Back to Journals » Transplant Research and Risk Management » Volume 11
BK virus in transplant recipients: current perspectives
Authors Muhsin SA , Wojciechowski D
Received 8 June 2019
Accepted for publication 29 August 2019
Published 9 September 2019 Volume 2019:11 Pages 47—58
DOI https://doi.org/10.2147/TRRM.S188021
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Professor Qing Yi
Saif A Muhsin, David Wojciechowski
Nephrology Division and Transplant Center, Massachusetts General Hospital, Boston, MA, USA
Correspondence: David Wojciechowski
UT Southwestern Medical Center, Dallas, TX USA
Tel +1 214 645 7846
Email [email protected]
Abstract: Since its discovery in 1971, BK Polyomavirus infection has been increasingly recognized, especially since the introduction of more effective immunosuppressive medications in the early 1990s. BK virus is believed to have a high seroprevalence in healthy individuals, entering a latent phase in the genitourinary that follows the primary infection. Reactivation may occur in immunocompromised hosts, with significant effects seen especially in kidney and hematopoietic stem cell transplant recipients. Screening methods have been developed and are implemented as early detection may allow for the prevention of irreversible tissue damage. Reduction of immunosuppression remains the cornerstone of therapy for BKV infection, and adjunctive therapies have shown variable results. Newer cellular-based therapeutics might provide a more targeted treatment for BKV infection but are still in need of more randomized human studies.
Keywords: kidney transplantation, BK polyomavirus, immunosuppression
Introduction
Human polyomavirus was first reported in 1971 in a kidney transplant patient who developed graft dysfunction with ureteral obstruction 5 months following living-related kidney transplantation. It was named BK virus (BKV), after the initials of that first patient in whom it was described.1 BKV is thought to lay dormant in the urinary system of 80–90% of the general population, with no disease manifestations in immunocompetent individuals. Despite the initial discovery of BKV in 1971, it was not until the early 1990s with the introduction of more potent immunosuppression and widespread use of antibody induction agents that BKV infections have become increasingly recognized and found to be associated with graft dysfunction or failure in kidney transplant recipients and hemorrhagic cystitis in bone marrow transplant recipients. In this review, we will discuss the natural history and clinical features of BK polyomavirus infection in transplant recipients, the current understanding about the risk factors and pathogenesis, as well as the approach to treatment focusing mainly on management in kidney transplant recipients.
Virology
BK Polyomavirus is one of the more than 10 human polyomaviruses within the Polyomaviridae family.2 It is a non-enveloped DNA virus that has a diameter of 40–45 nanometers. The double-stranded DNA genome is composed of 5153 base pairs that are wound around histones of host cell origin. The DNA genome is functionally divided into 3 regions. The noncoding control region (NCCR) regulates the expression of the viral early and late genes in concert with the differentiation and activation of the host cell. The early viral gene region (EVGR) encodes the regulatory nonstructural proteins called small T and large T antigen, which help shifting the host cell into S phase to use the cell’s DNA polymerase for viral replication. The late viral gene region (LVGR) encodes the capsid proteins VP-1, VP-2, and VP-3. These are assembled in the nucleus. This region also encodes a small cytoplasmic protein called agnoprotein, which has multiple regulatory roles.3
Epidemiology
BKV is noted to have a high seroprevalence in healthy human individuals, with rates up to 91% in ages 5–9 years.4 In 400 healthy blood donors, the seroprevalence decreased from 87% in the younger age group (20–29 years) to 71% in the oldest age group (50–59 years). Urinary shedding of BKV was detected in 7% of those healthy donors while BKV DNA was not detected in the blood of these patients.5 Another study of 51 healthy adults showed urinary viral shedding in about 16% of the individuals. Twenty-eight of these individuals were followed for 180 days and the urinary shedding was found to be occasional in the majority (one positive sample out of the six samples that were obtained).6 Another study of 1501 healthy blood donors found that BKV seroprevalence was 82%.7
There appears to be a natural progression of viral replication and detection in kidney transplant recipients. Manifestations of BKV infection occur in a step-wise manner. BKV is initially detected in the urine at a median of 16 weeks. This precedes detection in the plasma which occurs at a median of 23 weeks. This eventually results in BKV nephropathy that occurs at a median of 28 weeks.8 Urinary shedding of the virus was found to be very sensitive but had a low positive predictive value for BKV nephropathy. In one study, urinary shedding was strongly associated with the development of viremia, nephropathy, and graft loss. Kidney transplant patients who were found to have urinary decoy cells (N=103) underwent urinary PCR testing that revealed BKV shedding in 58 (56.3%) patients. BKV viremia was detected in 93%, and BKV nephropathy was evident on histopathological examination in 48% of these patients. BKV viremia of more than 10,000 copies was significantly associated with biopsy-proven BKV nephropathy (P<0.0001).9
BKV has four major subtypes, based on variations in VP-1 major capsid protein with variable geographical distributions that might suggest the migration patterns of human populations.10 The most frequent subtype is BKV subtype I.11
There has been no animal reservoir identified that contributes to the transmission of BKV. The possible modes of transmission are through contact with mucosal surfaces in the oropharynx, the respiratory tract, and the gastrointestinal tract. The fecal–oral transmission is supported by the finding of polyomaviruses in stool and urban sewage samples.12–16 BKV has also been detected in tonsillar tissue, which supports the possibility of respiratory transmission of the virus.17 BKV then reaches the urinary tract that serves as the main site for latent infection.18
Pathogenesis and risk factors
BK virus expresses the small and large T antigen after infecting the host cell. Active viral replication follows. The large T antigen colocalizes in the nucleus which increases in size and capsid assembly leads to the generation of intranuclear inclusions. The infected cells subsequently increase in size and detach from the basement membrane. Tubular cell lysis eventually occurs, leading to the release of the infectious progeny.3 The primary viral infection and reactivation in immunocompetent individuals are controlled by the effect of cytotoxic T cell responses that target the replicating infected host cells. In immunosuppressed individuals, the unchecked replication of the virus will lead to progression of local tissue damage and subsequent spread to the bloodstream.
