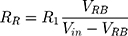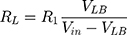Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Breast Cancer Detection Using Low-Frequency Bioimpedance Device
Authors Mansouri S , Alhadidi T, Ben Azouz M
Received 28 July 2020
Accepted for publication 1 September 2020
Published 18 September 2020 Volume 2020:12 Pages 109—116
DOI https://doi.org/10.2147/BCTT.S274421
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Sofiene Mansouri,1,2 Tareq Alhadidi,1 Marwa Ben Azouz2
1College of Applied Medical Sciences, Department of Biomedical Technology, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia; 2University of Tunis El Manar, Higher Institute of Medical Technologies of Tunis, Laboratory of Biophysics and Medical Technologies, Tunis 1009, Tunisia
Correspondence: Sofiene Mansouri
College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
Tel +966533791123
Email [email protected]
Introduction: Early detection of breast cancer saves lives. Existing detecting techniques are invasive. Electrical bioimpedance is a noninvasive technique and has a high diagnostic potential.
Methods: An impedance value different from the normal can predict a physiological abnormality. The idea is to use a designed bioimpedance device to early detect breast cancer. A low-frequency current (1 kHz, 0.9 mA) is injected to each breast to measure the extracellular resistances. The resistances of the two breasts are then measured, and if there is a significant difference, warning is displayed. The performance was tested on a set of reference resistors, and the validation was done in vitro on (Na+Cl-) solutions and in vivo on a group of forty volunteer women.
Results: The results confirm that the electrical conductivity of an ionic solution is proportional to its concentration. The concentration and the resistance are strongly correlated (correlation coefficient of 0.97). The accuracy and the repeatability of the measures were satisfactory. Early detection means that we can detect small extracellular concentration variations into the breast (from 0.6 g/l). In vivo measurements made it possible to set the threshold at 50 ohm. If the difference between the two measured breast resistances is greater than this threshold, we advise the patient to consult a doctor promptly.
Conclusion: The difference between measured resistances of the right and left breast is a pertinent parameter to early detect the presence of a cancer. The lowest resistance value (RR or RL) can provide information on the breast affected by the cancer (right or left). Various improvements in the system are possible but already the results are encouraging. In the future, this system could be integrated into a bra.
Keywords: biomedical engineering, breast cancer detection, electrical bioimpedance, FPGA Virtex-5 LX30, LabVIEW FPGA, NI PXI-7841R
Introduction
Breast cancer is a serious condition that threatens the lives of most women. The reduction of the mortality rate and the improvement of the chances of cure are only possible if the tumor is managed at the first stages of its appearance. The mammary gland is an organ in permanent evolution. The cells are still growing and differentiating, making it more susceptible to cancerous transformations. In case of breast cancer, the cells can remain in the breast or disperse in the body through the blood or lymphatic vessels. The progression and/or dispersion of breast cancer take, most of the time, several months or even a few years.1 Breast cancer usually develops in the milk ducts and lobules. The carcinogenesis process has four phases: initiation, promotion, progression and invasion.2 The most common sign is the presence of a mass in the breast. It is irregular in shape and very different from the rest of the breast tissue. Parallel to the site where they develop, we also distinguish cancers according to their stage of evolution. The stage is defined by the size of the tumor (T0 to T4). T0, the cancer is confined within the ducts or lobules of the breast tissue and has not spread into the surrounding tissue of the breast. T1, the tumor in the breast is 2 centimeters (cm) or smaller in size at its widest area. T2, the tumor is larger than 2 cm but not larger than 5 cm. T3, the tumor is larger than 5 cm. T4, the tumor has grown into the chest wall or into the skin or the chest wall and the skin or inflammatory breast cancer. In this case, breast cancer is said to be infiltrating.3 In Tunisia, the average tumor size diagnosed is 3 cm (Stage 2). Different screening methods such as mammography, ultrasound imaging and magnetic resonance imaging are used.4–8 They differ in terms of efficiency, invasiveness, cost, availability and ease of use. The different screening techniques have advantages and disadvantages.
