpedance vector analysis (BIVA), and blood urea nitrogen/creatinine ratio (BUN/Cr) could provide independent and complex prognostic information in patients with HF and, importantly, in combination with them, explain the 40% risk of death in these patients regardless of the acute or chronic HF.76
There is a need for large, well-controlled studies to determine the most appropriate approach in cardiac cachexia. There is still limited evidence that anabolic therapies, combined with a caloric-protein supplement, have better outcomes.13
Summary
Despite new treatment options in HF, the survival of chronic HF patients is still lacking. Moreover, many affected patients, especially those with more advanced chronic HF, also suffer from metabolic disorders. A combination of chronic HF and metabolic disorders increase the risk of muscle wasting, sarcopenia, and eventually cachexia. A patient’s nutritional status and cardiac cachexia require more attention from medical professionals as these conditions are associated with worse prognosis of chronic HF patients. Impaired absorption of nutrients due to HF may lead to malnutrition, anemia, and microelement deficiency. More effort is needed to improve our understanding of the role of metabolic disorders in muscle wasting and cachexia in HF patients. The search for specific therapies and methods of identification of patients at risk of cachexia is ongoing. At present, individualized therapy and a multidisciplinary approach are required to optimize the treatment of patients with HF and cachexia in everyday practice.
Abbreviations
BIVA, bioimpedance vector analysis; BNP, brain natriuretic peptide; BUN/Cr, blood urea nitrogen/creatinine ratio; CHF, chronic heart failure; CoQ10, co-enzyme Q10; CRH, corticoid releasing hormone; DXA, dual-energy X-ray; ePVS, estimated plasma volume status; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; hGH, human growth hormone; IGF-1, insulin-like growth factor; IL-1, interleukin 1; IL-6, interleukin 6; IL-10, interleukin 10; LVEF, left ventricular ejection fraction; NADPH, nicotinamide adenine dinucleotide phosphate; NPY, neuropeptide Y; NYHA, New York Heart Association; RAAs, renin-angiotensin-aldosterone system; TNF-α, tumor necrosis factor-alpha.
Acknowledgments
There were no other contributors to the article than the Authors as well as there was no writing assistance required. The certified English language services were provided.
Funding
This review paper was conducted as a theoretical part of the research project funded by the Ministry of Science and Higher Education in Poland as a part of a statutory grant of the Wroclaw Medical University for maintaining research potential (no. SUB.E020.19.003).
Disclosure
The authors have declared no conflict of interest.
References
1. Fang J, Mensah GA, Croft JB, Keenan NL. Heart failure-related hospitalization in the U.S., 1979 to 2004. J Am Coll Cardiol. 2008;52(6):428–434. doi:10.1016/j.jacc.2008.03.061
2. Ponikowski P, Anker SD, AlHabib KF, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1(1):4–25. doi:10.1002/ehf2.12005
3. von Haehling S, Doehner W, Anker SD. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res. 2007;73(2):298–309. doi:10.1016/j.cardiores.2006.08.018
4. Springer J, Anker SD. Publication trends in cachexia and sarcopenia in elderly heart failure patients. Wien Klin Wochenschr. 2016;128(Suppl S7):446–454. doi:10.1007/s00508-016-1126-2
5. von Haehling S, Anker SD. Prevalence, incidence and clinical impact of cachexia: facts and numbers—update 2014. J Cachexia Sarcopenia Muscle. 2014;5(4):261–263. doi:10.1007/s13539-014-0164-8
6. Anker SD, Chua TP, Ponikowski P, et al. Hormonal changes and catabolic/anabolic imbalance in chronic heart failure and their importance for cardiac cachexia. Circulation. 1997;96(2):526–534. doi:10.1161/01.cir.96.2.526
7. Okoshi MP, Capalbo RV, Romeiro FG, Okoshi K. Cardiac cachexia: perspectives for prevention and treatment. Arq Bras Cardiol. 2017;108(1):74–80. doi:10.5935/abc.20160142
8. Wleklik M, Uchmanowicz I, Jankowska-Polańska B, Andreae C, Regulska-Ilow B. The role of nutritional status in elderly patients with heart failure. J Nutr Health Aging. 2018;22(5):581–588. doi:10.1007/s12603-017-0985-1