Multiple studies have investigated the risk factors for developing BKV infections in kidney transplant recipients. These risk factors can be divided into donor, recipient, and transplant-related risk factors. Thangaraju et al evaluated 21,575 mate kidney pairs for the presence of a treatment code for BKV infection. Among the 1975 pairs that were discordant (one of the recipients had a treatment code and the other recipient did not), they found several factors that were associated with a higher odds of treatment. These factors included age younger than 18 or older than 60 years, male sex, HLA mismatch at 4 loci or more, acute rejection, and use of depleting antibody induction. Factors that were associated with a lower odds of treatment for BKV infection included diabetes and use of sirolimus. These authors also found a higher than expected rate of concordant treatment, suggesting a role for donor factors in the pathogenesis of BKV.19
Schold et al evaluated SRTR data to investigate the incidence of and risk factors for BKV infection in kidney transplant recipients. Significant independent risk factors included younger age, donors over the age of 65, recipients without diabetes as the primary diagnosis, male recipients, female donors, five- and six- HLA mismatched transplants, thymoglobulin induction, and tacrolimus maintenance at baseline.20
Human leukocyte antigens modulate immunity to BKV and successful immunity requires sharing of antigens between the anti-viral effector cell and the virus-infected target cell. Masutani et al evaluated 998 kidney transplant recipients and showed a lower risk of BK viremia in patients who received transplants from donors with matching HLA-A2, HLA-B44, and HLA-DR15.21
Studies by Sharif et al and Bentall et al showed a higher incidence of BKVAN in ABO-incompatible kidney transplant recipients. This might be related to the higher level of immunosuppression that these patients receive versus other immunologic factors associated with an ABO-incompatible transplant.22,23
Variation in cellular responses to BK virus is believed to play a role in the resolution of viral replication. Patients with BK viremia were found to have lower CD4, higher total CD8 proportions on pre-transplant samples, when compared to patients who did not develop BK viremia.24 Patients with more polyfunctional CD8+ T cells expressing multiple cytokines were more likely to clear BK infections within 3 months.25
Bohl et al studied urine samples from 20 recipient pairs of a deceased kidney transplant. Each of these pairs received 2 kidneys from the same deceased donor. Sixteen out of the 20 pairs were concordant for the presence of BKV viruria. Ten of the 16 recipient pairs did not have BKV infection, and 6 recipient pairs had BKV infection. Sequencing of the BKV NCCR and VP1 regions showed identical sequences in each pair of the 6 recipient pairs. This supported the theory of donor origin of BKV infection in kidney transplant recipients.26
Schmitt et al evaluated urine samples from living kidney donor and recipient pairs for the presence of BKV. In 20 out of the 249 donor/recipient pairs that were evaluated, post-transplant sequencing was successful in the donor and both recipients. The typing region sequences were found to be completely identical between the donor and both recipients in each of these 20 pairs. Pre-transplant sequencing was not available for all the pairs, making it difficult to exclude presence of identical BKV strains in the donor and the recipients resulting from intra-family transmission.27
Schwarz et al evaluated blood and urine samples from 214 living kidney donor–recipient pairs for the presence of BKV using quantitative PCR. They compared the BKV subtypes of the donor and recipients before transplant with the BKV subtype of the infected recipient after transplantation. The subtype comparisons suggested donor-derived infection in 24 of the 28 pairs studied.28 A more recent study by Wunderink et al evaluated 386 living kidney donor–recipient pairs for BKV serotypes before and after transplantation. Donor serotype was significantly correlated with the replicating genotype in the viremic recipient.29
Clinical features
Primary infection in immunocompetent individuals is thought to include nonspecific symptoms such as influenza-like upper respiratory symptoms, acute cystitis, with or without hematuria.30 Following the primary infection, the virus then enters a latent phase, usually in the urogenital system. Reactivation of the virus can occur with increasing age, pregnancy, diabetes mellitus, immunosuppressed states associated with congenital immunodeficiency, organ transplantation, or HIV infection.
BKV infection in kidney transplant recipients can manifest with ureteral stenosis in 3% of the patients who present with symptoms and signs of urinary tract obstruction and this usually occurs about 2–4 months post-transplant.31–33 BKV more commonly causes a tubulointerstitial nephritis that frequently manifests as an asymptomatic rise in serum creatinine on routine testing.
The prevalence of BK virus infections in non-kidney solid organ transplant recipients has not been very well established. Reports of incidence of BK viremia in liver transplant recipients ranged between 3% and 18% in different studies. Splendiani et al prospectively studied 37 liver transplant recipients and found BK viruria in 5 (13.5%) patients and BK viremia in 1 (2.7%) patient. Muñoz et al prospectively studied 64 liver transplant recipients with samples obtained at a median of 559 days post-transplant and detected BK viruria in 7.8%. None of the patients had BK viremia.34 The same study followed 43 heart transplant recipients and detected BK viruria and BK viremia in 11 (25.6%) and 3 (7%) patients, respectively. Razonable et al performed a longitudinal surveillance study of 121 liver transplant recipients and found BK viremia in 5 (4.1%) patients.35 The same study followed 45 heart transplant recipients and detected BK viremia in 3 (6.7%) patients. All these occurred following treatment for acute rejection, and they did not show evidence of kidney dysfunction within a year of detection. Loeches et al reported the incidence of BKV infection in 62 liver transplant recipients with samples obtained at 1 week, 4 weeks, and quarterly thereafter till 2 years post-transplant. BK viremia was detected in 11 patients (18%). Almost all cases of viremia were detected in the first 3 months after liver transplantation. BKV infection was more common in patients who developed rejection episodes and was associated with renal dysfunction.36
In patients with bone marrow transplants, BK viruria occurs in about 50% of the patients within the first few months, and this has been associated with hematuria, ureteral stenosis, and interstitial nephritis. The most common clinical symptom/manifestation, however, is hemorrhagic cystitis, which occurs in 10–25% of these patients.37
Screening and diagnosis
BKV infection progresses in a step-wise manner. The virus initially replicates and can be detected in the urinary space, followed by viremia, and finally, can be found in the kidney. The detection in urine is thought to precede viremia by several weeks. Patients are usually asymptomatic until there is allograft dysfunction that becomes evident in routine biochemical testing. This provides a window of opportunity for early detection and intervention before there is structural damage that might be otherwise irreversible.
