Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 14
Both Prevalence and Severity of Pruritus are Associated with Age in Chinese Patients with Skin Diseases
Authors Wang X, Lai Q, Zheng B, Ye L, Wen S, Yan Y, Yang B , Man MQ
Received 5 January 2021
Accepted for publication 12 February 2021
Published 4 March 2021 Volume 2021:14 Pages 217—223
DOI https://doi.org/10.2147/CCID.S300458
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Xiaohua Wang,1,* Qingsong Lai,2,* Baoqing Zheng,1 Li Ye,1 Si Wen,1 Yunling Yan,1 Bin Yang,1 Mao-Qiang Man1
1Dermatology Hospital, Southern Medical University, Guangdong, 510091, People’s Republic of China; 2Center for Chronic Disease Prevention and Control of Puning City, Puning, Guangdong, 515300, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mao-Qiang Man; Bin Yang
Dermatology Hospital, Southern Medical University, 2 Lujing Road, Yuexiu District, Guangzhou, Guangdong, 510091, People’s Republic of China
Email [email protected]; [email protected]
Background: Although the characteristics of pruritus in some skin diseases are documented, characteristics of pruritus related to gender-, age-, and skin disorder have not yet been well defined.
Objective: To characterize dermatosis-associated pruritus in Chinese patients.
Methods: A cross-sectional study was carried out in a single center. The intensity of pruritus was evaluated using a 0– 10 visual analog scale (VAS). Skin disorders were diagnosed by dermatologists. The prevalence and intensity of pruritus were compared among skin disorders, and between males and females.
Results: Valid questionnaires were obtained from 1,246 female and 864 male patients. Patients with acne, eczematous dermatitis, and urticaria accounted for 18%, 17%, and 14%, respectively. Both the prevalence and severity of pruritus varied greatly with skin disorders (p< 0.0001). Patients with either urticaria or eczematous dermatitis displayed a higher prevalence of pruritus (92% and 82%, respectively), while subjects with urticaria exhibited the highest VAS in comparison to those with other skin disorders (p< 0.05 to p< 0.001 vs the others). Moreover, both the prevalence and severity of pruritus were positively associated with age in both males and females (p< 0.0001). Furthermore, 60 out of 77 patients (78%) with topical glucocorticoid-induced dermatitis experienced pruritus, with a VAS of 2.03± 0.21. Finally, a lower VAS was found in subjects with oily skin than those with either dry or normal skin.
Conclusion: The prevalence and severity of pruritus vary with skin disorders, skin type, age, and gender in Chinese patients.
Keywords: skin disorder, pruritus, prevalence, severity, gender, age
Introduction
Pruritus is defined as an unpleasant sensation of the skin, provoking a desire to scratch. Pruritus can negatively impact the quality of patients’ lives and exacerbate cutaneous and extracutaneous conditions.1,2 Both cutaneous and extracutaneous disorders can provoke pruritus.3,4 Almost 80% of patients with skin disorders experience pruritus with visual analog scales (VAS)>2.3 Prevalence of pruritus is higher in subjects with prurigo, urticaria, and inflammatory skin disorders such as acne, eczematous dermatitis, and psoriasis than other skin disorders.3,4 Female patients display higher prevalence of pruritus than males.4,5 Although these sporadic studies provided some information of pruritus in skin disorders, it is hard to compare the characteristics of dermatosis-associated pruritus without side-by-side comparison among dermatoses. Moreover, the majority of previous studies were done in Europeans. But skin pigment differs between Chinese (Fitzpatrick skin phototypes III to V) and Europeans (Fitzpatrick skin phototypes I to IV),6,7 while skin pigment can influence epidermal function and cutaneous inflammation, which can affect pruritus.8,9 Thus, dermatosis-associated pruritus in Chinese could differ from that in Europeans. Therefore, we assessed here the association of prevalence and intensity of pruritus with skin disorders, age, and gender in a large cohort of Chinese patients.
