Back to Journals » OncoTargets and Therapy » Volume 12
BRMS1 downregulation is a poor prognostic biomarker in anaplastic thyroid carcinoma patients
Authors Wu Y, Wang H, Zhi J, Hu L, Hou X, Ruan X, Zheng X, Liu H, Gao M
Received 14 June 2019
Accepted for publication 10 August 2019
Published 28 August 2019 Volume 2019:12 Pages 6937—6945
DOI https://doi.org/10.2147/OTT.S219506
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Gaetano Romano
Yu Wu,1–5,* Huijuan Wang,1–4,* Jingtai Zhi,1–4,* Linfei Hu,1–4 Xiukun Hou,1–4 Xianhui Ruan,1–4 Xiangqian Zheng,1–4 Hui Liu,5 Ming Gao1–4
1Department of Thyroid and Neck Tumor, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300600, People’s Republic of China; 2Department of National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300600, People’s Republic of China; 3Department of Tianjin’s Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300600, People’s Republic of China; 4Department of Key Laboratory of Cancer Prevention and Therapy, Tianjin Medical University Cancer Institute and Hospital, Tianjin Medical University, Tianjin 300600, People’s Republic of China; 5Department of Head and Neck Surgery, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, Fujian 350014, People’s Republic of China
Correspondence: Hui Liu
Department of Head and Neck Surgery, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, Fujian 350014, People’s Republic of China
Tel +86 1 380 506 9511
Email [email protected]
Ming Gao
Department of Thyroid and Neck Tumor, National Clinical Research Center for Cancer, Tianjin Medical University Cancer Institute and Hospital, Tianjin 300060, People’s Republic of China
Tel +86 1 382 088 1516
Email [email protected]
*These authors contributed equally to this work
Background: Anaplastic thyroid carcinoma (ATC) is the most aggressive cancer in humans with no optimal treatment strategy available. The molecular mechanisms of ATC remain unclear. The aim of this study was to investigate the prognostic value and role of BRMS1 in the progression of ATC.
Methods: BRMS1 expression was examined in thyroid cell lines using Western blot analysis. Immunohistochemistry was also performed to assess BRMS1 expression in ATC and papillary thyroid cancer (PTC) tissue. Cell proliferation assays, colony formation analysis, cell migration assays, cell apoptosis analysis, and animal studies were used to examine the effects of BRMS1 expression on ATC progression.
Results: The expression of BRMS1 was significantly lower in ATC than in PTC and was associated with poor prognosis in ATC patients. Downregulation of BRMS1 expression promoted the proliferation and migration of 8505C cells and decreased their expression of CX43. Over-expressed BRMS1 promoted the apoptosis and impaired the proliferation and migration of CAL-62 cells via upregulated CX43. In vivo, BRMS1 significantly promoted apoptosis and impaired cell proliferation.
Conclusion: Taken together, these findings demonstrate that decreased expression of BRMS1 is a poor prognostic biomarker in ATC patients. BRMS1 significantly promoted apoptosis and impaired cell proliferation via CX43 and P53. Loss of BRMS1 expression is therefore, one of the key pathomechanisms in ATC.
Keywords: BRMS1, anaplastic thyroid carcinoma, prognosis, CX43
Introduction
Thyroid cancer (TC) is the most common malignancy of the endocrine system.1 For most TC patients, the standard treatment (surgery followed by either radioactive iodine or observation) can promise an excellent prognosis.2 Anaplastic thyroid carcinoma (ATC) is a rare subtype of TC, only accounting for 1–2% of TC. However, the mortality rate of ATC approaches 100% with an average survival time of 3–6 months, rendering it the most aggressive cancer in humans.3 Because of the rarity of this subtype, the management of ATC patients is based on clinical experience and published case series. ATCs often develop into inoperable and metastatic tumors, and the conventional treatment for ATC has failed to prevent ATC progression. Although the optimal treatment strategy remains unclear, patients usually undergo multimodal therapy, including tumor debulking surgery, external beam radiation, combination chemotherapy, systemic therapy, and palliative care.4 Unfortunately, despite improvements in every component of multimodal therapy, the prognosis of ATC and the prevention of recurrent disease are still poor; over the past 30 years, the survival rate of ATC patients has remained unchanged.5 In order to develop effective therapeutic interventions, we urgently need to understand the molecular mechanisms of ATC.6
Breast cancer metastasis suppressor 1 (BRMS1), which maps to chromosome 11q13, was first identified as a metastasis suppressor gene in breast cancer cell lines.7 The expression of BRMS1 is now regarded as a biomarker for predicting the prognosis of patients with various kinds of cancer. In nasopharyngeal carcinoma (NPC), the overexpression of BRMS1 significantly reverses the metastatic phenotype, and low expression of BRMS1 is associated with the poor prognosis of NPC patients.8 In osteosarcoma, low BRMS1 is correlated with metastasis and poor patient survival.9 These studies indicate that BRMS1 plays an important role in the progression of tumors. However, the prognostic value of its expression and the pathways regulated by BRMS1 in ATC are still unknown. The aim of this study was to investigate the prognostic value and role of altered BRMS1 expression in ATC.
