Back to Journals » OncoTargets and Therapy » Volume 11
Biosimilar filgrastim vs filgrastim: a multicenter nationwide observational bioequivalence study in patients with chemotherapy-induced neutropenia
Authors Sevinç A, Özkan M, Özet A, Dane F, Öksüzoğlu B, Işıkdoğan A, Özdemir F, Uncu D, Gümüş M, Evrensel T, Yaren A, Kara O, Tekin SB
Received 13 February 2016
Accepted for publication 10 August 2017
Published 18 January 2018 Volume 2018:11 Pages 419—426
DOI https://doi.org/10.2147/OTT.S106342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr William C. Cho
Video abstract presented by Alper Sevinç.
Views: 3738
Alper Sevinç,1 Metin Özkan,2 Ahmet Özet,3 Faysal Dane,4 Berna Öksüzoğlu,5 Abdurrahman Işıkdoğan,6 Feyyaz Özdemir,7 Doğan Uncu,8 Mahmut Gümüş,9 Türkkan Evrensel,10 Arzu Yaren,11 Oğuz Kara,12 Salim Başol Tekin13
1Department of Medical Oncology, Medical Park Gaziantep Hospital, Gaziantep, 2Department of Medical Oncology, Erciyes University Faculty of Medicine, Kayseri, 3Department of Medical Oncology, Gazi University Faculty of Medicine, Ankara, 4Department of Medical Oncology, Marmara University Faculty of Medicine, Istanbul, 5Department of Medical Oncology, Dr Abdurrahman Yurtaslan Ankara Oncology Training and Research Hospital, Ankara, 6Department of Medical Oncology, Dicle University Faculty of Medicine, Diyarbakır, 7Department of Medical Oncology, Karadeniz Technical University Faculty of Medicine, Trabzon, 8Department of Medical Oncology, Ankara Numune Hospital, Ankara, 9Department of Medical Oncology, Istanbul Medeniyet University, Istanbul, 10Department of Medical Oncology, Uludağ University Faculty of Medicine, Bursa, 11Department of Medical Oncology, Pamukkale University Faculty of Medicine, Denizli, 12Department of Medical Oncology, Çukurova University Faculty of Medicine, Adana, 13Department of Medical Oncology, Atatürk University Faculty of Medicine, Erzurum, Turkey
Background: We studied the comparative effectiveness of biosimilar filgrastim vs original filgrastim in patients with chemotherapy-induced neutropenia.
Patients and methods: This multicenter, observational study was conducted at 14 centers. The study included 337 patients experiencing neutropenia under chemotherapy. Patients were given either filgrastim 30 MIU or 48 MIU (Neupogen®) or biosimilar filgrastim 30 MIU (Leucostim®). Data regarding age, chemotherapeutic agents used, number of chemotherapy courses, previous diagnosis of neutropenia, neutrophil count of patients after treatment, medications used for the treatment of neutropenia, and duration of neutropenia were collected. Time to absolute neutrophil count (ANC) recovery was the primary efficacy measure.
Results: Ambulatory and hospitalized patients comprised 11.3% and 45.1% of the enrolled patients, respectively, and a previous diagnosis of neutropenia was reported in 49.3% of the patients, as well. Neutropenia occurred in 13.7% (n=41), 45.5% (n=136), 27.4% (n=82), 11.4% (n=34), and 2.0% (n=6) of the patients during the first, second, third, fourth, and fifth cycles of chemotherapy, respectively. While the mean neutrophil count was 0.53±0.48 before treatment, a significant increase to 2.44±0.66 was observed after treatment (p=0.0001). While 90.3% of patients had a neutrophil count <1.49 before treatment, all patients had a neutrophil count ≥1.50 after treatment. Neutropenia resolved within ≤4 days of filgrastim therapy in 60.1%, 56.7%, and 52.6% of the patients receiving biosimilar filgrastim 30 MIU, original filgrastim 30 MIU, and original filgrastim 48 MIU, respectively. However, there was no significant difference between the three arms (p=0.468). Similarly, time to ANC recovery was comparable between the treatment arms (p=0.332).
