Back to Journals » Neuropsychiatric Disease and Treatment » Volume 16
Can Maternal Autoantibodies Play an Etiological Role in ASD Development?
Authors Dudova I, Horackova K, Hrdlicka M , Balastik M
Received 21 November 2019
Accepted for publication 10 April 2020
Published 3 June 2020 Volume 2020:16 Pages 1391—1398
DOI https://doi.org/10.2147/NDT.S239504
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Roger Pinder
Iva Dudova,1 Klara Horackova,2 Michal Hrdlicka,1 Martin Balastik3
1Department of Child Psychiatry, Charles University Second Faculty of Medicine, Prague, Czech Republic; 2Department of Psychiatry, Charles University First Faculty of Medicine, Prague, Czech Republic; 3Laboratory of Molecular Neurobiology, Institute of Physiology of the Czech Academy of Sciences, Prague, Czech Republic
Correspondence: Iva Dudova
Department of Child Psychiatry, Charles University Second Faculty of Medicine, University Hospital Motol, V Uvalu 84, Prague 15006, Czech Republic
Tel +420 224 433 458
Fax +420 224 433 420
Email [email protected]
Abstract: Autism spectrum disorder (ASD) is a heterogeneous condition with multiple etiologies and risk factors – both genetic and environmental. Recent data demonstrate that the immune system plays an important role in prenatal brain development. Deregulation of the immune system during embryonic development can lead to neurodevelopmental changes resulting in ASD. One of the potential etiologic factors in the development of ASD has been identified as the presence of maternal autoantibodies targeting fetal brain proteins. The type of ASD associated with the presence of maternal autoantibodies has been referred to as maternal antibodies related to ASD (MAR ASD). The link between maternal autoantibodies and ASD has been demonstrated in both clinical studies and animal models, but the exact mechanism of their action in the pathogenesis of ASD has not been clarified yet. Several protein targets of ASD-related maternal autoantibodies have been identified. Here, we discuss the role of microtubule-associated proteins of the collapsin response mediator protein (CRMP) family in neurodevelopment and ASD. CRMPs have been shown to integrate multiple signaling cascades regulating neuron growth, guidance or migration. Their targeting by maternal autoantibodies could change CRMP levels or distribution in the developing nervous system, leading to defects in axon growth/guidance, cortical migration, or dendritic projection, which could play an etiological role in ASD development. In addition, we discuss the future possibilities of MAR ASD treatment.
Keywords: maternal autoantibodies, autism spectrum disorder, animal models, CRMP2, therapy
Introduction
Autism spectrum disorder (ASD) is a heterogeneous neurodevelopmental condition characterized by persistent deficits in social interaction, communication, and the presence of restricted, repetitive behaviors, interests, and activities.1,2 The etiology of ASD is considered to combine both genetic predispositions and environmental impacts.3 Estimates of ASD heritability, using twins studies, have ranged from 74% to 93%.4 Sibling studies indicated that ASD occurs in 7–20% of subsequent children after an older child is diagnosed with ASD.5 A significantly increased incidence of autism among twins and siblings attests to the influence of genetic factors in the pathogenesis of autism and is further supported by evidence of genetic variants includes genes involved in intellectual disability and neuropsychiatric disorders, common pathway genes and various ASD-risk genes, multigenic contributions from rare or common variations, and DNA mutations, as well as environmental effects on gene expression and/or protein function.3 ASD can occur in the context of a clinically defined genetic syndrome, or as a feature of molecularly defined syndrome.6 Common single-gene disorders associated with ASD are fragile X syndrome, neurofibromatosis-1, tuberous sclerosis complex, Rett syndrome, Angelman syndrome, and PTEN hamartoma tumor syndrome.1 ASD associated with a genetically defined syndrome is referred to as syndromic ASD. Despite extensive research, the genetic etiology for the vast majority of ASD cases remains unknown.3
While the mechanism for the pathogenesis of ASD is not yet known, it likely includes changes in brain development immediately after birth. ASD apparently begins with a cascade of pathological phenomena that are significantly influenced by environmental factors. Many prenatal, neonatal, and perinatal risk factors for ASD have been suggested. One of the important factors seems to be immune system dysregulation during critical periods of brain development.