Bioimpedance can offer a new and effective alternative for early detection of breast cancer. Bioimpedance is suggested as another method of detecting cancer because cancerous tissues have different electrical characteristics than normal tissues.9 By applying a current to an object through a finite number of electrodes, an electrical impedance system produces a precise impedance value of the object according to the electrical characteristics of the different tissues throughout the volume of the object. Numerous studies have shown that these systems can distinguish between cancerous and normal tissues.10–15
Materials and Methods
Bioimpedance corresponds to the opposition of tissues to the passage of a current. Various researchers have performed impedance measurements on healthy normal tissues as well as cancerous tissues of the human breast. However, some representative results are found.6,16–18 From these impedance measurements it can be deduced that compared to normal tissues, malignant tumors showed a higher current conductivity.17,18
The conductance G, (in Siemens) reflects the ability of the electrolytic solution to pass the electric current. It is the inverse of the resistance R (in Ω). It can be concluded that malignant breast tumors have lower electrical impedance than normal tissues. Morimoto et al13,14 suggested that impedance measurement can be used for the differential diagnosis of malignant and benign tumors. From the previous results, the mammary impedance measurement shows that malignant breast tumors have lower electrical impedance than normal tissues. Therefore, electrical impedance can be used as an indicator for the detection of breast cancer. There are significant differences between benign and malignant breast tumors9,11,16 and.19 Electrical impedance can therefore also be used to separate benign tumors from malignant tumors and thus reduce benign biopsies.17,20
To use this method in the early detection of breast cancer, we designed a new bioimpedance device. We aimed to have a noninvasive and efficient device which can be easily integrated into a bra in the future. The new bioimpedance device has been tested in vitro and in vivo. The use of alternating current in bioimpedance-based measurement systems aims to track the change in electrical impedance produced by structural or functional changes in the system under test. The different researchers had used a low amplitude AC current and a variable frequency for their impedance measurements on breast tissue.12,21 When applying a low-frequency current, the cell membrane prevents the current from entering the cell, limiting it to pass only in the extracellular fluid. Since the extracellular resistance of a tumor is less than that of the healthy tissue, then a weak low-frequency current is sufficient to determine the extracellular resistance of the mammary tissues.11 The use of low-frequency current has allowed us to simplify Fricke’s model and reduce it to a simple extracellular resistance, since the low-frequency current cannot penetrate the intracellular medium, Figure 1. In the extracellular medium, we do not have the capacitive effect of the membrane. The impedance is purely resistive and therefore the impedance Z can be equated with the resistance R.
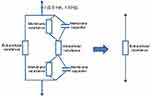 |
Figure 1 Simplification of the Frick’s model following the use of a low-frequency current. |
Currents are considered low frequency if they are less than 5 kHz.22 The majority of the lines of the electric field pass through the extracellular medium without being able to penetrate inside the cells. On the other hand, currents of high frequencies arrive easily to cross the capacitive membrane and the lines of the field thus pass by the two mediums intra and extra cellular indifferently.
The Designed Device
The prototype is composed by an acquisition card, the NI PXI-7841R, a chassis the NI PXI-1033, a shielded connector blocks the NI SCB-68 and development software, LabVIEW (LabVIEW Professional Development System with MathScript 8.6) with its LabVIEW FPGA module. The core of the NI PXI-7841R is an FPGA target, Xilinx’s Virtex-5 LX30. The configuration of the FPGA was not done by the VHDL language but rather by graphical programming thanks to LabVIEW FPGA. The design prototype satisfies the electrical safety requirements of biomedical equipment.23
Injection Module
We just change the frequency of the injection signal. The injection current is a 1 kHz square wave, with 5 V amplitude and 0.9 mA intensity. When injecting current into the mammary glands, two considerations must be taken into account: the protection of the patient and the obligation to have two symmetrical outlets directed towards the two mammary glands.
Acquisition Module
Measurement of the impedance of each breast is performed by two voltage dividers connected to the FPGA prototype. The impedance of each breast is measured using one voltage divider. The acquisition module is connected to each breast by two electrodes.
From these two voltage dividers, the designed system determines the right breast resistance (RR) by using (1) and the left breast resistance (RL) by using (2) and then calculates the difference between the two measurements.
VRB represents the voltage tension collected between the electrodes placed on the right breast and VLB is the tension collected in the left breast (Vin = 5 V).