Conclusion: The results indicate that original filgrastim and biosimilar filgrastim have comparable efficacy in treating neutropenia. Biosimilar filgrastim provides a valuable alternative; however, there is need for further studies comparing the two products in different patient subpopulations.
Keywords: chemotherapy, febrile neutropenia, neutrophil, ANC recovery, supportive care, myelosuppressive
Introduction
Despite improvements in supportive care, neutropenia and its complications remain a major issue for patients receiving myelosuppressive chemotherapy.1–3 The most serious complication of neutropenia is febrile neutropenia (FN), which is associated with an increased risk of morbidity and mortality as well as substantial cost of hospitalization and antibiotics.4 Dose reductions and delays are common consequences of neutropenic events and may compromise the efficacy of chemotherapy in cancer patients for whom completion of all planned cycles is essential to achieve a maximum chance of treatment success.5
Endogenous human G-CSF is a single polypeptide chain protein of 174 amino acids with O-glycosylation at one threonine residue (molecular weight 18 kDa, carbohydrate moiety 4% of total weight). It contains one free cysteinyl residue and two disulfide bonds. Cellular sources of G-CSF are monocytes, fibroblasts, and endothelial cells. The physiological role of G-CSF is to maintain neutrophil production during steady-state conditions and to increase production during acute situations such as infection.6 The common mode of action is to mobilize hematopoietic progenitor cells into the peripheral circulation. Fully differentiated neutrophils are functionally activated by G-CSF.7 Owing to its hematopoietic activity, G-CSF was identified as potentially useful for the prevention and treatment of neutropenia and associated complications.8
Neupogen® (filgrastim; F. Hoffman-La Roche Ltd. [under licence of Amgen]-Basel-Switzerland) was the first therapeutic recombinant G-CSF product approved by the US Food and Drug Administration (FDA). Filgrastim differs in structure from human G-CSF by lacking O-glycosylation and having an additional N-terminal methionine group as a result of bacterial expression, but it shows the same in vitro and in vivo activity as endogenous G-CSF. The main indication of filgrastim is to reduce the duration of neutropenia and the incidence of FN in patients receiving myelosuppressive chemotherapy.9 Filgrastim is also approved to reduce the duration of neutropenia in patients undergoing myeloablative therapy followed by bone marrow transplantation who are considered to be at increased risk of prolonged severe neutropenia, to mobilize peripheral blood progenitor cells (PBPCs), to increase neutrophil count, and to reduce the incidence and duration of infection-related events in neutropenic patients with an absolute neutrophil count (ANC) of <0.5×109/L.8,9
After the expiration of the patent of filgrastim in 2006 in Europe, several biosimilar versions of filgrastim have been approved by the European Medicines Agency (EMA) based on comparable quality, efficacy, and safety with the originator product.9 These products offer potential benefits by reducing health care costs and expanding access to these medications.8,10 Leucostim® (biosimilar filgrastim; Dong-A ST Co. Ltd., Daegu, South Korea) was the first biosimilar product approved by the Ministry of Health in Turkey and has been widely used across the country, since its launch in November 12, 2009. Biosimilar filgrastim has been on the market for >7 years and surpassed the use of the originator product in many hematology and oncology centers.
In the present study, as all biosimilars are unique, we aimed to investigate the bioequivalence of original filgrastim (Neupogen®) and biosimilar filgrastim (Leucostim®) by means of efficacy in treating neutropenia in cancer patients receiving chemotherapy. This study was partly presented as a poster presentation at the 9th National Medical Oncology Congress, September 12–16, 2012, Cyprus, and First Near East and Middle Asia Medical Oncology Associations (NEMA) Congress, November 29–December 2, 2012, Istanbul, Turkey.