Immune Considerations
Interaction between the immune and nervous systems begins during the prenatal period, and the successful development of the nervous system depends on balanced immune reactions. As such, immune system dysregulation can be an etiological factor in the pathogenesis of ASD. The relationship between maternal immune activation and neurodevelopment has been explored in several preclinical studies in which inflammation was induced in pregnant mice, rats, and nonhuman primates. Injection of synthetic viral RNA (Poly(I:C)) and bacterial endotoxin lipopolysaccharide (LPS), which evoke antiviral or antibacterial immune responses, respectively, into pregnant females, induced substantial behavioral changes in the offspring that were reminiscent of ASD and schizophrenia.7
Additional studies have since shown the effect of immune system deregulation on the pathogenesis of ASD, including familial autoimmune disorders, maternal viral or bacterial infections during pregnancy, dysregulation of cytokines and chemokines, and presence of autoantibodies in children with ASD.8 A registry-based Swedish study from 2010 found an association between children with ASD and autoimmune disease diagnosis in the parents. Several specific diagnoses among the mothers have been identified as ASD risk factors including type-1 diabetes, idiopathic thrombocytopenic purpura, myasthenia gravis, and rheumatic fever. For fathers, rheumatic fever was associated with autism spectrum disorders. The authors observed nearly 50% higher odds of being diagnosed with autism by age 10 years among children whose parents had any kind of autoimmune disease.9 A meta-analysis from 2015 showed that a family history of all autoimmune diseases combined was associated with a 28% higher risk of autism in children.10 For some specific autoimmune diseases, the pooled results showed that a family history of type 1 diabetes was associated with a 49% higher risk of autism in children, 59% for psoriasis, 51% for rheumatoid arthritis, and 64% for hypothyroidism.10 A large, exploratory, population-based study from Denmark revealed an association between a diagnosis of ASD in the child and hospitalization of the mother for either a viral infection during the first trimester of pregnancy or bacterial infection during the second trimester of pregnancy. This study emphasized the presence of many specific factors like pathogens, severity, and the timing of the mother’s infection.11 Several other studies found atypical cytokine and chemokine profiles in children with ASD.12 Most recently, studies have demonstrated that elevated mid-gestational levels of inflammatory cytokines and chemokines are associated with ASD with intellectual disability.8 Finally, prenatal exposure to maternal autoantibodies interacting with fetal brain proteins has been suggested as a factor inducing changes in the trajectory of neurodevelopment13 and a substantial risk factor for the development of ASD.14 For this specific type of ASD, the term MAR autism (maternal autoantibody related autism) or MAR ASD (maternal autoantibody related autism spectrum disorder) is used.
Maternal Autoantibodies and Neurodevelopment
During gestation, the fetal immune system is immature. Maternal IgG antibodies readily cross the placenta and enter the fetal circulation. These antibodies are highly protective against a variety of possible pathogens. However, along with the protective IgG antibodies, some maternal IgG autoantibodies can react with fetal brain tissue and impact neural development.15,16 It is still unclear how and when are these maternal autoantibodies generated in the maternal blood and whether/how the neuroantigens passage from the fetal brain to enter the maternal circulation. The release of neuroantigens may be more relevant during the early stages of fetal brain development with regards to the pathogenesis of ASD.17 Maternal autoantibodies, directed against fetal brain antigens, have been detected in mothers of children with ASD in several studies. Recent MAR ASD studies have identified some of the important targets as proteins involved in neurodevelopment. Seven candidate proteins, against which maternal antibodies related to ASD have been detected, include lactate dehydrogenase (LDH) A and B (subunits of enzyme catalysing lactate interconversion, used as a markers of necrotic cell damage15), stress-induced phosphoprotein 1 (STIP1) involved in neuritogenesis and neuronal survival16, guanine deaminase (GDA), also known by the name cypin regulating dendritic number and arborization in neuronal development15,16, collapsing response mediator protein (CRMP) 1 and 2 (discussed later), and Y-box binding protein (YBX1) promoting neuronal motility and migration.15 These proteins are crucial for normal brain development and play an important role in neurogenesis.17,18 Moreover, approximately 30–70% of autistic patients have circulating anti-brain autoantibodies targeting anticardiolipin, β2-Glycoprotein 1, anti-phosphoserine antibodies, anti-double-stranded DNA antibodies, and anti-nucleosome specific antibodies.19 Children with ASD not only have autoantibodies to