If the difference is greater than a threshold, we display a warning message, asking the patient to promptly consult a doctor. If the difference is less than the threshold, we memorize the two resistance values for a possible comparison with future measured values. The program flowchart is presented in Figure 2.
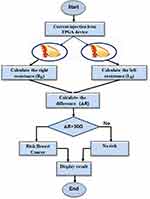 |
Figure 2 Program flowchart. |
To validate the designed system, we tested first the precision of the resistance determination and then we made in vitro measurements on (Na+Cl−) solutions and in vivo on a group of forty women volunteers. All subjects were voluntary and signed a consent document which specified the nature of the measures to be taken. In addition, the measures are non-invasive, non-dangerous and the current used is very low and has no effect on women volunteers. The biological tissues are composed of about 60% of water: two-thirds is inside the cells (cytoplasm) and the remaining third in the extracellular medium (interstitial medium). For low frequencies, the ionic conductivity of a tissue is highly dependent on the extracellular fluid present. The conductivity of biological tissues also depends on the temperature of about 2%/°C which is similar to the temperature coefficient of a saline solution. We have chosen (Na+Cl−) solutions because, extracellular liquids can be considered as aqueous solutions and the most numerous ions are by far the Na+ and Cl− ions, Table 1.24
 |
Table 1 Intracellular and Extracellular Concentrations of Ions |
Breast Phantoms
To perform in vitro measurements, we designed two identical hemispheric phantoms, made of plastic. The shape of a mammary gland is hemispheric in European and Asian women, rather conical in African women. The 2 nipples are spaced about 20 cm apart. The breast phantom size is 12 cm in height and 12 cm in diameter. Each phantom is connected to the designed system by four conductor wires (two for current injection and two for voltage measurement). To ensure that the conductor wires are immersed in the aqueous solutions of Na+Cl−, the phantom must be filled with a volume of water equal to 340 mL. Aqueous solutions of sodium chloride are prepared under the same conditions, only the amount of dissolved (Na+Cl−) which varies from one solution to another. The different concentrations prepared are presented in Figure 3. In the Na+Cl− solutions, in the absence of an electric current, the charges are immobile. When we apply an electric current, we observe a displacement of the charges and thus ionic conduction. The ionic solution behaves like an ohmic conductor. The aqueous solution of sodium chloride is a strong electrolyte. The presence of ions makes the solution a good conductor of electric current. We validated first the performance of the system designed in vitro using the two breast phantoms and the Na+Cl− solutions. Then in vivo on a group of forty volunteer women.
 |
Figure 3 Different solutions of Na+Cl− with water volume = 340 mL. |
Results
The performance of the system designed was tested on a set of reference resistors and then validated in vitro and in vivo. In this section, we presented the results obtained. For in vitro measurements, we used the two breast phantoms filled by Na+Cl− solutions with different concentrations. In vivo measurements were made on a group of forty volunteered women.
Resistors Measurements
The designed system has been tested on a set of resistors. We measured a set of resistors by a UT33C digital multimeter and the designed device. Figure 4 presents the Bland-Altman analysis for resistor measurement accuracy. The percent error equals 0.31%.
 |
Figure 4 Bland-Altman analysis for resistors measurement accuracy, with Bias = 5.09, upper LOA= 20.36 and lower LOA= −10.18. |
In vitro Measurements
In this section, we simultaneously measured into the two breast phantoms the resistances of the different Na+Cl− solutions. Then we studied the correlation between the resistance and the concentration of the solution. Finally, we studied the repeatability of the measures. We used the designed system, the two breast phantoms and the fourteen different solutions. Figure 5 presents the resistances obtained in the two phantoms, RR for the phantom placed on the right (by analogy to the right breast) and RL for the phantom placed on the left (left breast).