Patients and methods
Study design and patient characteristics
This multicenter, observational study was conducted at 14 centers in Turkey after receiving approval from the clinical trials ethics committee of Gaziantep University. The study included 337 patients who were receiving chemotherapy for cancer and experienced neutropenia under chemotherapy during June 2011 and April 2012. Written informed consent has been obtained from all patients. For the treatment of neutropenia, patients were given either filgrastim 30 MIU or 48 MIU (Neupogen®) or biosimilar filgrastim 30 MIU (Leucostim®) until neutrophil recovery. Doses were calculated based on patients’ weight; however, it was at the discretion of the physician to administer original filgrastim 30 MIU or 48 MIU. Since this was an observational and multicenter study, medications given by the treating physicians were selected based on their own choices, according to the 2:1 ratio rule (66% vs 33%). At the end of the study, percentage allocations of patients into biosimilar filgrastim and original filgrastim treatments were 61.9% and 38.1%, respectively. Furthermore, as this was an observational study, the study reflects routine clinical practice of the treating physician. Therefore, there were no exclusion criteria in the present study, except patients with comorbid diseases. Data regarding age, gender, weight, chemotherapeutic agents used, the number of chemotherapy courses, previous diagnosis of neutropenia, neutrophil count of the patients before and after the treatment, medications used for the treatment of neutropenia, and the duration of neutropenia were collected. Patient demographics and blood counts at baseline are given in Tables 1 and 2. Time to ANC recovery was the primary efficacy measure. ANC recovery is defined as an ANC of ≥0.5×109/L (500 mm3) for three consecutive laboratory values obtained on different days. Date of ANC recovery is the date of the first of three consecutive laboratory values where ANC is ≥0.5×109/L (detailed information on ANC calculation can be obtained from the following website: https://www.cibmtr.org/manuals/fim/1/en/topic/q8-11-initial-anc-recovery).
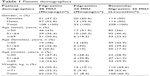  | Table 1 Patient demographics |
  | Table 2 Blood counts at baseline |
Definition of primary prophylaxis
Primary prophylaxis is defined as patients receiving myelotoxic chemotherapy with curative intent and which has a documented incidence rate of febrile neutropenia (FN) of >20% or patients receiving myelotoxic chemotherapy with curative intent and which has a documented incidence rate of FN of 10%–20%, and one or more of the following pre-disposing patients’ risk factors: pre-existing neutropenia due to disease infiltration of bone marrow or other etiology, age >65 years, advanced disease stage, poor performance status, previous episodes of FN whilst receiving earlier chemotherapy of a similar or lower dose intensity, extensive prior chemotherapy, previous irradiation to large volume of bone marrow, poor nutritional status, active infections or increased risk of infections, or serious co-morbidities.27
Statistical analyses of data
Data were analyzed using the Statistical Package for the Social Sciences (SPSS) for Windows (SPSS Inc., Chicago, IL, USA). Descriptive statistics were presented as mean ± standard deviation or n (%), where applicable. While categorical independent variables were compared using the Pearson’s chi-square test, numerical independent variables were compared using the nonparametric Mann–Whitney U test. The nonparametric Wilcoxon signed-rank test was used for the comparison of neutrophil values before and after treatment. The Kruskal–Wallis one-way analysis of variance was used to test the difference between the three treatment arms. A p-value <0.05 was considered as statistically significant.
Ethical approval
The observational study was approved by the Turkish Ministry of Health under the number 048146.
Results
A total of 337 patients from 14 centers in Turkey were included in this observational study. The mean age of the patients was 53.15±14.10 years, and 51.0% were males. There was a significant difference between the age groups in terms of gender (p=0.021). The difference was attributable to the higher number of males in the ≥65 years of age group; the majority of the patients ≥65 years of age were males (Table 1). Of the patients, 11.3% were hospitalized and 45.1% were ambulatory patients, and 49.3% had a previous diagnosis of neutropenia. Cancer types in enrolled patients are presented in Table 3. Chemotherapy courses administered to patients enrolled are presented in Table 4. Allocation of chemotherapy courses per number of patients during the entire study is given in Table 5.
  | Table 5 Allocation of chemotherapy courses per number of patients during the entire study |
Neutropenia occurred during the first cycle of chemotherapy in 13.7% (n=41) of the patients, during the second cycle of chemotherapy in 45.5% (n=136) of the patients, during the third cycle of chemotherapy in 27.4% (n=82) of the patients, during the fourth cycle of chemotherapy in 11.4% (n=34) of the patients, and during the fifth cycle of chemotherapy in 2.0% (n=6) of the patients. The most commonly used chemotherapeutic agent was fluorouracil (14.6%), followed by cisplatin (13.3%) and doxorubicin (8.3%). All patients had a neutrophil count <2.19 before filgrastim treatment. While the mean neutrophil count was 0.53±0.48 before treatment, a significant increase to 2.44±0.66 was observed after treatment (p=0.0001). While 90.3% of the patients had a neutrophil count <1.49 before treatment, all patients had a neutrophil count ≥1.50 after treatment. Patients received either filgrastim 30 MIU, filgrastim 48 MIU, or biosimilar filgrastim 30 MIU for the treatment of their neutropenia (Table 6).