The KDIGO 2009 guidelines suggest screening for BKV with quantitative nucleic acid testing at least monthly for the first 3–6 months followed by every 3 months until the end of the first year post-transplant, whenever there is an unexplained rise in serum creatinine, and after treatment for acute rejection.38 Our transplant center has implemented a standardized protocol for screening of BKV infection that uses nucleic acid amplification testing every month for the first 6 months post-transplant and then every 2 months until 24 months post-transplant (Figure 1).
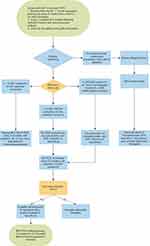 |
Figure 1 Screening and management protocol for BKV infection in kidney transplant recipients. Abbreviation: PCR, polymerase chain reaction. |
BKV infection can be detected using several methods. The virus-infected urinary epithelial cells have enlarged nuclei with large intranuclear inclusions and can be detected by cytological examination. These are called decoy cells. Positive cytology is suggestive of BKV nephropathy. Quantitative polymerase chain reaction (PCR) testing of BK viral replication in urine or plasma is more sensitive and can provide presumptive diagnosis of BKV nephropathy especially if associated with kidney allograft dysfunction. Detection of viral replication in urine can be detected in 20–60% of the kidney transplant recipients and precedes BK viremia by about 4 weeks.39 There are currently no established international lab standards for BK viral load assessment; hence, there is marked variability in results among different laboratories. There are, however, efforts to establish quantitative BKV DNA load cutoffs for clinical practice by introducing a common calibrator that would enable comparisons of biological measurements between different laboratories worldwide.40
A definite diagnosis of BKV nephropathy can be established by histological examination of a kidney allograft biopsy. The characteristic features include intranuclear basophilic viral inclusions, interstitial mononuclear/polymorphonuclear cell infiltrates, tubular injury, and tubulitis.41,42 A recent working group suggested a morphologic classification scheme for BKV nephropathy into 3 classes (I–III) based on the intrarenal polyomavirus load and Banff interstitial fibrosis ci score. These were found to be the most significant two independent histologic variables that were associated with clinical presentation. The different classes correlated with presentation at the time of biopsy, serum creatinine levels over 2 years of follow-up, and allograft failure.43
More non-invasive methods to assess for BK virus nephropathy have recently been investigated. A study by Brennan et al correlated kidney transplant biopsies of patients with BK viremia with the level of donor-derived cell-free DNA in their blood. They found that lower levels of dd-cfDNA may suggest that BK viremia is not associated with nephropathy. Higher dd-cfDNA levels were noted in the subset of patients who had concomitant biopsy features that met Banff criteria for T cell-mediated rejection.44 This test will require further clinical validation to establish its usefulness.
Treatment
No completed randomized trials exist to this date that evaluate therapies for BKV infections. A systematic review by Johnston et al in 2010 reviewed studies of adult kidney transplant recipients with BKV infections comparing reduction of immunosuppression alone or in combination with cidofovir, leflunomide, or both. The authors concluded that more adequately randomized trials are needed to provide evidence for this question.45 Some of the adjunctive therapies reported to be used for BKV infection are listed with their proposed mechanism of action in Table 1.
 |
Table 1 Adjunctive therapies for BKV infection |
Reduction of immunosuppression therapy
Saad et al performed a single-center analysis of 24 patients who developed BK viremia. Sixteen (66%) of those patients had evidence of BKVAN. With mean reductions in mycophenolate and tacrolimus of 44% and 41%, respectively, clearance of BK viremia was over a mean period of 5.8 months (range, 1–9.5 months). At a mean follow-up period of 43.5 months, all patients were alive, and 23 of them had a functioning graft. One patient had recurrent BKVAN during pregnancy and lost her graft. Three (13%) patients developed acute cellular rejection and received pulse steroid therapy.46
Brennan et al randomized 200 adult renal transplant recipients to either tacrolimus or cyclosporine and performed interval BKV screening with urine and blood BKV PCR for 1 year. Of the 200 patients, 35% developed viruria and 11.5% developed viremia. They found no independent association between development of viruria or viremia and any of the immunosuppressants individually. They also found that reduction of immunosuppression alone was associated with resolution of viremia in 95% of the infected patients.47 In a 5-year follow-up retrospective study of the same patient cohort, Hardinger et al found that minimization of immunosuppression was associated with excellent 5-year graft survival (84%), with low rejection rates (12%) noting a higher rate of rejection in the patients receiving cyclosporine (18%) versus those receiving tacrolimus (9%) (P=0.082).48
Schaub et al followed 38 kidney transplant patients who developed BK viremia over a median period of 34 months after detection of BK viremia. All but one of the patients were on a tacrolimus-based regimen at the time of diagnosis. Immunosuppression was lowered for all the patients, initially with reduction of the tacrolimus trough goal in two steps. If the BK viremia persisted, the antimetabolite dose was reduced by half. Thirty-five (92%) patients achieved clearance of BK viremia over the follow-up period. Three (8.2%) patients developed biopsy-proven Banff class I rejection. These patients received treatment for acute rejection. The authors noted that at least one kidney allograft biopsy was performed in another 15 patients, 7 of whom showed signs of subclinical rejection (6 with tubulitis and 1 with antibody-mediated rejection) Four of these 7 patients received treatment for rejection. Of the patients treated for rejection, 2 had low-level BK viremia that cleared. The creatinine levels at the end of the follow-up period were not significantly higher compared to baseline levels.49
Bischof et al followed 105 patients who developed BK viremia (including 48 patients with presumptive and 33 patients with proven BKVAN).50 The authors established a standard operating procedure whereby the calcineurin inhibitor was reduced. The immunosuppression regimen was adjusted such that the tacrolimus trough goal was reduced first. If the BK viremia persisted after 4 weeks, the tacrolimus goal trough was lowered further. If the BK viremia persisted after 4 weeks, the antimetabolite dose was reduced by half. The patients were followed for 6 years post-transplant. BK viremia cleared in 101 (96%) patients after a median of 137 days. In the patients who had clearance of the virus, 39% required tacrolimus reduction only, 43% required additional reduction of mycophenolic acid in 43% of the patients. Twelve of the 101 patients (12%) had recurrence of the BK viremia. At a median of 5 years of follow-up after clearance of the BK viremia, the rejection incidence rate was 11%.