Methods
Ethics statement
This study was approved by the Research Ethics Committee of tianjin medical university cancer institute and hospital. The informed written consents were collected from all enrolled patients and the entire study was performed based on the Declaration of Helsinki.
Cell culture and transfection
Human ATC cell lines (CAL-62 and 8505C) were obtained from the American Type Culture Collection. 8505C was maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). CAL-62 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS. Both cell lines were authenticated by short tandem repeat (STR) analysis. Cells were transfected using RNAiMix (Invitrogen) according to the manufacturer’s instructions. To investigate the role of BRMS1 in ATC progression and whether regulating CX43 expression was the key downstream mechanism, we used siRNA to decrease BRMS1 and CX43 expression. The siRNA-BRMS1 (siBRMS1), siRNA-CX43 (siCX43), and siNC were synthesized by Sigma-Aldrich (St. Louis, MO, USA). SiRNA ID was listed (Table 1) as follows:
 |
Table 1 The siRNA-BRMS1 (siBRMS1), siRNA-CX43 (siCX43), and siNC were synthesized by Sigma-Aldrich |
Plasmid pcDNA3-Flag-BRMS1 and were obtained from syngentech (Beijing, China).
Immunoblot analyses and antibodies
One normal thyroid cell line (N1), four PTC cell lines (TPC-1, BCPAP, K1and IHH4), three ATC cell lines (CAL-62, 8305C and 8505C) and transfected cell lines were lysated for immunoblot analyses. Cell lysates were measured by BCA protein assay reagent (Solarbio, Beijing, China). Cell lysates were resolved by SDS–PAGE and immunoblotted with indicated antibodies. The related antibodies we used includes anti-BRMS1 (ab134968, abcam), anti-CX43 (3512, Cell Signaling), anti-P53 (2527, Cell Signaling), anti-Bcl2 (15071, Cell Signaling), anti-Bax (5023, Cell Signaling) and anti-GAPDH (5174, Cell Signaling).
Immunohistochemical staining
ATC tissue specimens were obtained from patients who underwent surgery at Tianjin Medical University Cancer Institute and Hospital. Two experienced pathologists established the diagnosis of ATC and other characteristic pathological parameters. Briefly, 27 cases of ATC tissue specimens were blocked with 3% H2O2 for 30 mins. Tissue sections were incubated with primary antibodies against BRMS1, CX43 and CC3 (9664, Cell Signaling Technology). Sections were incubated with anti-mouse/rabbit horseradish peroxidase at room temperature for 30 min followed by staining with DAB substrate. Samples were counterstained with hematoxylin for three minutes. Two pathologists blinded to the clinicopathological information independently examined the stained slide.
Cellular migration assays
Cell migration was assessed by plated in a 24-well plate 8.0-μm chamber insert according to the manufacturer’s protocol. 30,000 cells (8505C, CAL-62, and transfected cells, respectively) were seeded in serum-free medium on the top of insert. After incubation at 37 °C for 6 hrs, the cells in the upper chamber were carefully scraped with a cotton swab, and the cells passing through the membrane were fixed in methanol and stained with crystal violet blue. Five separate areas under the filter were photographed and counted with microscope.
Cell proliferation assay
CCK-8 (Beyotime, Nantong, China) was used to analyze the cell proliferation. 1000 cells (8505C, CAL-62, and transfected cells, respectively) suspended in 100 µl DMEM medium containing 10% fetal bovine serum were inoculated into 96-well plates for 0, 24, 48, 72 and 96 hrs, and 10 µl CCK-8 solution was added into each hole, and the culture was incubated at 37 °C for 2 hrs. Absorption at 450 nm was measured on the ELX-800 spectrometer reader.
Colony forming assay
For colony forming assay, cells were seeded in a 6-well plate at 500 cells per well in duplicates and cultured for 8 days. Cells were then stained with 0.05% crystal violet before photographing.