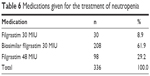  | Table 6 Medications given for the treatment of neutropenia |
Neutropenia resolved within ≤4 days of filgrastim therapy in 60.1%, 56.7%, and 52.6% of the patients receiving biosimilar filgrastim 30 MIU, original filgrastim 30 MIU, and original filgrastim 48 MIU, respectively. However, there was no significant difference between the three arms in this respect (p=0.468; Table 7). Similarly, time to ANC recovery was comparable between the treatment arms (p=0.332; Table 8).
  | Table 8 Time to ANC recovery |
Although there was no significant difference between ambulatory patients receiving biosimilar filgrastim 30 MIU, original filgrastim 30 MIU and original filgrastim 48 MIU with respect to the time to ANC recovery (p=0.985), hospitalized patients receiving filgrastim 48 MIU had a significantly longer recovery period than those receiving biosimilar filgrastim 30 MIU and those receiving filgrastim 30 MIU (p=0.001; Table 9).
The mean duration of neutropenia was 5.75±4.92 days, 5.26±3.89 days, and 5.62±4.83 days in the ≤50 years of age, 51–64 years of age, and ≥65 years of age groups, respectively (p=0.99). The mean duration of neutropenia was 5.08±3.70 days in male patients, while it was 6.02±5.25 days in female patients. There was no significant difference between genders in terms of the duration of neutropenia (p=0.57).
Among patients ≤70 kg, the percentage of those receiving biosimilar filgrastim 30 MIU was higher than the percentage of those receiving original filgrastim 30 MIU and 48 MIU. Similarly, the majority of patients ≥71 kg were receiving biosimilar filgrastim 30 MIU. However, the difference between the groups did not reach statistical significance (p=0.06).
The duration of neutropenia was 5.62±4.63 days, 6.00±5.75 days, and 5.67±3.75 days in patients ≤70 kg receiving biosimilar filgrastim 30 MIU, original filgrastim 30 MIU, and original filgrastim 48 MIU, respectively. The duration of neutropenia was comparable between the three treatment arms (p=0.39; Table 10).
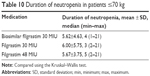  | Table 10 Duration of neutropenia in patients ≤70 kg |
Discussion
Biosimilars are biological medicinal products derived from recombinant human DNA and expressed by genetically engineered organisms to produce the target therapeutic proteins in large quantities. During the last few decades, including cytokines such as hematopoietic growth factors, interferons, and interleukins have received marketing authorization throughout the world. The term biosimilar is defined by the EMA along with the implementation of a specific regulatory framework for the marketing authorization of these products that are not identical to the innovator product because of inherent variability in the biological system and their complex manufacturing process. Because of the lack of chemical identity due to minor differences in amino acid sequence or posttranscriptional glycosylation pattern, profile of different impurities, and excipients, some concerns have been raised by the medical community over the safety and efficacy of biosimilars as these may lead to increased immunogenicity, which in turn results in safety and efficacy problems.11,12 Therefore, it must be shown by preapproval non-clinical and clinical studies that any differences in quality attributes have no impact on the efficacy and safety of the product.11
Clinical studies on filgrastim biosimilars have shown that these products are as safe and effective as original filgrastim in reducing the duration of severe neutropenia and the incidence of FN in different cancer settings.13–15 In their study on breast cancer patients receiving docetaxel/doxorubicin chemotherapy, del Giglio et al13 reported the mean duration of severe neutropenia to be 1.1 days during the first cycle of chemotherapy regimen both in Tevagrastim® (XM02) and filgrastim groups when the drugs were administered 24 hours after chemotherapy for at least 5 days and a maximum of 14 days. In the first cycle of chemotherapy, the incidence of FN was 12.1% and 12.5% in the XM02 and filgrastim groups, respectively. The authors observed no significant differences between the two treatment groups in