brain-specific antigens such as myelin basic protein, serotonin receptors, brain endothelium cerebellar tissue, and glutamic acid decarboxylase, but also to non-brain specific antigens such as folate receptor alpha (FRα) and mitochondria.20 Recent studies have shown a significant association between folate receptor autoantibodies (FRAA) and ASD both in children and their parents.21 A study by Zhou et al found that serum FRAA are more prevalent in children with ASD (77.5%) than in children with typical development (54.8%).22 An association between FRAA and autism was further supported by a study from 2018 analyzing the families of ASD children. Overall, 76% of the affected children, 75% of the unaffected siblings, 69% of fathers, and 59% of mothers were positive for either blocking or binding FRAA, whereas the prevalence of these autoantibodies in the normal controls was 29%. Thus the presence of FRAA autoantibodies appears to be one of the heritable risk factors that can contribute to ASD pathogenesis.21
The effect of maternal autoantibodies on neurodevelopment has been analyzed using animal models. In the late 1950s, it was demonstrated in mice that maternal autoantibodies directly influenced the brain and nervous system of embryos leading to significant changes in the brains of the offsprings.23 In the 1970s, researchers found evidence of maternal IgG antibodies in fetal cerebrospinal fluid and their transfer through the blood-brain barrier into the fetal brain during pregnancy.18 Animal experiments showed that antibodies against fetal brain proteins could induce changes in the behavior of exposed offspring.24,25 The first studies implicating the presence of maternal antibodies as a risk factor in the etiology of ASD were carried out in the 1990s.18
Maternal Autoantibodies and ASD
Animal Models
Various non-human primate and rodent studies have been conducted to provide support for the role of maternal autoantibodies in the pathogenesis of neurodevelopmental disorders, especially ASD. In the first, non-human primate study carried out by Martin et al, purified neuronal antibodies from the mothers of children with ASD were passively transferred to pregnant rhesus macaque monkeys. The offspring from this group of monkeys demonstrated increased whole-body stereotypies and higher levels of motor activity than control autoantibodies-unexposed monkeys. Transfer of IgG purified autoantibodies from mothers of typically developing children did not induce stereotypical or hyperactive behaviors.26 This study was followed by a larger, more targeted study in which specific IgG antibodies against major proteins with molecular weights of 37 and 73 kDa from mothers of children with ASD were given to female macaque rhesus monkeys. Macaques prenatally exposed to the 37/73 kDa maternal IgG showed abnormal behavior that included unreciprocated social approaches and inappropriate vocalizations compared with control offspring. Female macaques who were exposed to the effects of the antibodies from mothers of children with ASD demonstrated heightened maternal protectiveness during the early development of their offspring.27 Concurrently with the non-human primate studies, research was also conducted on rodents. Like the primates, pregnant mice were exposed to IgG from mothers of children with ASD. The offspring of IgG exposed mice displayed anxiety-like behavior and altered sociability, along with impaired motor and sensory development, which was in contrast to the mice offspring that received plasma from mothers of typically developing children.28,29 In a study by Brimberg et al, contactin-associated protein-like 2 (Caspr2) reactive antibody cloned from a mother of an ASD child mediated an ASD-like phenotype in mice, which displayed abnormal cortical development as well as impairments in sociability, flexible learning, and repetitive behavior.30 In a more targeted study, mouse embryos received a single intraventricular injection of maternal human plasma with autoantibodies reacting with proteins having a molecular weight of 37 and 73 kDa. The exposed mice demonstrated atypical behaviors, including stereotypical self-grooming and increased repetitive behaviors, relative to mice similarly injected with maternal IgG from mothers of neurotypical controls.31 The mice exposed to the plasma of mothers of children with ASD thus showed some ASD-like behavioral changes. Subsequent mouse studies demonstrated that prenatal exposure to autism-specific maternal autoantibodies led to neuroanatomical changes in the offspring, including increased radial glial cell proliferation along with accelerated migration, reduced numbers of cortical dendritic spines, as well as increased brain and neuron size.13 The most recent study using mice tried to create the first endogenous preclinical model of MAR ASD. Through immunization with the peptide epitope sequences of seven antigenic proteins that are targeted by maternal autoantibodies reactive to fetal brain proteins, the authors were able to create an endogenous, antigen-driven mouse model. Prenatally exposed MAR-ASD male and female offspring displayed a range of ASD-relevant behaviors throughout life, including aberrant social interactions, higher repetitive self-grooming behaviors and reduced vocalizations.13