Cell apoptosis analysis
The cell apoptosis was evaluated by flow cytometry by using a fluorescein isothiocyanate (FITC) Annexin V apoptosis kit (BD Pharmingen, Franklin Lakes, NJ, USA) according to manufacturer’s instructions.
Animal studies
6-week-old female BALB/C nude mice (Charles River Laboratories, Beijing, China) were subcutaneously injected with 2×106 control or BRMS1-overexpressed CAL-62 cell suspension. The formula for calculating the volume of tumors is V=0.5 x long, x wide.2 The tumors were measured every three days. After harvesting, the tumors were fixed overnight in formalin for immunohistochemical analysis. All animal studies were approved by the institutional ethical committee of Tianjin Medical University (permit # SYXK 2009-0001), and were in compliance with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals.
Statistical analysis
Statistical analyses were performed using SPSS v22.0 (IBM, NY, USA) or GraphPad Prism v6.02 (GraphPad Software, La Jolla, CA). The results were repeated in at least three times and are shown as the mean±standard deviation. An unpaired t-test was used to calculate P-values between different treatment cohorts. P<0.05 was considered as statistically significant.
Results
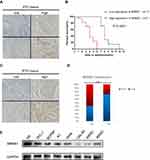
BRMS1 is associated with the poor prognosis of patients with ATC
We first investigated the clinical relevance of BRMS1 expression in ATC patients. BRMS1 protein expression was examined by immunohistochemical staining of tissue samples derived from ATC (a total of 27 cases). All ATC tissues were divided into two groups: low BRMS1 expression and high BRMS1 expression (Figure 1A). Both groups were subsequently assessed for associations with the survival outcomes of ATC patients. Low BRMS1 expression correlated significantly with a decreased survival time (Figure 1B). BRMS1 staining was further performed in 185 PTC patient samples. All PTC tissues were divided into two groups: low BRMS1 expression and high BRMS1 expression (Figure 1C). As shown in Figure 1D, BRMS1 expression was significantly higher in ATC samples compared to PTC samples (73% vs 41%). Next, we tested the expression of BRMS1 in one thyroid cell line, four PTC cell lines, and three ATC cell lines. The expression of BRMS1 in the ATC cell lines was significantly lower than in the thyroid cell line and PTC cell lines (Figure 1E). The above data indicate that decreased BRMS1 expression could be a candidate biomarker of poor clinical prognosis of patients with ATC.
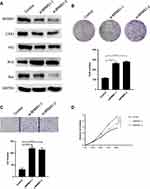
Downregulation of BRMS1 expression promotes the proliferation and migration of 8505C cells
As cell proliferation and migration are important factors in tumor progression, and BRMS1 expression is significantly reduced in ATC, we investigated the role of BRMS1 in ATC cell proliferation and migration. First, we transfected the ATC cell line 8505C with siRNA targeted toward BRMS1 and found that BRMS1 was downregulated in this cell line compared with the control cells (Figure 2A). Upregulation of CX43 and p53 by BRMS1 has been confirmed in other cancers; therefore, we investigated the impact of decreased BRMS1 on the expression of CX43 and p53. As expected, decreased expression of BRMS1 significantly downregulated the expression of CX43 and p53. Expression of BCL2 was increased while that of BAX remained contrast. Decreased expression of BRMS1 increased cell viability, colony formation, and migration of the 8505C cell line after transfection with the siRNA (Figure 2B–D). These results indicated that downregulated BRMS1 expression affected CX43 and p53 expression and was significantly correlated with the increased cell proliferation and migration of 8505C cells.
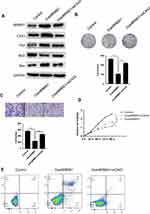
Over-expressed BRMS1 promotes the apoptosis and impairs the proliferation and migration of CAL-62 cells through upregulated CX43
We transfected ATC cell line CAL-62 with plasmid pcDNA3-Flag-BRMS1 and found that BRMS1 was upregulated in this cell line compared with the control cells (Figure 3A), which significantly upregulated the expression of CX43 and p53. Expression of BCL2 was decreased while that of BAX remained constant (Figure 3A). Overexpression of BRMS1 reduced the cell proliferation, migration capacity, and colony formation of the CAL-62 cell line (Figure 3B–D). Flow cytometry showed that overexpression of BRMS1 induced massive cell apoptosis compared with the control group (Figure 3E). To investigate whether BRMS1 downregulates p53 expression via CX43, we transfected BRMS1-overexpressing CAL-62 cells with siRNA to downregulate CX43 expression. Along with restored p53, BAX, and BCL2 expression, cell proliferation, migration, and colony formation also increased as expected (Figure 3A–D). The rate of apoptosis also declined in the transfected cell line (Figure 3E). These data show that upregulated BRMS1 induced apoptosis and decreased the proliferation and migration of ATC cells, through downregulation of p53 expression via upregulated CX43.