Sood et al prospectively followed 240 kidney transplant recipients and performed serial screening for BKV over 1 year. They performed blood and urine BKV DNA screening at 1, 3, 6, 12, and 24 months after transplantation. Sixty-five (27%) patients developed BK viremia. Twenty-eight (12%) had BK viremia levels>10,000 viral copies/mL. Twenty-three of the 28 patients underwent a kidney allograft biopsy and 5 of these showed subclinical BKVAN. The 28 patients were treated with concurrent reductions in doses of both tacrolimus and mycophenolate mofetil. The mean reductions in mycophenolate mofetil dose and tacrolimus trough levels were 45% and 39%, respectively (from 1 month after transplantation to 6 months after peak viremia). Four of the 28 patients developed acute cellular rejection. The mean eGFR for all patients at 12 months after reduction of immunosuppression was not significantly different from 1 month after transplantation.51
Randhawa et al identified 22 patients with BKVAN on biopsies performed for elevated creatinine levels. The initial 12 patients received steroid therapy out of concern for rejection as the biopsy demonstrated evidence of tubulitis. These patients had a high rate of graft loss (8 out of 12). The other 8 patients in this series who were managed with reduction of immunosuppression had preserved graft function.41
Current practice among many transplant centers has been to decrease or stop the antimetabolite upon detection of BK viremia. Persistence of the viremia after 4 weeks should prompt further discontinuation of the antimetabolite or reduction of the CNI dose. The KDIGO 2009 guidelines suggest reducing immunosuppressive medications when the BK viral load in blood is persistently above 10,000 copies/mL.38 Our center implements a standardized protocol for the management of BKV infections in kidney transplant recipients that aims for specific reductions in immunosuppressive medications based on the BK viral load with follow up monitoring to assess for response and need for adjunctive therapy (Figure 1).
IVIG
Human immunoglobulin preparations have been used in the kidney transplant recipients who develop BK viremia. Sener et al studied the outcome of BKVAN patients who received IVIG therapy over a period of 1 year. Eight patients were treated by reduction of immunosuppression and received 2 g/kg of IVIG. Over a mean follow-up period of 15 months, all but one of the patients were off dialysis but with impaired graft function.52 Randhawa et al showed that commercially available immunoglobulin preparations contain antibodies that neutralize all major subtypes of BKV.53
Shah et al reported 30 patients with BKVAN who already had their immunosuppression reduced and were on leflunomide but had persistent BK viremia after 8 weeks of therapy. These patients were given IVIG therapy. Twenty-seven (90%) of the patients had a positive response in clearing the viremia. Twelve-month patient and graft survival rates were 100% and 96.7%, respectively.54
Sharma et al reported a case of a young boy with had persistent BKVAN despite reduction of immunosuppression and cidofovir therapy. He was then treated with 5 doses of IVIG with follow-up biopsy showing no evidence of BKAVN and reduction in BKV viremia from 20,800 copies/mL after the first dose of IVIG to 1000–2000 copies/mL after completion of the IVIG course.55
Kable et al conducted a retrospective study looking at the impact of adjunct use of IVIG in 22 versus 28 mostly historic control patients with proven BKVAN (A 6%, B 92%, C 2%) and allograft dysfunction in 46%. The patients received reduction in immunosuppression therapy such as switching from tacrolimus to reduced cyclosporine in 56% of the patients, switching from mycophenolate to leflunomide in 83% of the patients, ciprofloxacin addition in 89% of the patients, and intravenous cidofovir in 85% of the patients. The overall clearance of BKV from the blood was seen in 65% of the cases. Therapy with IVIG and switching to cyclosporine were associated with faster clearance rates.56
Wojciechowski et al are currently conducting a multicenter prospective, randomized, placebo-controlled trial to compare IVIG (Privigen, 1 mg/kg monthly for 2 doses) with protocolized immunosuppression reduction to placebo and immunosuppression reduction in patients with BK viremia post kidney-transplantation. The hypothesis of this study is that IVIG could rapidly improve clearance of BK viremia thereby decreasing the potential of formation of alloantibodies in patients that have had immunosuppression reduction due to BK viremia. The primary outcome of this study is resolution of BK viremia (defined as a decreased in BK viral load<1000 copies/mL) by 3 months post enrollment. The secondary outcomes are presence of donor-specific anti-HLA antibodies, kidney allograft survival, and acute rejection.
Leflunomide
Leflunomide is an immunomodulatory agent that inhibits pyrimidine synthesis, with antiproliferative and anti-inflammatory effects. It has been found to reduce BK viral replication in animal and in vitro studies.
Josephson et al reported a case series of 26 patients with biopsy-proven BK nephropathy who were switched from mycophenolate to either leflunomide alone (17 patients) or in addition to cidofovir (9 patients). These patients also had reductions in their tacrolimus dosing to goal trough levels of 4–6 ng/mL. The BK viral loads decreased in all patients who sustained target blood levels of the active drug or had cidofovir added to the regimen.57
Liacini et al conducted experiments on renal epithelial cells that showed inhibition by leflunomide of the BK virus genome replication and early gene expression.58 Krisl et al conducted a single-center retrospective analysis of 76 patients with BK viremia to evaluate the effect of leflunomide on BKV clearance and showed no lack of association on multivariate analysis.59
Guasch et al conducted a randomized, open-label study comparing the effect of treatment with a leflunomide derivative (FK778) (30 patients) to standard reduction of immunosuppression (16 patients) on BKV infection. These patients were followed for 6 months after start of therapy. The reductions in BK viral loads seen with FK778 therapy were statistically significant (P=0.049) but these patients had numerically higher acute rejection rates.60
Fluoroquinolones
Fluoroquinolones were shown to have an inhibitory effect on the BK viral DNA replication as well as the helicase activity of the SV40 large tumor antigen.61 Gabardi et al retrospectively evaluated kidney transplant recipients who had at least one BK blood viral load check between 90 and 400 days post-transplant. They compared patients who received the standard sulfamethoxazole/trimethoprim prophylaxis (160 patients) with those who, due to intolerance to SMX/TMP, received atovaquone in addition to 1 month of either ciprofloxacin (250 mg daily) or levofloxacin (250 mg daily) (25 patients). They showed a higher rate of BK viremia at 1 year in the patients who received SMX/TMP compared to the patients who received fluoroquinolones (22.5% vs 4%, respectively; P=0.03). They also performed a subgroup analysis of the group of patients who received SMX/TMP. Out of the 160 patients who received SMX/TMP, 40 patients received a course of fluoroquinolones for a bacterial infection within the first 3 months post-transplant. When compared with the 120 patients who did not receive any fluoroquinolones, these 40 patients were found to have a lower rate of BK viremia at 1 year (7.5% vs 27.5%, respectively; P=0.008).62
Wojciechowski et al retrospectively evaluated the effect of change from no BK prophylaxis (106 patients) to BK prophylaxis with ciprofloxacin 250 mg twice daily for 30 days (in 130 patients) on the rate of BKV infection in the first year following kidney transplantation. They found that ciprofloxacin was associated with a lower rate of BK viremia (0.161 vs 0.065, P=0.0378) and viruria (0.303 vs 0.146, P=0.0067) at 3 months but not 12 months.63 This suggested that a longer prophylaxis course might help prevent BKV infections.