Subjects and Methods
Patients who visited a dermatology clinic (a tier 3 hospital) were asked to self-declare whether they had pruritus within the last 24 hours, and the intensity of pruritus, using a visual analog scale (VAS) (0=no pruritus, 10=very severe pruritus).10 In addition, patients were also asked to self-report their skin types (ie, normal, dry, or oily skin). Diagnoses of skin disorders were made by trained dermatologists, according to the clinical features. No pathology was performed. No information of extracutaneous conditions was collected. If a total of ≥15 males and females had the same disease, the overall prevalence and severity for this disease were separately analyzed. If both males and females each had ≥10 patients who had the same disorder, the prevalence and severity of pruritus of this disorder were also analyzed separately in males and females. When the number of subjects with each dermatosis was <10 or subjects had more than one dermatosis, they were included in the category of others. Eczematous dermatitis included both atopic dermatitis and contact dermatitis. After being fully informed about this study, all patients provided written informed consent. For the under-aged patients (<18 years old), written informed consent was obtained from their parent or legal guardian. This work was approved by the Institutional Review Board of Dermatology Hospital, Southern Medical University (020–83027645) and carried out in accordance with the Declaration of Helsinki.
Data Analyses
GraphPad Prism 5 software was used for all statistical analyses. A chi-square test was used to assess the significance of differences in frequency between or among groups. Significance of correlation between two variables was determined using Pearson r analysis. One-way ANOVA was used to determine the significance of quantitative data among groups, while unpaired t-test was used to determine the significance between two groups of quantitative data. Statistical methods are indicated in respective Tables and Figure legends. Data are expressed as mean±stand error of mean (SEM).
Results
Demographic Characteristics of Subjects
Questionnaires were collected from a total of 2,110 patients, including 1,246 females and 864 males, from March to May, 2019. All patients were Chinese Han, aged from 13–94 years (overall 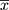 ±SEM=33.71±0.37; females=33.44±0.44, 95% CI=32.85–35.35; males=34.99±0.64, 95% CI=32.84–35.35).
±SEM=33.71±0.37; females=33.44±0.44, 95% CI=32.85–35.35; males=34.99±0.64, 95% CI=32.84–35.35).
Age Distribution Varies with Gender and Skin Disorders
Among the 2,110 patients, the top three common diseases were acne (17.58%), eczematous dermatitis (17.39%), and urticaria (13.98%) (Table 1). Subjects with either herpes zoster or eczema were older than those with other skin disorders, whereas patients with acne were younger than those with most other diseases. Moreover, females with acne, tinea, and verruca vulgaris were older than male patients (p<0.05 for tinea and verruca vulgaris; p<0.0001 for acne). In contrast, female patients with eczematous dermatitis were younger than males (p<0.0001) (Table 1). These results indicate that age distribution varies with gender and skin disorder.
 |
Table 1 Age Distribution of Skin Disorders |
Prevalence and Severity of Pruritus
In the entire cohort, the overall prevalence of pruritus was 62%, while VAS of pruritus was 2.75±0.06 (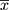 ±SEM) (range=0–9, 95% CI=2.635–2.865). Overall VAS of pruritus correlated positively with age in both males (Pearson r=0.2540, p<0.0001) and females (Pearson r=0.2141, p<0.0001). Because pruritus can be associated with epidermal function, which declines after age 50 years, next we analyzed the prevalence and VAS of pruritus in patients aged ≤50 vs compared to those >50 years old. VAS of pruritus was 50% higher in patients aged >50 years old than those ≤50 years (3.77±0.14 vs 2.52±0.06, Mann–Whitney U=240,800, p<0.0001). Similarly, the prevalence of pruritus was also higher in patients aged over 50 years than those ≤50 years (77.13% vs 59.34%, X2=40.95, p<0.0001). However, neither the overall prevalence nor VAS of pruritus differed significantly between males and females (63% vs 62% for prevalence; 2.79±0.09 vs 2.72±0.08 for VAS) (Table 2).