terms of the incidence of FN, neither in the first cycle nor across all cycles of chemotherapy. Similarly, the mean time to ANC recovery in the first cycle was 8.0 and 7.8 days in the XM02 and filgrastim groups, respectively.13 Another study under the same clinical development program on patients with non-Hodgkin’s (NH) lymphoma receiving CHOP (Cyclophosphamide Doxorubicin [Hydroxydaunomycin] Vincristine Prednisolone) therapy reported the mean duration of severe neutropenia to be 0.5 and 0.9 days in the first cycle in the XM02 and filgrastim groups, respectively.16 In that particular study, the incidence of FN in the first chemotherapy cycle was 11.1% and 20.7% in the XM02 and filgrastim groups, respectively. The third study in the program conducted on cancer patients with small cell or non-small-cell lung cancer receiving platinum-based chemotherapy reported a mean duration of severe neutropenia in the first cycle of chemotherapy of 0.5 and 0.3 days in the XM02 and filgrastim groups, respectively.14 Similar to the abovementioned studies, the two treatment groups were comparable in terms of the incidence of FN in the first cycle of chemotherapy (15.0% vs 8.8%, p=0.2347). A meta-analysis was conducted using the data of these three studies to compare XM02 and filgrastim in terms of their prophylactic effect on the development of FN during the first cycle of chemotherapy in relation to the myelotoxic potency of chemotherapy regimen.16 Patients were allocated to one of the following chemotherapy categories: docetaxel–doxorubicin, CHOP/Pt-vinorelbine or Pt-vinblastine/Pt-etoposide, and Pt-gemcitabine/Pt-docetaxel or Pt-paclitaxel. The incidence of FN in the first cycle of chemotherapy was low (ranged between 12% and 16%), and the authors concluded that the incidence of FN was not directly correlated with the myelotoxic potency of chemotherapy regimen.15
A more recent study comparing filgrastim and its biosimilar Nivestim® (Hospira Zagreb, Prigorje Brdovecko, Crotia) in a breast cancer setting reported the mean duration of severe neutropenia in the first cycle of chemotherapy to be 1.6 and 1.3 days in the Nivestim® and filgrastim groups, respectively. Furthermore, the incidence of severe neutropenia in the first cycle was comparable between the two groups (77.6% and 68.2%). During the subsequent cycles of chemotherapy, the incidence of severe neutropenia remained similar between the two groups. The incidence of FN in cycles 1–3 was 2.4% in both treatment groups. The Nivestim® and filgrastim groups were also comparable with respect to the mean duration to ANC recovery. The mean time to ANC recovery in the first chemotherapy cycle was 7.8 days in both groups.8
In the present study, secondary prophylaxis with biosimilar filgrastim provided a similar degree of hemopoietic support with respect to the time to ANC recovery as compared with original filgrastim. The mean time to ANC recovery was 5.41±4.54 days, 5.66±4.83 days, and 5.63±4.32 days in the biosimilar filgrastim 30 MIU, original filgrastim 30 MIU, and original filgrastim 48 MIU groups, respectively.
Although all patients have a risk for developing neutropenia and its complications while receiving chemotherapy, patients at greater risk can be predicted based on several risk factors such as the use of some specific classes of chemotherapy regimen and the phase of therapy. A study on elderly patients with aggressive NH lymphoma receiving doxorubicin-based chemotherapy reported that 63% of the toxic deaths occurred after the first cycle of chemotherapy.17 In a study on patients with intermediate-grade, NH lymphoma receiving CHOP chemotherapy, febrile neutropenic event occurred during the first cycle in one-half of 160 patients experiencing FN.18 Moreover, in a retrospective study on 1,355 patients with intermediate-grade NH lymphoma, more than one-half of all initial hospitalizations for FN occurred in the first or second cycle of chemotherapy.19 A more recent study reported that ~60% of febrile neutropenic events occurred during the first cycle of therapy.20 In accordance with the literature, ~60% of neutropenic events were noted during the first two cycles of chemotherapy in the present study.