Knoll et al conducted a multicenter, double-blind, placebo-controlled, randomized trial on 154 patients to determine the effect of a 3-month course of fluoroquinolones on prevention of BKV. Seventy-six patients received Levofloxacin (500 mg daily) that was started within 5 days after kidney transplantation. There was no significant difference in the incidence of BK viruria or viremia, rejection, and patient and allograft survival between the two groups. The lack of effect in these studies might have been related to the short duration of prophylaxis.
Our group retrospectively evaluated the 1-year incidence rate of BK viremia in patients who did (n=15) or did not (n=76) receive 6 months of fluoroquinolones post-transplant. These patients received either levofloxacin, 250 mg daily, or ciprofloxacin, 250–500 mg daily. The incidence rate of BKV at 1 year was 6.7% and 25% in the 6-month prophylaxis and no prophylaxis groups, respectively. Though there was a numerical difference, this was not statistically significant (P=0.1171).
Lee et al conducted a multicenter, randomized, placebo-controlled trial on 39 kidney transplant patients to evaluate the effect of a 1-month course of levofloxacin (500 mg daily) as treatment for BK viremia. There was no significant difference in BK viral reductions, at 3 months of follow up (70.3% and 69.1% in the treatment and placebo groups, respectively). There was no difference in allograft function between the two groups as well.64
Conversion of tacrolimus to cyclosporine
Cyclosporine was found to suppress primary BKV infection in vitro, and this effect was shown to be dose-independent and not related to cytotoxicity. However, cyclosporine in these experiments did not have any influence on the cells with high-level infection (>109copies/mL).65 Li et al conducted in vitro studies and showed that cyclosporine inhibits BKV replication in human renal proximal tubular cells and uroepithelial cells of the bladder.66
Conversion to mTORi
Wali et al reported 3 cases of BKVN who were switched from tacrolimus and mycophenolate to sirolimus and prednisone therapy. After a median follow-up period of 18 months, all patients had undetectable plasma BKV DNA with improvement in allograft function. They had no episodes of rejection.67
Experiments by Liacini et al on renal epithelial cells and human tubular epithelial cells showed that infection with BKV led to increase in the phosphorylation of mTOR, 70 kDa ribosomal protein S6 kinase (p70S6K), and other protein kinase pathways. Treatment with sirolimus led to inhibition of the p70S6K phosphorylation with reduced BK virus large T antigen expression.58
Hirsch et al compared the effects of mTOR inhibitors and calcineurin inhibitors on BKV replication in primary human renal tubular epithelial cells. They found that treatment with sirolimus led to rapid and effective inhibition during the early but not late gene expression. They also showed that tacrolimus activated BKV replication and reversed the inhibition by sirolimus. Both effects were shown to be through the FK binding protein 12kda (FKBP-12).68
Wojciechowski et al evaluated the effect of conversion from mycophenolate to everolimus on BKV infections. They randomized 40 patients with BKV infection to either conversion from mycophenolate to everolimus or reduction in the mycophenolate dose by 50%. The primary endpoint was more than 50% reduction in BKV viruria or clearance of viremia at 3 months. Eleven (55%) in the everolimus group and 8 (40%) in the mycophenolate group reached the primary endpoint (P=0.53). Among patients who had BK viremia, 8 of 16 (50%) cleared the virus in the everolimus group, and 5 of 15 (33.3%) cleared the virus in the MMF group at 3 months. These differences were not statistically significant (P=0.47).69
Mallat et al conducted a meta-analysis to compare the incidence rate of BKV infections in patients receiving mTOR inhibitor-based regimens compared to those receiving calcineurin inhibitor-based regimens (12 trials). They found no significant difference between the two groups with quality of evidence that was judged to be moderate due to imprecision.70
Cell-based therapies
Cellular immunotherapy has been used for BKV infections in hematopoietic stem cell recipients. Pello et al reported a case of a male recipient of a haploidentical hematopoietic cell transplant who developed BK viremia and hemorrhagic cystitis that was resistant to standard therapy. Adoptive transfer of donor cells enriched in BKV-specific T cells led to resolution of the symptoms and clearance of the viremia.71 Papdopoulou et al reported the use of rapidly generated virus-specific T cells that recognize 12 immunogenic antigens from five viruses (BKV, EBV, CMV, adenovirus, and HHV-6) in hematopoietic cell transplant patients. This was administered to 11 hematopoietic cell transplant recipients with, 7 of whom had evidence of BKV reactivation. Five out of the 7 patients achieved a complete response and 1 patient achieved a partial response. Three of those 7 patients had severe hemorrhagic cystitis that was resistant to cidofovir and achieved marked resolution of symptoms and reductions in viral load within 2–4 weeks of receiving the treatment.72
Conclusion and future directions
BKV infection in transplant recipients has become an increasingly recognized problem with the advent of more potent immunosuppressive regimens. The infection can cause significant deleterious effects, such as BKV nephropathy in kidney transplant recipients and hemorrhagic cystitis in hematopoietic stem cell transplant recipients. Multiple methods to detect the infection exist, but the most commonly used method is the detection of viral replication in blood with polymerase chain reaction testing. Screening protocols have been implemented to detect the infection early after transplant, providing an opportunity to reduce immunosuppression, which is the current cornerstone of therapy. There are no specific antiviral agents that are available for the treatment of BKV infection. Multiple agents have been evaluated for the treatment of BKV infection with mixed results in human studies. Cell-based therapies have shown some encouraging results in limited studies. There is a need for more multicenter randomized clinical trials to evaluate more specific therapeutic agents that can eradicate the virus effectively.