9. Wang C, Dong X, Wei L, et al. The relationship of appetite-regulating hormones in the development of cardiac cachexia. Int Heart J. 2019;60(2):384–391. doi:10.1536/ihj.18-131
10. von Haehling S, Anker MS, Anker SD. Prevalence and clinical impact of cachexia in chronic illness in Europe, USA, and Japan: facts and numbers update 2016. J Cachexia Sarcopenia Muscle. 2016;7(5):507–509. doi:10.1002/jcsm.12167
11. Anker SD, Negassa A, Coats AJS, et al. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: an observational study. Lancet. 2003;361(9363):1077–1083. doi:10.1016/S0140-6736(03)12892-9
12. Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle. 2016;7(3):246–260. doi:10.1002/jcsm.12118
13. Anker SD, Morley JE. Cachexia: a nutritional syndrome? J Cachexia Sarcopenia Muscle. 2015;6(4):269–271. doi:10.1002/jcsm.12088
14. von Haehling S. Casting the net broader to confirm our imaginations: the long road to treating wasting disorders. J Cachexia Sarcopenia Muscle. 2017;8(6):870–880. doi:10.1002/jcsm.12256
15. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013
16. Lena A, Coats AJS, Anker MS. Metabolic disorders in heart failure and cancer. ESC Heart Fail. 2018;5(6):1092–1098. doi:10.1002/ehf2.12389
17. Lainscak M, von Haehling S, Anker SD. PURE muscle and more. Int J Cardiol. 2016;202:446–447. doi:10.1016/j.ijcard.2015.09.121
18. Araújo JP, Lourenço P, Rocha-Gonçalves F, Ferreira A, Bettencourt P. Nutritional markers and prognosis in cardiac cachexia. Int J Cardiol. 2011;146(3):359–363. doi:10.1016/j.ijcard.2009.07.042
19. Alsafwah S, Laguardia SP, Arroyo M, et al. Congestive heart failure is a systemic illness: a role for minerals and micronutrients. Clin Med Res. 2007;5(4):238–243. doi:10.3121/cmr.2007.737
20. Doenst T, Abel ED. Spotlight on metabolic remodelling in heart failure. Cardiovasc Res. 2011;90(2):191–193. doi:10.1093/cvr/cvr077
21. Sciatti E, Lombardi C, Ravera A, et al. Nutritional deficiency in patients with heart failure. Nutrients. 2016;8(7):442. doi:10.3390/nu8070442
22. McMurray JJV, Dunselman P, Wedel H, et al. Coenzyme Q10, rosuvastatin, and clinical outcomes in heart failure: a pre-specified substudy of CORONA (controlled rosuvastatin multinational study in heart failure). J Am Coll Cardiol. 2010;56(15):1196–1204. doi:10.1016/j.jacc.2010.02.075
23. Folkers K, Langsjoen P, Willis R, et al. Lovastatin decreases coenzyme Q levels in humans. Proc Natl Acad Sci U S A. 1990;87(22):8931–8934. doi:10.1073/pnas.87.22.8931
24. Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo MET. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail. 2010;3(2):195–201. doi:10.1161/CIRCHEARTFAILURE.109.907899
25. Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Piña IL. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail. 2013;1(1):84–90. doi:10.1016/j.jchf.2012.11.003
26. Cohen-Solal A, Leclercq C, Deray G, et al. Iron deficiency: an emerging therapeutic target in heart failure. Heart. 2014;100(18):1414–1420. doi:10.1136/heartjnl-2014-305669
27. Drozd M, Jankowska EA, Banasiak W, Ponikowski P. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. Am J Cardiovasc Drugs. 2017;17(3):183–201. doi:10.1007/s40256-016-0211-2
28. Kalantar-Zadeh K, Anker SD, Horwich TB, Fonarow GC. Nutritional and anti-inflammatory interventions in chronic heart failure. Am J Cardiol. 2008;101(11):89E–103E. doi:10.1016/j.amjcard.2008.03.007
29. Kamo T, Akazawa H, Suzuki J, Komuro I. Novel concept of a heart-gut axis in the pathophysiology of heart failure. Korean Circ J. 2017;47(5):663–669. doi:10.4070/kcj.2017.0028
30. Pasini E, Aquilani R, Corsetti G, Dioguardi FS. Malnutrition and gut flora dysbiosis: specific therapies for emerging comorbidities in heart failure. Biomed Res Int. 2015;2015:382585. doi:10.1155/2015/382585
31. Horwich TB, Fonarow GC, Clark AL. Obesity and the obesity paradox in heart failure. Prog Cardiovasc Dis. 2018;61(2):151–156. doi:10.1016/j.pcad.2018.05.005
32. Zamora E, Díez-López C, Lupón J, et al. Weight loss in obese patients with heart failure. J Am Heart Assoc. 2016;5(3):e002468. doi:10.1161/JAHA.115.002468