Imaging Studies
Studies using imaging methods have demonstrated the presence of abnormal brain growth in children with ASD. A study by Courchesne et al demonstrated that children suffering from autism have a normal overall brain volume at birth; this finding was based on the measure of neonatal head circumference. By ages 2–4 years, 90% of autistic boys in the sample had brain volumes larger than the normal average. Autistic 2- to 3-year-old boys had more cerebral and cerebellar white matter, and more cerebral cortical gray matter than normal, whereas older autistic children and adolescents did not have enlarged gray and white matter volumes.32 Abnormal brain enlargement in preschool-aged children with ASD has been found consistently33,34 in about 6% of cases.35 However, this early childhood acceleration in brain growth and enlargement of brain volume is not a general characteristic of all individuals with autism. For example, abnormal brain size was found in preschool-age boys with ASD with regression.36 Abnormal brain enlargement was also shown in boys with ASD born to mothers with reactivity to 37 and 73 kDa fetal brain proteins. In a study by Nordahl et al, while the group of all preschool-aged ASD children exhibited abnormal brain enlargement, which is commonly observed in this age range, the group of ASD children whose mothers had the 37/73 kDa IgG autoantibodies exhibited a more extreme 12.1% abnormal brain enlargement relative to the typically developing group. The remaining ASD children exhibited a smaller 4.4% abnormal brain enlargement relative to controls.26 Interestingly, male macaque rhesus monkeys exposed prenatally to the 37/73 kDa autoantibodies had enlarged brain volumes compared with controls.27 Prenatal exposure to ASD-specific maternal autoantibodies increase stem cell proliferation in the embryonic neocortex, enlarges the brain, and increases neuronal size in adult mice.38
Both human and animal studies provide support for the hypothesis that the 37/73 kDa autoantibodies, either directly or indirectly, affect brain development leading to abnormal enlargement and neurobiological alterations leading to autism spectrum disorder.37
Clinical Studies
In 2008, Braunschweig et al published a case-control study that included 61 mothers of children with autism, 40 mothers of children with delayed development, and 62 mothers of typically developing children. The study found a significant correlation between the paired reactivity of maternal antibodies to fetal brain proteins with a molecular weight of 37 and 73 kDa and a diagnosis of autism in the child.39 The population-based case-control study collected blood samples from mothers during mid-pregnancy and measured the maternal autoantibody reactivity to fetal proteins with a molecular weight of 39 and 73 kDa. The study found that reactivity to the 39 kDa band was more common among mothers of children later diagnosed with autism compared to mothers of children with delayed or typical development. Simultaneous reactivity to 39 kDa and 73 kD bands were found only among mothers of children with ASD.40 Another population-based study with a large cohort of mothers of children with autism and controls reported significant associations between the presence of IgG reactivity to fetal brain proteins with molecular weights of 37 and 73 kDa and a childhood diagnosis of autism, as well as the correlation of reactivity to 39 and 73 kDa proteins and a broader diagnosis of ASD. The study further supported ASD-related patterns of reactivity of maternal autoantibodies with specific fetal brain proteins.41 A subsequent study by Braunschweig, which included 246 mothers of children with ASD and 149 mothers of regularly developing children, uncovered some of the protein targets of ASD-related maternal autoantibodies, such as LDH, cypin, STIP1, CRMP1, CRMP2, and YBX1, alone and in combinations. The results showed a highly significant association between the presence of individual maternal autoantibodies to fetal brain proteins and an ASD diagnosis. When all antigen reactivity patterns were combined, a total of 23% of mothers of children with ASD had an autoantibody pattern containing two or more to the target proteins compared to only 1% of mothers with typically developing children.15 Importantly, while the identified protein targets of ASD-related maternal autoantibodies, ie, LDH, cypin, STIP1, CRMP1, CRMP2, and YBX1, are involved in various signaling cascades and cell processes, they are also all related to neurodevelopment.15 Both secreted and intracellular proteins appear to be targets of ASD-related maternal autoantibodies. The mechanism by which the antibodies influence these intracellular proteins is, so far, not well understood. The presence of CRMP1 and CRMP2, among the six identified protein targets of ASD-related maternal autoantibodies, point to the importance of the CRMP (collapsing response mediator protein) family of microtubule- associated proteins, in ASD pathogenesis.