Disclosure
Dr David Wojciechowski reports grants from CSL Behring, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Gardner S, Field A, Coleman D, Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971;1(7712):1253–1257. doi:10.1016/s0140-6736(71)91776-4
2. Calvignac-Spencer S, Feltkamp MCW, Daugherty MD, et al. A taxonomy update for the family polyomaviridae. Arch Virol. 2016;161(6):1739–1750. doi:10.1007/s00705-016-2794-y
3. Imperiale M, Major E. Polyomaviruses. In: Fields Virology.
4. Knowles WA, Pipkin P, Andrews N, et al. Population-based study of antibody to the human polyomaviruses BKV and JCV and the simian polyomavirus SV40. J Med Virol. 2003;71(1):115–123. doi:10.1002/jmv.10450
5. Egli A, Infanti L, Dumoulin A, et al. Prevalence of polyomavirus BK and JC infection and replication in 400 healthy blood donors. J Infect Dis. 2009;199(6):837–846. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19434930.
6. Polo C, Pérez JL, Mielnichuck A, Fedele CG, Niubó J, Tenorio A. Prevalence and patterns of polyomavirus urinary excretion in immunocompetent adults and children. Clin Microbiol Infect. 2004;10(7):640–644. doi:10.1111/j.1469-0691.2004.00882.x
7. Kean JM, Rao S, Wang M, Garcea RL. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009;5(3):e1000363. doi:10.1371/journal.ppat.1000706
8. Hirsch H, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal transplant recipients. N Engl J Med. 2002;347(7):488–496. doi:10.1056/NEJMoa020439
9. Drachenberg CB, Hirsch HH, Papadimitriou JC, et al. Polyomavirus BK versus JC replication and nephropathy in renal transplant recipients: a prospective evaluation. Transplantation. 2007;84(3):323–330. doi:10.1097/01.tp.0000269706.59977.a5
10. Zhong S, Randhawa PS, Ikegaya H, et al. Distribution patterns of BK polyomavirus (BKV) subtypes and subgroups in American, European and Asian populations suggest co-migration of BKV and the human race. J Gen Virol. 2009;90(1):144–152. doi:10.1099/vir.0.83611-0
11. Ikegaya H, Saukko PJ, Tertti R, et al. Identification of a genomic subgroup of BK polyomavirus spread in European populations. J Gen Virol. 2006;87(11):3201–3208. doi:10.1099/vir.0.82266-0
12. Vanchiere JA, Nicome RK, Greer JM, Demmler GJ, Butel JS. Frequent detection of polyomaviruses in stool samples from hospitalized children. J Infect Dis. 2005;192(4):658–664. doi:10.1086/432076
13. Vanchiere JA, Abudayyeh S, Copeland CM, Lu LB, Graham DY, Butel JS. Polyomavirus shedding in the stool of healthy adults. J Clin Microbiol. 2009;47(8):2388–2391. doi:10.1128/JCM.02472-08
14. Wong ASY, Cheng VCC, Yuen KY, Kwong YL, Leung AYH. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant. 2009;43(1):43–47. doi:10.1038/bmt.2008.266
15. Bofill-Mas S, Pina S, Girones R. Documenting the epidemiologic patterns of polyomaviruses in human populations by studying their presence in urban sewage. J Virol. 2000;66(1):238–245.
16. Bofill-mas L, Formiga-cruz M, Clemente-casares P, Calafell F, Girones R. Potential transmission of human polyomaviruses through the gastrointestinal tract after exposure to virions or viral DNA edited by Foxit reader. J Virol. 2008;2(C):10290–10299.
17. Goudsmit J, Dillen PW, van Strien A, van der Noordaa J. The role of BK virus in acute respiratory tract disease and the presence of BKV DNA in tonsils. J Med Virol. 1982;10(2):91–99.
18. Chesters PM, Heritage J, McCance DJ. Persistence of DNA sequences of BK virus and JC virus in normal human tissues and in diseased tissues. J Infect Dis. 2019;147(4):676–684. doi:10.1093/infdis/147.4.676
19. Thangaraju S, Gill J, Wright A, Dong J, Rose C, Gill J. Risk factors for BK polyoma virus treatment and association of treatment with kidney transplant failure: insights from a paired kidney analysis. Transplantation. 2016;100(4):854–861. doi:10.1097/TP.0000000000000890
20. Schold JD, Rehman S, Kayler LK, Magliocca J, Srinivas TR, Meier-Kriesche HU. Treatment for BK virus: incidence, risk factors and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22(6):626–634. doi:10.1111/j.1432-2277.2009.00842.x
21. Masutani K, Ninomiya T, Randhawa P. HLA-A2, HLA-B44 and HLA-DR15 are associated with lower risk of BK viremia. Nephrol Dial Transplant. 2013;28(12):3119–3126. doi:10.1093/ndt/gft298
22. Sharif A, Alachkar N, Bagnasco S, et al. Incidence and outcomes of BK virus allograft nephropathy among ABO- and HLA-incompatible kidney transplant recipients. Clin J Am Soc Nephrol. 2012;7(8):1320–1327. doi:10.2215/CJN.00770112
23. Bentall A, Neil D, Sharif A, Ball S. ABO-incompatible kidney transplantation is a novel risk factor for BK nephropathy. Transplantation. 2015;99(2):e8–e9. doi:10.1097/TP.0000000000000483
24. DeWolfe D, Gandhi J, Mackenzie MR, et al. Pre-transplant immune factors may be associated with BK polyomavirus reactivation in kidney transplant recipients. PLoS One. 2017;12(5):1–15. doi:10.1371/journal.pone.0177339
25. Schaenman JM, Korin Y, Sidwell T, et al. Increased frequency of BK virus-specific polyfunctional CD8+ T cells predict successful control of BK viremia after kidney transplantation. Transplantation. 2017;101(6):1479–1487. doi:10.1097/TP.0000000000001314
26. Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5(9):2213–2221. doi:10.1111/j.1600-6143.2005.01000.x
27. Schmitt C, Raggub L, Linnenweber-Held S, Adams O, Schwarz A, Heim A. Donor origin of BKV replication after kidney transplantation. J Clin Virol. 2014;59(2):120–125. doi:10.1016/j.jcv.2013.11.009
28. Schwarz A, Linnenweber-Held S, Heim A, Framke T, Haller H, Schmitt C. Viral origin, clinical course, and renal outcomes in patients with BK virus infection after living-donor renal transplantation. Transplantation. 2016;100(4):844–853. doi:10.1097/TP.0000000000001066
29. Wunderink HF, De Brouwer CS, Gard L, et al. Source and relevance of the BK polyomavirus genotype for infection after kidney transplantation. Pen Forum Infect Dis. 2019;6(3):1–6. doi:10.1093/ofid/ofz078