33. Mariotti R, Castrogiovanni F, Canale ML, Borelli G, Rondinini L. Weight loss and quality of life in chronic heart failure patients. J Cardiovasc Med (Hagerstown). 2008;9(6):576–580. doi:10.2459/JCM.0b013e3282f2de13
34. Padwal R, McAlister FA, McMurray JJV, et al. The obesity paradox in heart failure patients with preserved versus reduced ejection fraction: a meta-analysis of individual patient data. Int J Obes (Lond). 2014;38(8):1110–1114. doi:10.1038/ijo.2013.203
35. Oga EA, Eseyin OR. The obesity paradox and heart failure: a systematic review of a decade of evidence. J Obes. 2016;2016:1–9. doi:10.1155/2016/9040248
36. Martins T, Vitorino R, Moreira-Gonçalves D, Amado F, Duarte JA, Ferreira R. Recent insights on the molecular mechanisms and therapeutic approaches for cardiac cachexia. Clin Biochem. 2014;47(1–2):8–15. doi:10.1016/j.clinbiochem.2013.10.025
37. Attanasio P, Anker SD, Doehner W, von Haehling S. Hormonal consequences and prognosis of chronic heart failure. Curr Opin Endocrinol Diabetes Obes. 2011;18(3):224–230. doi:10.1097/MED.0b013e3283469505
38. Yoshida T, Tabony AM, Galvez S, et al. Molecular mechanisms and signaling pathways of angiotensin II-induced muscle wasting: potential therapeutic targets for cardiac cachexia. Int J Biochem Cell Biol. 2013;45(10):2322–2332. doi:10.1016/j.biocel.2013.05.035
39. Yoshida T, Delafontaine P. Mechanisms of cachexia in chronic disease states. Am J Med Sci. 2015;350(4):250–256. doi:10.1097/MAJ.0000000000000511
40. von Haehling S, Schefold JC, Lainscak M, Doehner W, Anker SD. Inflammatory biomarkers in heart failure revisited: much more than innocent bystanders. Heart Fail Clin. 2009;5(4):549–560. doi:10.1016/j.hfc.2009.04.001
41. Aukrust P, Ueland T, Lien E, et al. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83(3):376–382. doi:10.1016/s0002-9149(98)00872-8
42. Kistorp C, Faber J, Galatius S, et al. Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation. 2005;112(12):1756–1762. doi:10.1161/CIRCULATIONAHA.104.530972
43. Scherbakov N, Bauer M, Sandek A, et al. Insulin resistance in heart failure: differences between patients with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17(10):1015–1021. doi:10.1002/ejhf.317
44. Korek E, Krauss H, Gibas-Dorna M, Kupsz J, Piątek M, Piątek J. Fasting and postprandial levels of ghrelin, leptin and insulin in lean, obese and anorexic subjects. Prz Gastroenterol. 2013;8(6):383–389. doi:10.5114/pg.2013.39922
45. Yoshida T, Semprun-Prieto L, Sukhanov S, Delafontaine P. IGF-1 prevents ANG II-induced skeletal muscle atrophy via Akt- and Foxo-dependent inhibition of the ubiquitin ligase atrogin-1 expression. Am J Physiol Heart Circ Physiol. 2010;298(5):H1565–H1570. doi:10.1152/ajpheart.00146.2010
46. Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010;81(1):11–20. doi:10.1111/j.1740-0929.2009.00712.x
47. Sanders PM, Russell ST, Tisdale MJ. Angiotensin II directly induces muscle protein catabolism through the ubiquitin–proteasome proteolytic pathway and may play a role in cancer cachexia. Br J Cancer. 2005;93(4):425–434. doi:10.1038/sj.bjc.6602725
48. Morley JE. Treatment of sarcopenia: the road to the future. J Cachexia Sarcopenia Muscle. 2018;9(7):1196–1199. doi:10.1002/jcsm.12386
49. Saitoh M, Ishida J, Doehner W, et al. Sarcopenia, cachexia, and muscle performance in heart failure: review update 2016. Int J Cardiol. 2017;238:5–11. doi:10.1016/j.ijcard.2017.03.155
50. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
51. Tyrovolas S, Koyanagi A, Olaya B, et al. Factors associated with skeletal muscle mass, sarcopenia, and sarcopenic obesity in older adults: a multi‐continent study. J Cachexia Sarcopenia Muscle. 2016;7(3):312–321. doi:10.1002/jcsm.12076
52. Szulc P, Feyt C, Chapurlat R. High risk of fall, poor physical function, and low grip strength in men with fracture—the STRAMBO study. J Cachexia Sarcopenia Muscle. 2016;7(3):299–311. doi:10.1002/jcsm.12066
53. Uchmanowicz I, Łoboz-Rudnicka M, Szeląg P, Jankowska-Polańska B, Łoboz-Grudzień K. Frailty in heart failure. Curr Heart Fail Rep. 2014;11(3):266–273. doi:10.1007/s11897-014-0198-4
54. Yamada S, Kamiya K, Kono Y. Frailty may be a risk marker for adverse outcome in patients with congestive heart failure. ESC Heart Fail. 2015;2(3):168–170. doi:10.1002/ehf2.12052