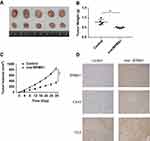
BRMS1 promotes apoptosis and impairs proliferation in vivo
Next, our study sought to examine the in vivo efficacy of BRMS1. For this purpose, a nude mice xenograft model was implanted with either control CAL-62 cells or BRMS1-overexpressing CAL-62 cells, and the expression of BRMS1 was tested by immunostaining. As shown in Figure 4A–C, overexpressed BRMS1 markedly reduced tumor growth in mice. Xenografts isolated from the BRMS1-overexpressed group had significantly more apoptotic cells than those derived from the control group, as measured by cleaved caspase-3 (CC3) staining (Figure 4D). Compared with the control group, the expression of CX43 was also increased in the BRMS1-overexpressed group. Altogether, these results demonstrate that BRMS1 promotes apoptosis and impairs cell proliferation through CX43 both in vitro and in vivo.
Discussion
BRMS1 is a component of multiple SIN3-HDAC complexes, which regulate various cellular pathways, including the STAT3, p53, NF-
Our study found that BRMS1 expression was significantly lower in ATC than in normal thyroid tissue or PTC. Furthermore, decreased BRMS1 expression was associated with poor patient prognosis, suggesting that loss of BRMS1 expression plays an important role in ATC progression. In vitro experiments showed that knocking down BRMS1 expression significantly increased the proliferation and migration of ATC cells. Overexpressing BRMS1 in CAL-62 cells caused the opposite phenotypes, inducing apoptosis of ATC cells, which was consistent with other experiments.
In breast cancer, Liu et al demonstrated that p53 acetylation, not p53 expression, was affected by BRMS1. BRMS1 interacts with DBC1 to mediate SIRT1-dependent p53 deacetylation.13 However, in our study, BRMS1 expression significantly affected p53 levels, suggesting BRMS1 may affect p53 activity through different mechanisms. As a ubiquitous mode of communication between cells, gap junctions are widely involved in regulating cell proliferation, differentiation, apoptosis, and homeostasis. As an important protein in gap junction-mediated intercellular communication, abnormal CX43 expression has been shown to be involved in the dysfunction of this form of intercellular communication, playing a role in the progression of many malignant tumors.14 Thus, CX43 protein is a useful biomarker for the early diagnosis of malignancies of the breast, lung, and other organs,15,16 as its expression level is correlated with tumor progression and patient prognosis. Jensen et al found that increased CX43 expression in thyroid cancer cells can significantly sensitize these malignant cells to anoikis, suggesting that decreased CX43 expression is an important mechanism in thyroid cancer progression.17 Saunders et al found that BRMS1 expression in MDA-MB-435 cells dysregulated CX43 expression, leading to a phenotype in gap junction activity that was similar to that of normal breast tissue.18 In head and neck squamous cell carcinomas, CX43 expression was positively correlated with p53 expression.19 Our study confirmed that BRMS1 expression modulated CX43 and p53 expression both in vivo and in vitro. In the 8505C cell line, decreasing BRMS1 expression with siRNA significantly dysregulated CX43 and p53 expression. In CAL-62 cells, overexpressing BRMS1 increased the expression of CX43 and p53, while knocking down CX43 in BRMS1-overpressing CAL-62 cells significantly decreased p53 expression. Simultaneously, cell proliferation and invasiveness were restored, and apoptotic cells were reduced after decreasing CX43 expression. In ATC, loss of BRMS1 induced increased proliferation and migration of cancer cells, along with downregulating p53 expression through CX43.
In conclusion, decreased BRMS1 expression is a poor prognostic biomarker in ATC patients. BRMS1 significantly promoted apoptosis and impaired proliferation via CX43 and P53. Therefore, loss of BRMS1 expression is one of the key pathomechanisms of ATC.
Ethics approval and informed consent
This study was approved by the Research Ethics Committee of Tianjin Medical University Cancer Institute and Hospital. The informed written consents were collected from all enrolled patients and the entire study was performed based on the Declaration of Helsinki.