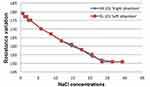 |
Figure 5 Resistance variation as a function of Na+Cl− concentration. |
The resistance R is inversely proportional to the Na+Cl− concentration C. In fact, the less concentrated solutions have greater resistance values compared to solutions of high concentrations. The two curves are decreasing, which shows that the resistance of an electrolyte is inversely proportional to its concentration. The measurements obtained confirm Kohlrausch’s law: the electrical conductivity of an electrolyte is proportional to its concentration. From the concentration value (C = 26.5 g/l), the solutions become saturated, the sodium chloride is no longer soluble in water. The curves are stable and the resistances measured remain invariable and equal to 151 Ω. For our application, breast cancer screening, this limit is not a problem, because when the solution is saturated this will correspond to an advanced stage of the cancer and not to an early detection stage. A correlation analysis was done; the concentration and the resistance are strongly correlated. We obtained a correlation coefficient of ‘0.97ʹ. The error between the adjusted values and the measured values is 0.969 Ω for the right phantom, and 0.972 for left phantom. The lowest concentration value we detected is 0.6g/l, certainly, we can go further but we must realize a more complex system. To study the repeatability, we used five different Na+Cl− solutions; we measured the resistance at each minute during 10 minutes (Figure 6). For each solution, from the fifth minute the resistance did not change any more. This observation allowed us to require a five-minute delay before each in vivo measurement.
 |
Figure 6 Evolution of the resistance as a function of time for different Na+Cl− concentrations. |
In vivo Measurements
The designed system has been tested on forty volunteer women of different ages. The present study and protocol were conducted in accordance with the Declaration of Helsinki and approved by the Research Ethics Committee in Health and Science Disciplines (REC-HSD) of the Prince Sattam bin Abdulaziz University (approval no. REC-HSD-013-2020). The adopted experimental protocol begins by placing the woman in a supine position on her back. Then, on each breast, we placed four electrodes: Two for the injection of the low-frequency current and two for the acquisition of the signal. We waited five minutes (Figure 6, temporal stabilization of the measurement); then, we recorded the resistances measured and deduced the difference between the right and left breast resistances. The measurements obtained are illustrated in Table 2.
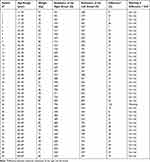 |
Table 2 In Vivo Measurements |
Discussion
The high resistance measured values indicate a denser breast,5.25,26 The appearance of micro-calcifications in a woman’s breasts alters the hydration of breast tissue and the permeability of the cell membrane, resulting in significant variations in electrical impedance,7,10,25–27 Jossinet and Schmitt,20 Morimoto et al14 confirmed that malignant breast tumors have lower electrical impedance than surrounding normal tissues. Then for an early detection of breast cancer, a significant difference between the extracellular resistances obtained from the right and left breast can be a relevant parameter. We computed the difference between the resistance measures of the right and left breast. We retained the difference value equal to 50 Ω as a threshold. If the difference is greater than 50 Ω, this means that there is a serious risk and a warning message is displayed.
The results show that the difference is small than 50 Ω in thirty-nine cases. Only patient number 26 presents a high difference, 120 Ω. We asked here to go to the hospital and make a breast cancer screening. The examinations carried out at the hospital confirmed that she had a stage 2 breast cancer in her right breast. Actually, the extracellular resistance of the right breast is lower than that of the left breast, RR = 730 Ω and RL = 850 Ω.
The results presented above, show the relevance of the choices selected. Indeed, in vitro and in vivo measurements have confirmed that the bioimpedance method can offer a serious alternative for the early detection of breast cancer. Early detection means that we can detect small extracellular concentration variations into the breast (from 0.6 g/l). Various improvements in the system are possible but already the results are encouraging. This method is non-invasive (no X-ray or any other ionizing radiation), low cost and can be used safely at home.
Conclusion
The designed system based on the use of a low-frequency current allowed us to realize a noninvasive device which can be integrated into a bra. The designed system is connected. Each woman can have it and perform a self-check whenever she wants (noninvasive and safe). Malignant breast tumors have lower electrical impedance than surrounding normal tissues. The use of low-frequency current has permit to focus only on the breast the extracellular resistance. The difference of the measured resistances of the right and left breast is a pertinent parameter to early detect the presence of a cancer. A difference greater than 50 Ω is sufficient to decide whether to visit a cancer center for further investigations. In this case, the lowest resistance value (RR or RL) can provide information on the breast affected by the cancer (Right or left). The in vitro study has permit to confirm the strong correlation between the concentration of the Na+Cl− and the resistance (r=0.97). After five minutes the measures became stable and the difference between the two phantoms is less than 1 Ω.