The Role of CRMPs in Neural Development and ASD
Microtubule-associated proteins (MAPs) are a large group of proteins that bind to microtubules and regulating their stability, dynamics, or microtubule-based transport. MAPs have been shown to regulate many steps in brain development, including neurogenesis, neuron migration, polarization, axon/dendrite growth and guidance, arborization, and synapse formation. Many ASD-linked brain abnormalities, ie, variations in mini-columnar and laminar cortical organization, synaptic abnormalities, and faulty links in neuronal circuits, have been linked to MAP gene mutations and changes.42
CRMPs are a 5-member family of MAPs originally identified as regulators of axon guidance and growth cone collapse in neurons.43 They are strongly expressed in the nervous system, in particular during discrete periods of neuronal development, while their expression in the adult nervous system is significantly lower. Among the CRMPs, CRMP2 was the first identified and remains the best characterized. CRMP2 interacts (1) with tubulin heterodimers and promotes polarization or (2) with assembled microtubules, via a taxol-sensitive interaction, and promotes stabilization.44 In adult brains, CRMP2 appears mainly in areas with higher neuroplasticity such as the hippocampus.45
The binding of CRMP2 to microtubules is tightly regulated by phosphorylation by CDK5, GSK-3β, ROCK, or other kinases. Stimulation of growth cones by Semaphorin 3A has been shown to induce CDK5 phosphorylation of CRMP2, which than looses it affinity to microtubules and mediates growth cone collapse.46,47 With numerous phosphorylation sites, CRMP2 serves as a hub, integrating multiple signaling cascades regulating neuron growth, guidance and migration, and conveying their signals to microtubules.48
The CRMP2 gene undergoes alternative splicing generating two isoforms CRMP2A and CRMP2B,49 which seem to play distinctive roles in neural development as they are differentially expressed in the nervous system, localized in neurons and are regulated by conformational changes.47
Deregulation of CRMP2 has also been associated with several neuropathological or psychiatric conditions in humans, including Alzheimer’s disease, schizophrenia, ASD, mood disorders, and epilepsy through genetic polymorphisms, changes in protein expression, post-translational modifications, and protein/protein interactions.50,51 Recently, a primary in vivo analysis of conditional knockout of all CRMP2 isoforms demonstrated that CRMP2 deficiency leads to neuronal development deficits and behavioral impairments in mice sharing similarity to schizophrenia (eg, changed dendritic spine density, behavioral changes, and a deficit of the prepulse inhibition).51 In contrast, a total deficiency of CRMP2 in full CRMP2 knockout mice has recently been shown to lead to morphological and behavioral alterations associated with ASD (ie, defects in axon and dendritic spine pruning, increased dendritic spine density in vivo and in vitro, defects in ultrasonic vocalization in early postnatal stages, as well as social and behavioral changes in adults).50 This suggests that even a minor difference in the spatio-temporal inactivation of CRMP2 during development can have a major impact on the development and severity of the resulting neurodevelopmental defects leading to schizophrenia-, or ASD-like phenotypes.
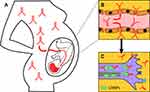
Considering the role of CRMP2 in the pathogenesis of ASD, the targeting of CRMP2 or CRMP1 (which shares large structural and functional similarities to CRMP2) by maternal autoantibodies in ASD patients, could change the amount and distribution of these proteins in the developing nervous system, thus leading to defects in axon growth/guidance, cortical migration, and dendritic projection, as well as playing an etiological role in ASD development (Figure 1). The exact role of CRMP2 isoforms in ASD pathogenesis is, so far, not known. Their differential regulation, distribution, and function in neurons,47 though, suggests that autoantibodies targeting different members of the CRMP family or their specific isoforms could trigger different changes in neural development and promote different neurodevelopmental disorders. Future studies will need to address these questions.
Therapy of MAR ASD
While there is evidence that maternal autoantibodies are important risk factors for developing ASD, little is known about potential options for intervention. However, the identification of maternal autoantibody targets increases therapeutic possibilities.14 If we can prove a causal connection between maternal autoantibodies and the development of ASD, then we have the potential for prevention or treatment of MAR ASD. Three main mechanisms can be employed to prevent pathogenic antibodies from entering the fetus: ex vivo antibody removal, in vivo antibody competition and removal, and inhibiting antibody generation.14
Removing pathogenic antibodies from maternal circulation is a relatively safe technique that includes therapeutic plasma exchange or plasmapheresis. However, this method is limited by the non-selective removal of all plasma components. Plasmapheresis is successfully used today to improve symptoms linked to a number of autoimmune disorders, such as antibody-mediated acute inflammatory demyelinating polyradiculoneuropathy, also known as a Guillain-Barré syndrome52 and immune-mediated cerebellar ataxias.53 In most autoimmune disorders, autoantibodies must be constantly removed from circulation throughout the life of the individual; however, MAR-ASD potentially pathogenic maternal autoantibodies need only be removed during gestation when maternal antibodies are transferred to the fetus. Thus, this could be a therapeutically, very promising treatment for MAR autism.14 Another potential therapeutic method increases the degradation of maternal anti-brain autoantibodies using intravenous immunoglobulin (IVIg) therapy or therapy using recombinant antibodies. Regrettably, these treatments, due to their non-specificity, could systemically induce the degradation of all IgGs.54 IVIg therapy may also be useful for individuals with ASD who demonstrate other immune abnormalities. Out of five studies that demonstrated the benefits of IVIg therapy for ASD patients, three involved individuals with immune system abnormalities.20 More research is needed to better understand (1) which subset of children with ASD can benefit from IVIg therapy and (2) the optimal dose and interval for treatment.20 The use of immunosuppressants could be another method for treat the impact of autoantibodies. Alternatively, the administration of proteasome inhibitors that reduce autoantibody levels prior to pregnancy could also be considered.14 However, evidence that this is safe for pregnant women and/or non-toxic to the developing fetus is still limited and will need to be analyzed before being used as a MAR ASD treatment.