30. Hirsch HH, Steiger J. Polyomavirus BK. Lancet Infect Dis. 2003;3(10):611–623.
31. Pahari A, Rees L. BK virus-associated renal problems - clinical implications. Pediatr Nephrol. 2003;18(8):743–748. doi:10.1007/s00467-003-1184-3
32. Coleman DV, Mackenzie EFD, Gardner SD, Poulding JM, Amer B, Russell WJ. Human polyomavirus (BK) infection and ureteric stenosis in renal allograft recipients. J Clin Pathol. 1978;31(4):338–347. doi:10.1136/jcp.31.4.338
33. van Gorder MA, Della Pelle P, Henson JW, Sachs DH, Cosimi AB, Colvin RB. Cynomolgus polyoma virus infection: a new member of the polyoma virus family causes interstitial nephritis, ureteritis, and enteritis in immunosuppressed cynomolgus monkeys. Am J Pathol. 2011;154(4):1273–1284. doi:10.1016/S0002-9440(10)65379-5
34. Muñoz P, Fogeda M, Bouza E, Verde E, Palomo J, Bañares R. Prevalence of BK virus replication among recipients of solid organ transplants. Clin Infect Dis. 2005;41(12):1720–1725. doi:10.1086/498118
35. Razonable R, Brown R, Humar A, Covington E, Alecock E, Paya C. A longitudinal molecular surveillance study of human polyomavirus viremia in heart, kidney, liver, and pancreas transplant patients. J Infect Dis. 2019;192(8):1349–1354. doi:10.1086/466532
36. Loeches B, Valerio M, Peréz M, et al. BK virus in liver transplant recipients: a prospective study. Transplant Proc. 2009;41(3):1033–1037. doi:10.1016/j.transproceed.2009.02.021
37. Dropulic L, Jones R. Polyomavirus BK infection in blood and marrow transplant recipients. Bone Marrow Transplant. 2008;41(1):58. doi:10.1038/sj.bmt.1705886
38. Group KDIGO (KDIGO) TW. Special issue: KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9:S1–S155. doi:10.1111/j.1600-6143.2009.02834.x
39. Hirsch HH, Brennan DC, Drachenberg CB, et al. Polyomavirus-associated nephropathy in renal transplantation: interdisciplinary analyses and recommendations. Transplantation. 2005;79(10):1277–1286. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15912088.
40. Tan SK, Milligan S, Sahoo MK, Taylor N, Pinsky BA. Calibration of BK virus nucleic acid amplification testing to the 1st WHO international standard for BK virus. J Clin Microbiol. 2017;55(3):923–930. doi:10.1128/JCM.02315-16
41. Randhawa P, Finkelstein S, Scantelbury V, et al. Human polyoma virus-associated interstitial nephritis in the allograft kidney. Transplantation. 1999;67(1):103–109. doi:10.1097/00007890-199901150-00018
42. Drachenberg C, Beskow C, Cangro CB, et al. Human polyoma virus in renal allograft biopsies : morphological findings and correlation with urine cytology. Hum Pathol. 1999;30(8):970–977. doi:10.1016/s0046-8177(99)90252-6
43. Nickeleit V, Singh HK, Randhawa P, et al. The banff working group classification of definitive polyomavirus nephropathy: morphologic definitions and clinical correlations. J Am Soc Nephrol. 2017;29(2):680–693. doi:10.1681/ASN.2017050477
44. Brennan D, Bromberg J, Yee J, Dholakia S, Haas M. Donor derived cell free DNA (dd-cfDNA) May aid in the diagnosis of BK virus nephropathy [abstract]. Am J Transplant. 2019;19(suppl 3). Available from: https://atcmeetingabstracts.com/abstract/donor-derived-cell-free-dna-dd-cfdna-may-aid-in-the-diagnosis-of-bk-virus-nephropathy.