In order to improve our work, we will first carry out a measurement campaign on subjects suffering from breast cancer as well as on normal subjects. This measurement campaign will help us to improve our system and choose the best threshold. Then, we will increase the number of electrodes to accurately locate the tumor in the affected breast and to create an image that will be displayed on the smartphone. This will allow having a smart mammograph by the electrical bioimpedance method.
Acknowledgments
Authors thank the Prince Sattam Bin Abdulaziz University. This project was supported by the Deanship of Scientific Research at Prince Sattam Bin Abdulaziz University under the research project # 2019/01/10096.
Author Information
Sofiene Mansouri was born in Ain Drahem Village, TUNISIA in 1967. He becomes an engineer in electronics engineering from University Mentouri of Constantine, Algeria and M.S. degrees in biomedical engineering from the University of Technology of Compiegne, France, in 1991 and the Ph.D. degree in electronics from AL-Manar University, Tunis, Tunisia, in 2011. From 2005 to 2011, he was a teaching assistant in biophysics and biomedical engineering Higher Institute of Medical Technologies of Tunis – ISTMT, Tunisia. From 2011 to 2018, he has been an Assistant Professor. Since 2018 he becomes assistant professor with College of medical applied sciences, Prince Sattam bin Abdelaziz University, Saudi Arabia. He is the author of one book, and coauthor of three books, more than 30 articles. Member of the research laboratory in biophysics and medical technologies.
Tareq Alhadidi was born in Jeddah – Saudi Arabia, in 1971. He received his bachelor of Electronic Electricity and Automatic-2005 and M.S. degree in Ultrasonic Acoustic & Signal processing from in 2006 and the Ph.D. degree in Models, Methods & Algorithms in Biology, Health & Environment from University Joseph Fourier, Grenoble 1- France in 2011. From 2013, he was Head of biomedical Technology Dep. and Assistant Professor at Prince Sattam Bin Abdulaziz University, KSA. He is the coauthor of a book and also author of a book chapter, more than 8 articles and more than 11 communications in international conferences. He was a member of the Technical Committee for the IEEE-HealthCom’ 2013, Lisbon, Portugal and at Natal, Brazil 2014. He was chair of 2nd IEEE International Workshop on Emerging Technologies for Pervasive Healthcare and Applications, IEEE-HealthCom’17, 12–15 October 2017, Dalian-China.
Marwa Ben Azouz was born in Menzel Bourguiba Village, TUNISIA in 1990. She obtained her diploma of applied science in biomedical engineering in 2012 and a professional master on biomedical engineering in 2015 and she obtained M.S. degrees in Biophysics, Medical Physics and Medical Imaging in 2017 from Tunis AL-Manar University, Tunisia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Cotterchio M, Mirea L, Ozcelik H, Kreiger N. Active cigarette smoking, variants in carcinogen metabolism genes and breast cancer risk among pre- and postmenopausal women in Ontario, Canada. Breast J. 2014;20(5):468–480. doi:10.1111/tbj.12304
2. Mombelli S. Cancers du sein et Immunologie anti-tumorale [Ph.D. dissertation]. France: Univ. Reims Champagne-Ardenne; 2014.
3. Le-Petross HT, Shetty MK. Magnetic resonance imaging and breast ultrasonography as an adjunct to mammographic screening in high-risk patients. Semin Ultrasound CT MR. 2011;32(4):266–272. doi:10.1053/j.sult.2011.03.005
4. Takkar N, Kochhar S, Garg P, et al. Screening methods (clinical breast examination and mammography) to detect breast cancer in women aged 40–49 years. J Midlife Health. 2017;8(1):2–10.