The chemotherapy regimen is one of the primary determinants of the risk of neutropenia, and some chemotherapy regimens are more myelotoxic than others.5,21,22 High doses of cyclophosphamide or the use of etoposide in patients with NH lymphoma,23,24 as well as high doses of anthracyclines in patients with early breast cancer,25 have been identified as significant predictors of neutropenic complications. Recently, a multivariate model of risk developed using the data of patients with solid tumors or malignant lymphoma showed that the use of several classes of chemotherapeutic agents, including the anthracyclines, taxanes (paclitaxel and docetaxel), certain alkylating agents (cyclophosphamide and ifosfamide), type I and type II topoisomerase inhibitors, platinums (cisplatin and carboplatin), gemcitabine, and vinorelbine, were associated with an increased risk of neutropenic events.26 However, a meta-analysis of three bioequivalence studies found no direct correlation between the development of neutropenic events and myelotoxic potency of chemotherapy regimen in patients with NH lymphoma.16 However, in that particular study, the comparison was made between three chemotherapy regimens that were associated with a high risk of neutropenic events. In this study, the most commonly used chemotherapeutic agent was fluorouracil, followed by cisplatin and doxorubicin. The common use of these agents might have contributed to the development of neutropenia in our patients.
Conclusion
The results of this observational study indicate that original filgrastim and biosimilar filgrastim have comparable efficacy in treating neutropenia in patients receiving cancer chemotherapy. Biosimilar filgrastim provides a valuable alternative to original filgrastim; however, there is need for further studies comparing the two products in different patient subpopulations.
Disclosure
The authors report no conflicts of interest in this work.
References
Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–4531. | ||
Kuderer NM. Meta-analysis of randomized controlled trials of granulocyte colony-stimulating factor prophylaxis in adult cancer patients receiving chemotherapy. Cancer Treat Res. 2011;157:127–143. | ||
Kuderer NM, Lyman GH. Personalized medicine and cancer supportive care: appropriate use of colony-stimulating factor support of chemotherapy. J Natl Cancer Inst. 2011;103(12):910–913. | ||
Komrokji RS, Lyman GH. The colony-stimulating factors: use to prevent and treat neutropenia and its complications. Expert Opin Biol Ther. 2004;4(12):1897–1910. | ||
Ria R, Reale A, Moschetta M, Dammacco F, Vacca A. Neutropenia and G-CSF in lymphoproliferative diseases. Hematology. 2013;18(3):131–137. | ||
Pizzo PA, Meyers J. Infections in the cancer patients. In: Devita VT Jr, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 3rd ed. Philadelphia, PA: JB Lippincott Co; 1989:2089–2098. | ||
Hong WS, et al. Clinical effects of Gracin (recombinant human granulocyte-colony stimulating factor) on leucopenia induced by VAP chemotherapy in patients with lung cancer. Korean J BRM. 1993;3(1):55–66. | ||
Waller CF, Semiglazov VF, Tjulandin S, Bentsion D, Chan S, Challand R. A phase III randomized equivalence study of biosimilar filgrastim versus Amgen filgrastim in patients receiving myelosuppressive chemotherapy for breast cancer. Onkologie. 2010;33(10):504–511. Erratum in: Onkologie. 2010;33(12):725. | ||
Abraham I, Tharmarajah S, MacDonald K. Clinical safety of biosimilar recombinant human granulocyte colony-stimulating factors. Expert Opin Drug Saf. 2013;12(2):235–246. | ||
Verpoort K, Möhler TM. A non-interventional study of biosimilar granulocyte colony-stimulating factor as prophylaxis for chemotherapy-induced neutropenia in a community oncology centre. Ther Adv Med Oncol. 2012;4(6):289–293. | ||