±SEM) (range=0–9, 95% CI=2.635–2.865). Overall VAS of pruritus correlated positively with age in both males (Pearson r=0.2540, p<0.0001) and females (Pearson r=0.2141, p<0.0001). Because pruritus can be associated with epidermal function, which declines after age 50 years, next we analyzed the prevalence and VAS of pruritus in patients aged ≤50 vs compared to those >50 years old. VAS of pruritus was 50% higher in patients aged >50 years old than those ≤50 years (3.77±0.14 vs 2.52±0.06, Mann–Whitney U=240,800, p<0.0001). Similarly, the prevalence of pruritus was also higher in patients aged over 50 years than those ≤50 years (77.13% vs 59.34%, X2=40.95, p<0.0001). However, neither the overall prevalence nor VAS of pruritus differed significantly between males and females (63% vs 62% for prevalence; 2.79±0.09 vs 2.72±0.08 for VAS) (Table 2).
 |
Table 2 Comparison of Pruritus Between Males and Females |
We analyzed next the prevalence and intensity of pruritus in each skin disorder. In this analysis, either number of patients with a skin disorder was fewer than 15 or patients with more than one skin disorders were grouped together as “others”. As shown in Figure 1A, the prevalence of pruritus varied greatly with skin disorders (p<0.0001), with the highest prevalence in patients with either prurigo or dermatitis aestivale (both 95%). Likewise, VAS of pruritus also differed significantly among skin disorders (p<0.0001) (Figure 1B). When each skin disorder in males and females was analyzed separately, higher VAS was found in both males and females with urticaria, followed by eczematous dermatitis (Table 2). Moreover, VAS of pruritus correlated positively with age in patients with either acne (Pearson r=0.2107, p<0.0001) or eczematous dermatitis (Pearson r=0.2070, p<0.0001) or in the group of “others” (Pearson r=0.1549, p<0.01). Interestingly, significant differences in intensity of pruritus were observed between males and females with either verruca vulgaris or “others” (Table 2). Prevalence of pruritus in verruca vulgaris tended to be higher in males than in females (41% vs 14%, p=0.0508). Collectively, these results demonstrate that the prevalence and intensity of pruritus vary with age, gender, and skin disorder.
 |
Figure 1 Prevalence and intensity of pruritus. (A) The prevalence of pruritus in common skin disorders. Chi-square test was used to analyze significance of differences in prevalence among skin disorders (X2=370.0, p<0.0001). (B) VAS of pruritus. Data are expressed as mean±SEM. One-way ANOVA analysis was used to determine the significance of variation in VAS among skin disorders, p<0.0001.Abbreviation: GC, Glucocorticoids. |
Finally, we determined the association of skin type with pruritus. The prevalence of pruritus in females did not differ significantly among skin type (X2=3.534). In contrast, the prevalence of pruritus in males varied greatly with skin type (X2=30.15, p<0.0001) (Figure 2A). The prevalence of pruritus in each type of skin did not differ significantly between males and females. VAS of pruritus was lower in both males and females with oily skin than with either dry or normal skin (p<0.001) (Figure 2B). Moreover, VAS of pruritus in subjects with dry skin was higher in males than in females (p<0.05). These results indicate that skin type determines the prevalence and intensity of pruritus.
Discussion
Although it is known that certain skin diseases are age-associated, information of age distribution in skin disorders is still limited, particularly in the Chinese patients. Surprisingly, the present study demonstrated gender-differences in patients’ age in some skin diseases such as eczematous dermatitis, tinea, and verruca vulgaris. The underlying mechanisms contributing to such gender-differences in patients’ age in some dermatoses are not clear.