5. Cheng HD, Shan J, Ju W, Guo Y, Zhang L. Automated breast cancer detection and classification using ultrasound images: a survey. Pattern Recognit. 2010;43(1):299–317. doi:10.1016/j.patcog.2009.05.012
6. Kuhl CK, Schrading S, Leutner CC, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol. 2005;23(33):8469–8476. doi:10.1200/JCO.2004.00.4960
7. Lehman CD, Isaacs C, Schnall MD. Cancer yield of mammography, MR, and US in high-risk women: prospective multi-institution breast cancer screening study. Radiology. 2007;244:381–388. doi:10.1148/radiol.2442060461
8. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. doi:10.3322/canjclin.57.2.75
9. Zou Y, Guo Z. A review of electrical impedance techniques for breast cancer detection. Med Eng Phys. 2003;25(2):79–90. doi:10.1016/S1350-4533(02)00194-7
10. Piperno G, Frei EH, Moshitzky M. Breast cancer screening by impedance measurements. Front Med Biol Eng. 1990;2:111–117.
11. Melloul M, Paz A, Ohana G, et al. Double phase 99mTc-sestamibi scintimammography and trans-scan in diagnosing breast cancer. J Nucl Med. 1999;40(3):376–380.
12. Cherepenin V, Karpov A, Korjenevsky A, et al. A 3D electrical impedance tomography [EIT] system for breast cancer detection. Physiol Meas. 2001;22:9–18. doi:10.1088/0967-3334/22/1/302
13. Morimoto T, Kinouchi Y, Iritani T, et al. Measurement of the electrical bio-impedance of breast tumors. Eur Surg Res. 1990;22(2):86–92. doi:10.1159/000129087
14. Morimoto T, Kimura S, Konishi Y, et al. A study of the electrical bioimpedance of tumors. J Invest Surg. 1993;6(1):25–32. doi:10.3109/08941939309141189
15. Osterman KS, Kerner TE, Williams DB, et al. Multifrequency electrical impedance imaging: preliminary in vivo experience in breast. Physiol Meas. 2013;21:99–109. doi:10.1088/0967-3334/21/1/313
16. Wolf I, Sadetzki S, Catane R, et al. Diabetes mellitus and breast cancer. Lancet Oncol. 2005;6(2):103–111. doi:10.1016/S1470-2045(05)01736-5
17. Jossinet J. The impedivity of freshly excised human breast tissue. Physiol Meas. 1998;19(1):61–75. doi:10.1088/0967-3334/19/1/006
18. Chen CM, Chou Y-H, Han K-C, et al. Breast lesions on sonograms: computer-aided diagnosis with nearly setting-independent features and artificial neural networks. Radiology. 2003;226:504–514. doi:10.1148/radiol.2262011843
19. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004;292(11):1317–1325. doi:10.1001/jama.292.11.1317
20. Jossinet J, Schmitt M. A review of parameters for the bioelectrical characterization of breast tissue. Ann NY Acad Sci. 1999;873:30–41.
21. Sardanelli F, Podo F, Santoro F, et al. Multicenter surveillance of women at high genetic breast cancer risk using mammography, ultrasonography, and contrast-enhanced magnetic resonance imaging [the high breast cancer risk Italian 1 study]: final results. Invest Radiol. 2011;46(2):94–105. doi:10.1097/RLI.0b013e3181f3fcdf
22. Nitenberg G, Leverve X, Chioléro R. Nutrition Artificielle De L’adulte En Reanimation. Elsevier; 2002:25.
23. Mansouri S, Mahjoubi H, Ben Salah R. Design of a peripheral bioimpedance prototype with an FPGA Virtex-5 LX30. IRBM. 2010;31(5–6):280–288. doi:10.1016/j.irbm.2010.10.002
24. Melkikh AV, Sutormina MI. Model of active transport of ions in cardiac cell. J Theor Biol. 2008;252:247–254. doi:10.1016/j.jtbi.2008.02.006
25. Chung D. Flexible conductive polymer electrodes for applications in tissue electrical impedance scanning (EIS) [Doctoral dissertation]. Applied Science: School of Engineering Science; 2012
26. Teh W, Wilson ARM. The role of ultrasound in breast cancer screening. A consensus statement by the European group for breast cancer screening. Eur J Cancer. 1998;34(4):449–450. doi:10.1016/S0959-8049(97)10066-1
27. Weinstein SP, Localio AR, Conant EF, et al. Multimodality screening of high-risk women: a prospective cohort study. J Clin Oncol. 2009;27(36):6124–6128. doi:10.1200/JCO.2009.24.4277
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.