Conclusion
Evidence of maternal autoantibodies that target fetal brain proteins offers new insights into the role of immune dysfunction in ASD. A significant number of mothers of ASD children carry maternal autoantibodies directed against various fetal brain proteins essential for the regulation of normal brain development. Among them, the collapsing response mediator proteins (in particular CRMP2 and CRMP1) have been shown to control neuron polarization, growth, guidance, and synapse refinement during the embryonic and early postnatal development of the nervous system. The role of CRMP2 in ASD pathogenesis was recently analyzed using different mouse models. Further research will be needed to clarify the causal role of maternal autoantibodies against different CRMP proteins in the pathogenesis of ASD. In the future, the elimination of specific pathological maternal autoantibodies could be an option for treating MAR ASD.
Acknowledgments
This study was supported by the Ministry of Health, Czech Republic, Grant No. NV18-04-00085. All rights reserved. The authors would like to thank Thomas Secrest, MSc., for his assistance with the English version of the manuscript. The abstract of this paper was presented at the 19th WPA World Congress of Psychiatry (Lisbon, 21–24 August 2019) as a poster presentation. The poster’s abstract was published in abstracts online: https://2019.wcp-congress.com/scientific-program/.
Disclosure
Dr Iva Dudova, Dr Klara Horackova, Prof. Dr. Michal Hrdlicka and Dr Martin Balastik report grants, personal fees from the Ministry of Health, Czech Republic, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. McDougle C, eds. Autism Spectrum Disorder. New York: Oxford University Press; 2016.
2. APA. Diagnostic and Statistical Manual of Mental Disorders: DSM – 5. Vol. 5th. Arlington, VA: American Psychiatric Association; 2013.
3. Masi A, DeMayo MM, Glozier N, Guastella AJ. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci Bull. 2017;33(2):183–193. doi:10.1007/s12264-017-0100-y
4. Tick B, Bolton P, Happé F, Rutter M, Rijsdijk F. Heritability of autism spectrum disorders: a meta-analysis of twin studies. J Child Psychol Psychiatry. 2016;57:585–595. doi:10.1111/jcpp.12499
5. Lord C, Elsabbagh M, Baird G, Veenstra-Vanderweele J. Autism spectrum disorder. Lancet. 2018;392:508–520. doi:10.1016/S0140-6736(18)31129-2
6. Fernandez BA, Scherer S. Syndromic autism spectrum disorders: moving form a clinically defined to a molecularly defined approach. Dialogues Clin Neurosci. 2017;19:353–371.
7. Solek CM, Farooqi N, Verly M, Lim TK, Ruthazer ES. Maternal immune activation in neurodevelopmental disorders. Dev Dyn. 2018;247:588–619.
8. Meltzer A, Van de Water J. The role of the immune system in autism spectrum disorder. Neuropsychopharmacology. 2017;42(1):284–298. doi:10.1038/npp.2016.158
9. Keil A, Daniels JL, Forssen U, et al. Parental autoimmune diseases associated with autism spectrum disorders in offspring. Epidemiology. 2010;21:805–808. doi:10.1097/EDE.0b013e3181f26e3f
10. Wu S, Ding Y, Wu F, et al. Family history of autoimmune diseases is associated with an increased risk of autism in children: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2015;55:322–332. doi:10.1016/j.neubiorev.2015.05.004
11. Atlandottir HO, Thorsen P, Ostergaard I, et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J Autism Dev Disord. 2010;40:1423–1430. doi:10.1007/s10803-010-1006-y
12. Matelski L, Van de Water J. Risk factors in autism: thinking outside the brain. J Autoimmun. 2016;67:1–7. doi:10.1016/j.jaut.2015.11.003
13. Jones KL, Pride MC, Edmiston E, et al. Autism-specific maternal autoantibodies produce behavioral abnormalities in an endogenous antigen-driven mouse model of autism. Mol Psychiatry. 2018. doi:10.1038/s41380-018-0126.1
14. Fox-Edmiston E, Van de Water J. Maternal anti-fetal brain IgG autoantibodies and autism spectrum disorder: current knowledge and its implications for potential therapeutics. CNS Drugs. 2015;29:715–724. doi:10.1007/s40263-015-0279-2
15. Braunschweig D, Krakowiak P, Duncanson P, et al. Autism-specific maternal autoantibodies recognize critical proteins in developing brain. Transl Psychiatry. 2013;3:e277. doi:10.1038/tp.2013.50
16. Jones KL, Van de Water J. Maternal autoantibody related autism: mechanisms and pathways. Mol Psychiatry. 2019;24:252–265. doi:10.1038/s41380-018-0099-0