The present study showed that both prevalence and intensity of pruritus were higher in patients with either eczematous dermatitis or urticaria in comparison to other skin diseases. This is not surprising because there are abundant inflammatory cytokines and histamine in the skin of eczematous dermatitis and urticaria, while these inflammatory mediators can activate purinergic receptors on C-fibers in the skin, leading to pruritic sensation.11,12 For example, in addition to increased sensitivity to pruritogens, both immune cell- and keratinocyte-derived factors such as IL‐31, thymic stromal lymphopoietin, and histamine, which all can provoke pruritus, are increased in the skin of atopic dermatitis.13 Thus, higher prevalence and VAS are observed in patients with eczematous dermatitis. However, folliculitis, another inflammatory skin condition, displayed low prevalence and intensity of pruritus. The discrepancy in pruritus between folliculitis and other inflammatory skin diseases can be ascribed to the differences in the pathophysiology. Folliculitis, commonly caused by bacterial infections or trauma, is featured mainly by neutrophilic infiltration. Although neutrophils can be involved in the development of pruritus,14 the levels of histamine and IL-31, major mediators of pruritus, produced by neutrophils may not be sufficient to provoke severe pruritus in folliculitis.15,16 Moreover, neutrophils can produce a number of cytokines such as IL-1β, IL-6, and TNFαα,16 which can directly and indirectly induce pain, while pain can inhibit pruritus.17,18 Correspondingly, patients with folliculitis often experience pain. Hence, the prevalence and intensity of pruritus is lower in patients with folliculitis than with other inflammatory skin disorders.
It is worth noting that both prevalence and intensity of pruritus were correlated positively with age. These age-associated characteristics of pruritus can be attributed, in part, to pre-existing conditions of the altered epidermal function in the aged humans. First, aged skin exhibits reduced stratum corneum hydration, resulting in increased cutaneous inflammation.19 Second, the elevated skin pH in aged skin can increase activity of protease-activated receptor 2 (PAR2), a trigger of pruritus.20 Third, epidermal permeability barrier recovery is delayed in the aged skin, which can cause prolonged release of cytokines and increased mast cells in the skin in response to barrier disruption induced by scratches or trauma.21 Moreover, the number of mast cells contacting nerve fibers are increased in the papillary dermis of the aged skin, which is linked to pruritus.22,23 Of course, contribution of aging-associated neurodegeneration to the development of pruritus cannot be excluded. Thus, these conditions can contribute to the high prevalence and intensity of pruritus in aged patients with skin disorders.
Regarding the association of skin type with pruritus, lower prevalence and VAS in oily skin could be due to a higher levels of stratum corneum hydration in comparison to normal and dry skin. Although stratum corneum hydration was not measured, previous studies showed that stratum corneum hydration levels correlate with sebum content.24,25 Reduced stratum corneum hydration can cause cutaneous inflammation.26 Conversely, improvements in stratum corneum hydration can lower expression levels of cytokines in the skin.19,26 Thus, patients with oily skin can exhibit lower prevalence and VAS of pruritus in comparison to either dry skin or normal skin. Although moisturizers can mitigate pruritus in some skin conditions such as dry skin and eczematous dermatitis, whether topical moisturizers can alleviate pruritus in other skin disorders remains to be explored.
The limitations of the present study include that a) we did not exclude whether pruritus in some patients was caused by extracutaneous conditions or medications, and b) we did not determine whether the pruritus in some disorders is induced by exogenous stimuli such as temperature changes and contact of clothing, because some skin diseases can increase sensitivity of the skin to stimuli. Nevertheless, the present study demonstrates that the prevalence and intensity of pruritus varies with age, gender, skin type, and skin disorders in the Chinese patients.
Ethics
This work was approved by the Institutional Review Board of Dermatology Hospital Southern Medical University (020-83027645).
Disclosure
The authors declare no conflicts of interest.
References
1. Zachariae R, Lei U, Haedersdal M, Zachariae C. Itch severity and quality of life in patients with pruritus: preliminary validity of a Danish adaptation of the itch severity scale. Acta Derm Venereol. 2012;92(5):508–514. doi:10.2340/00015555-1221
2. Swarna SS, Aziz K, Zubair T, et al. Pruritus associated with chronic kidney disease: a comprehensive literature review. Cureus. 2019;11(7):e5256.
3. Hawro T, Przybyłowicz K, Spindler M, et al. The characteristics and impact of pruritus in adult dermatologic patients: a prospective, cross-sectional study. J Am Acad Dermatol. 2020;S0190–9622(20):32426–32429.