Acknowledgments
We thank Natasha Beeton-Kempen, Ph.D., from Liwen Bianji, Edanz Editing China, for editing the English text of a draft of this manuscript. This research was supported by the Natural Science Foundation of Fujian Province (No. 2019J01190 and No. 2017J01258).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Wang Z, Chen JQ, Liu JL, et al. Clinical impact of BRAF mutation on the diagnosis and prognosis of papillary thyroid carcinoma: a systematic review and meta-analysis. Eur J Clin Invest. 2016;46(2):146–157. doi:10.1111/eci.12577
3. Cao X, Dang L, Zheng X, et al. Targeting super-enhancer-driven oncogenic transcription by CDK7 inhibition in anaplastic thyroid carcinoma. Thyroid. 2019;29:809–823. doi:10.1089/thy.2018.0550
4. Cabanillas ME, Zafereo M, Gunn GB, et al. Anaplastic thyroid carcinoma: treatment in the age of molecular targeted therapy. J Oncol Pract. 2016;12(6):511–518. doi:10.1200/JOP.2016.012013
5. Tiedje V, Stuschke M, Weber F, et al. Anaplastic thyroid carcinoma: review of treatment protocols. Endocr Relat Cancer. 2018;25(3):R153–R161. doi:10.1530/ERC-17-0435
6. Valerio L, Pieruzzi L, Giani C, et al. Targeted therapy in thyroid cancer: state of the art. Clin Oncol (R Coll Radiol). 2017;29(5):316–324. doi:10.1016/j.clon.2017.02.009
7. Kodura MA, Souchelnytskyi S. Breast carcinoma metastasis suppressor gene 1 (BRMS1): update on its role as the suppressor of cancer metastases. Cancer Metastasis Rev. 2015;34(4):611–618. doi:10.1007/s10555-015-9583-z
8. Cui RX, Liu N, He QM, et al. Low BRMS1 expression promotes nasopharyngeal carcinoma metastasis in vitro and in vivo and is associated with poor patient survival. BMC Cancer. 2012;12:376. doi:10.1186/1471-2407-12-376
9. Li G, Li L, Sun Q, et al. MicroRNA-3200-5p promotes osteosarcoma cell invasion via suppression of BRMS1. Mol Cells. 2018;41(6):523–531. doi:10.14348/molcells.2018.2200
10. Mei P, Bai J, Shi M, et al. BRMS1 suppresses glioma progression by regulating invasion, migration and adhesion of glioma cells. PLoS One. 2014;9(5):e98544. doi:10.1371/journal.pone.0098544
11. Wu J, Wang Y, Qiao X, et al. Cloning and characterization of a novel human BRMS1 transcript variant in hepatocellular carcinoma cells. Cancer Lett. 2013;337(2):266–275. doi:10.1016/j.canlet.2013.04.030
12. You J, He X, Ding H, et al. BRMS1 regulates apoptosis in non-small cell lung cancer cells. Cell Biochem Biophys. 2015;71(1):465–472. doi:10.1007/s12013-014-0226-8
13. Liu X, Ehmed E, Li B, et al. Breast cancer metastasis suppressor 1 modulates SIRT1-dependent p53 deacetylation through interacting with DBC1. Am J Cancer Res. 2016;6(6):1441–1449.
14. Bodenstine TM, Vaidya KS, Ismail A, et al. Homotypic gap junctional communication associated with metastasis suppression increases with PKA activity and is unaffected by PI3K inhibition. Cancer Res. 2010;70(23):10002–10011. doi:10.1158/0008-5472.CAN-10-2606
15. Plante I, Stewart MK, Barr K, et al. Cx43 suppresses mammary tumor metastasis to the lung in a Cx43 mutant mouse model of human disease. Oncogene. 2011;30(14):1681–1692. doi:10.1038/onc.2010.551
16. Bucciarelli PR, Tan KS, Chudgar NP, et al. BRMS1 expression in surgically resected lung adenocarcinoma predicts future metastases and is associated with a poor prognosis. J Thorac Oncol. 2018;13(1):73–84. doi:10.1016/j.jtho.2017.10.006
17. Jensen K, Patel A, Klubo-Gwiezdzinska J, et al. Inhibition of gap junction transfer sensitizes thyroid cancer cells to anoikis. Endocr Relat Cancer. 2011;18(5):613–626. doi:10.1530/ERC-10-0289
18. Saunders MM, Seraj MJ, Li Z, et al. Breast cancer metastatic potential correlates with a breakdown in homospecific and heterospecific gap junctional intercellular communication. Cancer Res. 2001;61(5):1765–1767.
19. Danos K, Brauswetter D, Birtalan E, et al. The potential prognostic value of connexin 43 expression in head and neck squamous cell carcinomas. Appl Immunohistochem Mol Morphol. 2016;24(7):476–481. doi:10.1097/PAI.0000000000000212
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.