17. Bagasra O, Heggen C. Autism and Environmental Factors. NJ: Wiley; 2018.
18. Edmiston E, Ashwood P, Van de Water J. Autoimmunity, autoantibodies, and autism spectrum disorders (ASD). Biol Psychiatry. 2017;81(5):383–390. doi:10.1016/j.biopsych.2016.08.031
19. Tzang R-F, Chang C-H, Chang Y-C, Lane H-Y. Autism associated with anti-NDMAR encephalitis: glutamate-related therapy. Front Psychiatry. 2019;10:440. doi:10.3389/fpsyt.2019.00440
20. Connery K, Tippett M, Delhey LM, et al. Intravenous immunoglobulin for the treatment of autoimmune encephalopathy in children with autism. Transl Psychiatry. 2018;8:148. doi:10.1038/s41398-018-0214-7
21. Quadros EV, Sequeira JM, Brown WT, et al. Folate receptor autoantibodies are prevalent in children diagnosed with autism spectrum disorder, their normal siblings and parents. Autism Res. 2018;11:707–712. doi:10.1002/aur.1934
22. Zhou J, Liu A, He F, et al. Hogh prevalence of serum folate receptor autoantibodies in children with autism spectrum disorders. Biomarkers. 2018;23(7):622–624.5276. doi:10.1080/1354750X.2018.1458152
23. Gluecksohn-Waelsh S. The effect of maternal immunization against organ tissues on embryonic differentiation in the mouse. Development. 1957;5:83–92.
24. Karpiak SE, Rapport MM. Behavioral changes 2-month-old rats following prenatal exposure to antibodies against synaptic membranes. Brain Res. 1975;92:405–413. doi:10.1016/0006-8993(75)90325-X
25. Rick JT, Gregson AN, Leibowitz S, Adinolfi M. Behavioural changes in adult rats following administration of antibodies again brain gangliosides. Dev Med Child Neurol. 1980;22:719–724. doi:10.1111/j.1469-8749.1980.tb03738.x
26. Martin LA, Ashwood P, Braunschweig D, et al. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immune. 2008;22(6):806–816. doi:10.1016/j.bbi.2007.12.007
27. Bauman MD, Iosif AM, Ashwood P, et al. Maternal antibodies from mothers of children with autism alter brain growth and social behavior development in the rhesus monkey. Transl Psychiatry. 2013;3:e278. doi:10.1038/tp.2013.47
28. Singer HS, Morris C, Gause C, et al. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: a pregnant dam mouse model. J Neuroimmunol. 2009;211(1–2):39–48. doi:10.1016/j.jneuroim.2009.03.011
29. Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. J Neuroimmunol. 2012;252(1–2):56–65. doi:10.1016/j.jneuroim.2012.08.002
30. Brimberg L, Mader S, Jeganathan V, et al. Caspr2-reactive antibody cloned from a mother of an ASD child mediates as ASD-like phenotype in mice. Mol Psychiatry. 2016;21(12):1663–1671. doi:10.1038/mp.2016.165
31. Camacho J, Jones KL, Miller E, et al. Embryonic intraventricular exposure to autism-specific maternal autoantibodies produces alterations in autistic-like stereotypical behaviors in offspring mice. Behav Brain Res. 2014;266:46–51. doi:10.1016/j.bbr.2014.02.045
32. Courchesne E, Karns CM, Davis HR, et al. Unusual brain growth patterns in early life in patients with autistic disorder: an MRI study. Neurology. 2001;57:245–254. doi:10.1212/WNL.57.2.245
33. Hazlett HC, Poe M, Gerig G, et al. Magnetic resonance imaging and head circumference study of brain size in autism: birth through age 2 years. Arch Gen Psychiatry. 2005;62:1366–1376. doi:10.1001/archpsyc.62.12.1366
34. Schumann CM, Bloss CS, Carter Barnes C, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30(12):4419–4427. doi:10.1523/JNEUROSCI.5714-09.2010
35. Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31:137–145. doi:10.1016/j.tins.2007.12.005
36. Nordahl CW, Lange N, Li DD, et al. Brain enlargement is associated with regression in preschool-age boys with autism spectrum disorders. Proc Natl Acad Sci U S A. 2011;108:20195–20200. doi:10.1073/pnas.1107560108