4. Schut C, Dalgard FJ, Halvorsen JA, et al. Occurrence, chronicity and intensity of Itch in a clinical consecutive sample of patients with skin diseases: a multi-centre study in 13 European countries. Acta Derm Venereol. 2019;99(2):146–151.
5. Dalgard F, Stern R, Lien L, Hauser S. Itch, stress and self-efficacy among 18-year-old boys and girls: a Norwegian population-based cross-sectional study. Acta Derm Venereol. 2012;92(5):547–552.
6. Ho SG, Chan HH. The Asian dermatologic patient: review of common pigmentary disorders and cutaneous diseases. Am J Clin Dermatol. 2009;10(3):153–168.
7. Trakatelli M, Bylaite-Bucinskiene M, Correia O, et al. Clinical assessment of skin phototypes: watch your words! Eur J Dermatol. 2017;27(6):615–619.
8. Man MQ, Lin TK, Santiago JL, et al. Basis for enhanced barrier function of pigmented skin. J Invest Dermatol. 2014;134(9):2399–2407.
9. Lin TK, Man MQ, Abuabara K, et al. By protecting against cutaneous inflammation, epidermal pigmentation provided an additional advantage for ancestral humans. Evol Appl. 2019;12(10):1960–1970.
10. Reich A, Heisig M, Phan NQ, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Derm Venereol. 2012;92(5):
11. Potenzieri C, Undem BJ. Basic mechanisms of itch. Clin Exp Allergy. 2012;42(1):8–19.
12. Yosipovitch G, Rosen JD, Hashimoto T. Itch: from mechanism to (novel) therapeutic approaches. J Allergy Clin Immunol. 2018;142(5):1375–1390.
13. Yosipovitch G, Berger T, Fassett MS. Neuroimmune interactions in chronic itch of atopic dermatitis. J Eur Acad Dermatol Venereol. 2020;34(2):239–250.
14. Hashimoto T, Rosen JD, Sanders KM, Yosipovitch G. Possible role of neutrophils in itch. Itch. 2018;3(4):e17.
15. Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Front Immunol. 2019;10:1383.
16. Tecchio C, Micheletti A, Cassatella MA. Neutrophil-derived cytokines: facts beyond expression. Front Immunol. 2014;5:508.
17. Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75(2):125–131.
18. Bardoni R, Shen KF, Li H, et al. Pain Inhibits GRPR neurons via GABAergic signaling in the spinal cord. Sci Rep. 2019;9(1):15804.
19. Hu L, Mauro TM, Dang E, et al. Epidermal dysfunction leads to an age-associated increase in levels of serum inflammatory cytokines. J Invest Dermatol. 2017;137(6):
20. Jang H, Matsuda A, Jung K, et al. Skin pH is the master switch of kallikrein 5-mediated skin barrier destruction in a murine atopic dermatitis model. J Invest Dermatol. 2016;136(1):127–135.
21. Lin TK, Man MQ, Santiago JL, et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol. 2013;133(2):469–478.
22. Pilkington SM, Barron MJ, Watson REB, et al. Aged human skin accumulates mast cells with altered functionality that localize to macrophages and vasoactive intestinal peptide-positive nerve fibres. Br J Dermatol. 2019;180(4):849–858.
23. Jin X, Zhao W, Kirabo A, et al. Elevated levels of mast cells are involved in pruritus associated with polycythemia vera in JAK2V617F transgenic mice. J Immunol. 2014;193(2):477–484.
24. Fluhr JW, Mao-Qiang M, Brown BE, et al. Glycerol regulates stratum corneum hydration in sebaceous gland deficient (asebia) mice. J Invest Dermatol. 2003;120(5):728–737.
25. Choi EH, Man MQ, Wang F, et al. Is endogenous glycerol a determinant of stratum corneum hydration in humans? J Invest Dermatol. 2005;125(2):288–293.
26. Kikuchi K, Kobayashi H, Hirao T, et al. Improvement of mild inflammatory changes of the facial skin induced by winter environment with daily applications of a moisturizing cream. A half-side test of biophysical skin parameters, cytokine expression pattern and the formation of cornified envelope. Dermatology. 2003;207(3):269–275.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