45. Johnston O, Jaswal D, Gill JS, Doucette S, Fergusson DA, Knoll GA. Treatment of polyomavirus infection in kidney transplant recipients: a systematic review. Transplantation. 2010;89(9):1057–1070. doi:10.1097/TP.0b013e3181d0e15e
46. Saad ER, Bresnahan BA, Cohen EP, et al. Successful treatment of BK viremia using reduction in immunosuppression without antiviral therapy. Transplantation. 2008;85(6):850–854. doi:10.1097/TP.0b013e318166cba8
47. Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5(3):582–594. doi:10.1111/j.1600-6143.2005.00742.x
48. Hardinger KL, Koch MJ, Bohl DJ, Storch GA, Brennan DC. BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. Am J Transplant. 2010;10(2):407–415. doi:10.1111/j.1600-6143.2009.02952.x
49. Schaub S, Hirsch HH, Dickenmann M, et al. Reducing immunosuppression preserves allograft function in presumptive and definitive polyomavirus-associated nephropathy. Am J Transplant. 2010;10(12):2615–2623. doi:10.1111/j.1600-6143.2010.03310.x
50. Schaub S, Steiger J, Hirt-Minkowski P, et al. Reducing calcineurin inhibitor first for treating BK polyomavirus replication after kidney transplantation: long-term outcomes. Nephrol Dial Transplant. 2018:1–11. doi:10.1093/ndt/gfy346
51. Sood P, Senanayake S, Sujeet K, et al. Management and outcome of BK viremia in renal transplant recipients: a prospective single-center study. Transplantation. 2012;94(8):814–821. doi:10.1097/TP.0b013e31826690c6
52. Sener A, House AA, Jevnikar AM, et al. Intravenous immunoglobulin as a treatment for BK virus associated nephropathy: one-year follow-up of renal allograft recipients. Transplantation. 2006;81(1):117–120. doi:10.1097/01.tp.0000181096.14257.c2
53. Randhawa P, Pastrana DV, Zeng G, et al. Commercially available immunoglobulins contain virus neutralizing antibodies against all major genotypes of polyomavirus BK. Am J Transplant. 2015;15(4):1014–1020. doi:10.1111/ajt.13083
54. Shah T, Vu D, Naraghi R, Campbell A, Min D. Efficacy of intravenous immunoglobulin in the treatment of persistent BK viremia and BK virus nephropathy in renal transplant recipients. Clin Transpl. 2014;109–116.
55. Sharma AP, Moussa M, Casier S, Rehman F, Filler G, Grimmer J. Intravenous immunoglobulin as rescue therapy for BK virus nephropathy. Pediatr Transplant. 2009;13(1):123–129. doi:10.1111/j.1399-3046.2008.00958.x
56. Nankivell BJ, Davies CD, Kable K, Oʼconnell PJ, Chapman JR. Clearance of BK virus nephropathy by combination antiviral therapy with intravenous immunoglobulin. Transplant Direct. 2017;3(4):e142. doi:10.1097/txd.0000000000000641
57. Josephson MA, Gillen D, Javaid B, et al. Treatment of renal allograft polyoma BK virus infection with leflunomide. Transplantation. 2006;81(5):704–710. doi:10.1097/01.tp.0000181149.76113.50
58. Liacini A, Seamone ME, Muruve DA, Tibbles LA. Anti-BK virus mechanisms of sirolimus and leflunomide alone and in combination: toward a new therapy for BK virus infection. Transplantation. 2010;90(12):1450–1457. doi:10.1097/TP.0b013e3182007be2
59. Krisl JC, Taber DJ, Pilch N, et al. Leflunomide efficacy and pharmacodynamics for the treatment of BK viral infection. Clin J Am Soc Nephrol. 2012;7(6):1003–1009. doi:10.2215/CJN.12531211
60. Guasch A, Roy-Chaudhury P, Woodle ES, Fitzsimmons W, Holman J, First MR. Assessment of efficacy and safety of FK778 in comparison with standard care in renal transplant recipients with untreated BK nephropathy. Transplantation. 2010;90(8):891–897. doi:10.1097/TP.0b013e3181f2c94b
61. Ali SH, Chandraker A, DeCaprio JA. Inhibition of simian virus 40 large T antigen helicase activity by fluoroquinolones. Antivir Ther. 2007;12(1):1–6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17503741.
62. Gabardi S, Waikar SS, Martin S, et al. Evaluation of fluoroquinolones for the prevention of BK viremia after renal transplantation. Clin J Am Soc Nephrol. 2010;5(7):1298–1304. doi:10.2215/CJN.08261109
63. Wojciechowski D, Chanda R, Chandran S, et al. Ciprofloxacin prophylaxis in kidney transplant recipients reduces BK virus infection at 3 months but not at 1 year. Transplantation. 2012;94(11):1117–1123. doi:10.1097/TP.0b013e31826ec74e
64. Lee BT, Gabardi S, Grafals M, et al. Efficacy of levofloxacin in the treatment of BK viremia: a multicenter, double-blinded, randomized, placebo-controlled trial. Clin J Am Soc Nephrol. 2014;9(3):583–589. doi:10.2215/CJN.04230413
65. Acott PD, O’Regan PA, Lee SH, Crocker JFS. In vitro effect of cyclosporin A on primary and chronic BK polyoma virus infection in Vero E6 cells. Transpl Infect Dis. 2008;10(6):385–390. doi:10.1111/j.1399-3062.2008.00330.x
66. Li Y-J, Weng C-H, Lai W-C, et al. A suppressive effect of cyclosporine A on replication and noncoding control region activation of polyomavirus BK virus. Transplantation. 2010;89(3):299–306. doi:10.1097/tp.0b013e3181c9b51c
67. Wali RK, Drachenberg C, Hirsch HH, et al. BK virus-associated nephropathy in renal alloqraft recipients: rescue therapy by sirolimus-based immunosuppression. Transplantation. 2004;78(7):1069–1073. doi:10.1097/01.TP.0000142127.84497.50
68. Hirsch HH, Yakhontova K, Lu M, Manzetti J. BK polyomavirus replication in renal tubular epithelial cells is inhibited by sirolimus, but activated by tacrolimus through a pathway involving FKBP-12. Am J Transplant. 2016;16(3):821–832. doi:10.1111/ajt.13541
69. Wojciechowski D, Chandran S, Webber A, Hirose R, Vincenti F. Mycophenolate mofetil withdrawal with conversion to everolimus to treat BK virus infection in kidney transplant recipients. Transplant Proc. 2017;49(8):1773–1778. doi:10.1016/j.transproceed.2017.06.030
70. Mallat SG, Tanios BY, Itani HS, et al. CMV and BKPyV infections in renal transplant recipients receiving an mtor inhibitor–based regimen versus a cni-based regimen: a systematic review and meta-analysis of randomized, controlled trials. Clin J Am Soc Nephrol. 2017;12(8):1321–1336. doi:10.2215/CJN.13221216
71. Pello OM, Innes AJ, Bradshaw A, et al. BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur J Haematol. 2017;98(6):632–634. doi:10.1111/ejh.12848
72. Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 Infections after HSCT. Sci Transl Med. 2014;6(242):1–12. doi:10.1126/scitranslmed.3008825
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