37. Nordahl CW, Braunschweig D, Iosif AM, et al. Maternal autoantibodies are associated with abnormal brain enlargement in a subgroup of children with autism spectrum disorder. Brain Behav Immun. 2013;30:61–65. doi:10.1016/j.bbi.2013.01.084
38. Martínez-Cerdeño V, Camacho J, Fox E, et al. Prenatal exposure to autism-specific maternal autoantibodies alters proliferation of cortical neural precursor cells, enlarges brain, and increases neuronal size in adult animals. Cereb Cortex. 2016;26:374–383. doi:10.1093/cercor/bhu291
39. Braunschweig D, Ashwood P, Krakowiak P, et al. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi:10.1016/j.neuro.2007.10.010
40. Croen LA, Braunschweig D, Haapanen L. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–588. doi:10.1016/j.biopsych.2008.05.006
41. Braunschweig D, Duncanson P, Boyce R, et al. Behavioral correlates of maternal antibody status among children with autism. J Autism Dev Disord. 2012;42:1435–1445. doi:10.1007/s10803-011-1378-7
42. Chang Q, Yang H, Wang M, Wei H, Hu F. Role of microtubule-associated protein in autism spectrum disorder. Neurosci Bull. 2018;34(6):1119–1126. doi:10.1007/s12264-018-0246-2
43. Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. In: Pasterkamp RJ, editor. Semaphorins: Receptor and Intracellular Signaling Mechanisms. Advances in Experimental Medicine and Biology. Vol. 600. New York: Springer; 2007:1–11.
44. Lin PC, Chan PM, Hall C, Manser E. Collapsin response mediator proteins (CRMPs) are a new class of microtubule-associated protein (MAP) that selectively interacts with assembled microtubules via a taxol-sensitive binding interaction. J Biol Chem. 2011;286:41466–41478. doi:10.1074/jbc.M111.283580
45. Wang LH, Strittmatter SM. A family of rat CRMP genes is differentially expressed in the nervous system. J Neurosci. 1996;16:6197–6207. doi:10.1523/JNEUROSCI.16-19-06197.1996
46. Yoshimura T, Kawano Y, Arimura N, et al. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell. 2005;120(1):137–149. doi:10.1016/j.cell.2004.11.012
47. Balastik M, Zhou XZ, Alberich-Jorda M, et al. Prolyl isomerase Pin1 regulates axon guidance by stabilizing CRMP2A selectively in distal axons. Cell Rep. 2015;13(4):812–828. doi:10.1016/j.celrep.2015.09.026
48. Ip JP, Fu AK, Ip NY. CRMP2: functional roles in neural development and therapeutic potential in neurological diseases. Neuroscientist. 2014;20(6):589–598. doi:10.1177/1073858413514278
49. Yuasa-Kawada J, Suzuki R, Kano F, et al. Axonal morphogenesis controlled by antagonistic roles of two CRMP subtypes in microtubule organization. Eur J Neurosci. 2003;17:2329–2343. doi:10.1046/j.1460-9568.2003.02664.x
50. Ziak J, Weissova R, Jeřábková K, et al. CRMP2 mediates Sema3F-dependent axon pruning and dendritic spine remodeling. EMBO Rep. 2020;e48512. doi:10.15252/embr.201948512
51. Zhang H, Kang E, Wang Y, et al. Brain-specific Crmp2 deletion leads to neuronal development deficits and behavioral impairments in mice. Nat Commun. 2016;7. doi:10.1038/ncomms11773
52. Buzzigoli SB, Genovesi M, Lambelet P, Logi C, Raffaelli S, Cattano D. Plasmapheresis treatment in Guillain-Barré syndrome: potential benefit over intravenous immunoglobulin. Anaesth Intensive Care. 2010;38(2):387–389. doi:10.1177/0310057X1003800225
53. Mitoma H, Manto M, Hampe CS. Immune-mediated cerebellar ataxias: practical guidelines and therapeutic challenges. Curr Neuropharmacol. 2019;17(1):33–58. doi:10.2174/1570159X16666180917105033
54. Vaccaro C, Zhou J, Ober RJ, Ward ES. Engineering the Fc region of immunoglobulin G to modulate in vivo antibody levels. Nat Biotechnol. 2005;23(10):1283–1288. doi:10.1038/nbt1143
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.