Pani L, Montilla S, Pimpinella G, Bertini Malgarini R. Biosimilars: the paradox of sharing the same pharmacological action without full chemical identity. Expert Opin Biol Ther. 2013;13(10):1343–1346. | ||
Rinaudo-Gaujous M, Paul S, Tedesco ED, Genin C, Roblin X, Peyrin-Biroulet L. Review article: biosimilars are the next generation of drugs for liver and gastrointestinal diseases. Aliment Pharmacol Ther. 2013;38(8):914–924. | ||
del Giglio A, Eniu A, Ganea-Motan D, Topuzov E, Lubenau H. XM02 is superior to placebo and equivalent to Neupogen in reducing the duration of severe neutropenia and the incidence of febrile neutropenia in cycle 1 in breast cancer patients receiving docetaxel/doxorubicin chemotherapy. BMC Cancer. 2008;8:332. | ||
Gatzemeier U, Ciuleanu T, Dediu M, Ganea-Motan E, Lubenau H, Del Giglio A. XM02, the first biosimilar G-CSF, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with small cell or non-small cell lung cancer receiving platinum-based chemotherapy. J Thorac Oncol. 2009;4(6):736–740. | ||
Engert A, del Giglio A, Bias P, Lubenau H, Gatzemeier U, Heigener D. Incidence of febrile neutropenia and myelotoxicity of chemotherapy: a meta-analysis of biosimilar G-CSF studies in breast cancer, lung cancer, and non-Hodgkin’s lymphoma. Onkologie. 2009;32(10):599–604. | ||
Engert A, Griskevicius L, Zyuzgin Y, Lubenau H, del Giglio A. XM02, the first granulocyte colony-stimulating factor biosimilar, is safe and effective in reducing the duration of severe neutropenia and incidence of febrile neutropenia in patients with non-Hodgkin lymphoma receiving chemotherapy. Leuk Lymphoma. 2009;50(3):374–379. | ||
Gómez H, Hidalgo M, Casanova L, et al. Risk factors for treatment-related death in elderly patients with aggressive non-Hodgkin’s lymphoma: results of a multivariate analysis. J Clin Oncol. 1998;16(6):2065–2069. | ||
Lyman GH, Morrison VA, Dale DC, et al; OPPS Working Group; ANC Study Group. Risk of febrile neutropenia among patients with intermediate-grade non-Hodgkin’s lymphoma receiving CHOP chemotherapy. Leuk Lymphoma. 2003;44(12):2069–2076. | ||
Lyman GH, Delgado DJ. Risk and timing of hospitalization for febrile neutropenia in patients receiving CHOP, CHOP-R, or CNOP chemotherapy for intermediate-grade non-Hodgkin lymphoma. Cancer. 2003;98(11):2402–2409. | ||
Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–118. | ||
Crawford J, Dale DC, Lyman GH. Chemotherapy-induced neutropenia: risks, consequences, and new directions for its management. Cancer. 2004;100(2):228–237. Review. Erratum in: Cancer. 2004;100(9):1993–1994. | ||
Pettengell R, Schwenkglenks M, Leonard R, et al; Impact of Neutropenia in Chemotherapy-European Study Group (INC-EU). Neutropenia occurrence and predictors of reduced chemotherapy delivery: results from the INC-EU prospective observational European neutropenia study. Support Care Cancer. 2008;16(11):1299–1309. | ||
Voog E, Bienvenu J, Warzocha K, et al. Factors that predict chemotherapy-induced myelosuppression in lymphoma patients: role of the tumor necrosis factor ligand-receptor system. J Clin Oncol. 2000;18(2):325–331. | ||
Wunderlich A, Kloess M, Reiser M, et al. Practicability and acute haematological toxicity of 2- and 3-weekly CHOP and CHOEP chemotherapy for aggressive non-Hodgkin’s lymphoma: results from the NHL-B trial of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol. 2003;14(6):881–893. | ||
Lyman GH, Kuderer N, Agboola O, Balducci L. Evidence-based use of colony-stimulating factors in elderly cancer patients. Cancer Control. 2003;10(6):487–499. | ||
Lyman GH, Kuderer NM, Crawford J, et al. Predicting individual risk of neutropenic complications in patients receiving cancer chemotherapy. Cancer. 2011;117(9):1917–1927. | ||
Carr J. Guideline for the Use of Granulocyte-Colony Stimulating Factor (G-CSF) in Adult Patients. Avon, Somerset and Wiltshire Cancer Services; 2012:9. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