55. Lomivorotov VV, Efremov SM, Boboshko VA, et al. Evaluation of nutritional screening tools for patients scheduled for cardiac surgery. Nutrition. 2013;29(2):436–442. doi:10.1016/j.nut.2012.08.006
56. Suzuki T, Palus S, Springer J. Skeletal muscle wasting in chronic heart failure. ESC Heart Fail. 2018;5(6):1099–1107. doi:10.1002/ehf2.12387
57. Harris R, Chang Y, Beavers K, et al. Risk of fracture in women with sarcopenia, low bone mass, or both. J Am Geriatr Soc. 2017;65(12):2673–2678. doi:10.1111/jgs.15050
58. Ebner N, von Haehling S. Unlocking the wasting enigma: highlights from the 8th cachexia conference. J Cachexia Sarcopenia Muscle. 2016;7(1):90–94. doi:10.1002/jcsm.12106
59. Brown JC, Harhay MO, Harhay MN. Sarcopenia and mortality among a population‐based sample of community‐dwelling older adults. J Cachexia Sarcopenia Muscle. 2016;7(3):290–298. doi:10.1002/jcsm.12073
60. Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle. 2018;9(1):3–19. doi:10.1002/jcsm.12238
61. Bean N, Bennett KM, Lehmann AB. Habitus and hip fracture revisited: skeletal size, strength and cognition rather than thinness? Age Ageing. 1995;24(6):481–484. doi:10.1093/ageing/24.6.481
62. Gonzalez MC, Heymsfield SB. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: what are we really estimating? J Cachexia Sarcopenia Muscle. 2017;8(2):187–189. doi:10.1002/jcsm.12159
63. Malmstrom TK, Miller DK, Simonsick EM, Ferrucci L, Morley JE. SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle. 2016;7(1):28–36. doi:10.1002/jcsm.12048
64. Morley JE, Bauer JM. Editorial: the future of sarcopenia. Curr Opin Clin Nutr Metab Care. 2019;22(1):1–3. doi:10.1097/MCO.0000000000000531
65. Minaglia C, Giannotti C, Boccardi V, et al. Cachexia and advanced dementia. J Cachexia Sarcopenia Muscle. 2019;10(2):263–277. doi:10.1002/jcsm.12380
66. Vest AR, Chan M, Deswal A, et al. Nutrition, obesity, and cachexia in patients with heart failure: a consensus statement from the Heart Failure Society of America Scientific Statements Committee. J Card Fail. 2019;25(5):380–400. doi:10.1016/j.cardfail.2019.03.007
67. Carbone S, Billingsley HE, Rodriguez-Miguelez P, et al. Lean mass abnormalities in heart failure: the role of sarcopenia, sarcopenic obesity, and cachexia. Curr Probl Cardiol. 2019;100417. doi:10.1016/j.cpcardiol.2019.03.006.
68. Rolfe M, Kamel A, Ahmed MM, Kramer J. Pharmacological management of cardiac cachexia: a review of potential therapy options. Heart Fail Rev. 2019;24(5):617–623. doi:10.1007/s10741-019-09784-3
69. Rozentryt P, von Haehling S, Lainscak M, et al. The effects of a high-caloric protein-rich oral nutritional supplement in patients with chronic heart failure and cachexia on quality of life, body composition, and inflammation markers: a randomized, double-blind pilot study. J Cachexia Sarcopenia Muscle. 2010;1(1):35–42. doi:10.1007/s13539-010-0008-0
70. Fumagalli S, Fattirolli F, Guarducci L, et al. Coenzyme Q10 terclatrate and creatine in chronic heart failure: a randomized, placebo-controlled, double-blind study. Clin Cardiol. 2011;34(4):211–217. doi:10.1002/clc.20846
71. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi:10.1093/eurheartj/ehw128
72. Dalla Libera L, Ravara B, Angelini A, et al. Beneficial effects on skeletal muscle of the angiotensin II type 1 receptor blocker irbesartan in experimental heart failure. Circulation. 2001;103(17):2195–2200. doi:10.1161/01.cir.103.17.2195
73. Clark AL, Coats AJS, Krum H, et al. Effect of beta‐adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: results from the COPERNICUS trial. J Cachexia Sarcopenia Muscle. 2017;8(4):549–556. doi:10.1002/jcsm.12191
74. von Haehling S, Anker SD. The times they are a-changin’: the cachexia conference goes annual. J Cachexia Sarcopenia Muscle. 2016;7(1):3–4. doi:10.1002/jcsm.12110
75. Meleleo D, Bartolomeo N, Cassano L, et al. Evaluation of body composition with bioimpedance. A comparison between athletic and non-athletic children. Eur J Sport Sci. 2017;17(6):710–719. doi:10.1080/17461391.2017.1291750
76. Massari F, Scicchitano P, Iacoviello M, et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J Cardiol. 2020;75(1):47–52. doi:10.1016/j.jjcc.2019.05.017
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.