Back to Journals » Cancer Management and Research » Volume 12
Biliverdin Reductase A (BLVRA) Promotes Colorectal Cancer Cell Progression by Activating the Wnt/β-Catenin Signaling Pathway
Authors Mao H, Xu Y, Zhang Z, Sun G, Wang Z, Qiao D, Yin X, Liu S, Bo P
Received 16 December 2019
Accepted for publication 6 March 2020
Published 23 April 2020 Volume 2020:12 Pages 2697—2709
DOI https://doi.org/10.2147/CMAR.S242531
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Haiyan Mao,1,2 Yuan Xu,1 Zhengrong Zhang,1 Guozhuang Sun,3 Zhu Wang,1 Dawei Qiao,2 Xudong Yin,1 Siping Liu,4 Ping Bo2
1Oncology Department, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, Jiangsu Province 225000, People’s Republic of China; 2Department of Integrative Chinese and Western Medicine, Medical College of Yangzhou University, Yangzhou, Jiangsu Province 225009, People’s Republic of China; 3Laboratory Department, Xuyi People’s Hospital, Huai’an, Jiangsu Province 211700, People’s Republic of China; 4Imaging Department, Yangzhou Hospital of Traditional Chinese Medicine Affiliated to Nanjing University of Chinese Medicine, Yangzhou 225000, People’s Republic of China
Correspondence: Xudong Yin
Oncology Department, The Affiliated Hospital of Yangzhou University, Yangzhou University, Yangzhou, Jiangsu Province 225000, People’s Republic of China
Email [email protected]
Ping Bo
Department of Integrative Chinese and Western Medicine, Medical College of Yangzhou University, Yangzhou, Jiangsu Province 225009, People’s Republic of China
Email [email protected]
Purpose: Biliverdin reductase A (BLVRA) is a pleiotropic enzyme that converts biliverdin-IX-alpha into the antioxidant and anti-nitrosative compound, bilirubin-IX-alpha. It is related to various diseases, including cancer. It is overexpressed in many types of cancers and promotes cancer development and metastasis, but the effects of BLVRA in colorectal cancer have not been researched at present. This study was aimed to investigate the effects of biliverdin reductase A (BLVRA) in vivo and vitro experiments and its possible mechanism.
Methods: The clinical samples of CRC patients and CRC cell lines HT-29 and SW620 were chosen to perform the experiments. ELISA and Immunohistochemistry (IHC) were applied to test the level of BLVRA in patients. HT-29 knockdown of BLVRA and SW620 overexpression of BLVRA was established by the lentiviral vector transfection. Reverse transcription-quantitative real-time polymerase chain reaction and Western blotting were performed to examine the expression of BLVRA. MTT was used to detect the proliferation of CRC cells. Flow cytometry was applied to assess the rate of apoptosis. Transwell assay was performed to examine the capacity of migration and invasion. Immunofluorescence staining was adopted to assess the expression of E-cadherin and vimentin. Western blotting was utilized to detect the expression of apoptosis-related proteins, EMT-related proteins and target proteins of Wnt/β-catenin signaling pathway.
Results: Analysis of the clinical samples revealed that BLVRA was overexpressed in CRC patients and implied poor prognosis. BLVRA overexpression in the in vitro studies revealed that it increased the potential of CRC cells for proliferation, migration and invasion; augmented EMT; and hindered apoptosis. In addition, BLVRA overexpression was found to upregulate positive target genes and downregulate negative target genes of the Wnt/β-catenin signaling pathway, which implied that the biological effects of BLVRA in CRC were mediated by this pathway. In contrast, knockdown of BLVRA manifested the opposite effects.
Conclusion: Our results suggested that BLVRA might be a promising prognostic marker and a potential therapeutic target in CRC.
Keywords: biliverdin reductase A, colorectal cancer, cancer progression, Wnt/β-catenin signaling pathway
Introduction
Colorectal cancer (CRC) is a common malignancy of the digestive tract.1 Among all types of malignant tumors, it has the third-highest incidence, both worldwide and in China, and the second- and fifth-highest death rates, respectively.2,3 CRC is characterized by occult onset, easy metastasis, and poor prognosis. Although early diagnosis techniques and clinical treatment methods have greatly progressed, the overall treatment efficacy and survival rate of CRC are still rather poor.4 Therefore, the discovery of new targets and the development of additional therapies are urgently needed.
Biliverdin reductase (BVLR or BVR) is a well-characterized enzyme of the heme degradation pathway. Its name-giving activity is the conversion of biliverdin-IX-alpha into bilirubin-IX-alpha.5 As the latter is a potent antioxidant, BLVRA is important in maintaining the cellular redox balance. Biliverdin reductase A (BLVRA), the major isoform of BVR, is a pleiotropic enzyme. In addition to catalyzing the aforementioned reaction, it can also modulate signal transduction, either directly through its serine/threonine/tyrosine kinase activities or indirectly by acting as a scaffold/bridge and intracellular transporter of kinases that mediate cell growth and proliferation. This multitude of activities allows it to influence thousands of genes, including genes involved in signaling pathways and apoptosis, as well as cyclins.5–8
Overexpression of BLVRA has been reported in many diseases, including cancers. The level of BLVRA is rather high in many cancers, including liver cancer,6,9 lung cancer,7 breast cancer,10 skin cancer,11 and esophageal cancer.12 However, the role of BLVRA in CRC has not yet been fully elucidated.
In this study, we first demonstrated that CRC patients displayed increased BLVRA levels. Moreover, we used CRC lines to investigate the biological functions of BLVRA in CRC cells. Finally, we established that BLVRA exerted its actions by modulating the Wnt/β-catenin signaling pathway.
Materials and Methods
Clinical Samples
The study was carried out using samples from 110 patients who were histopathologically diagnosed as colorectal adenocarcinoma cases at the Affiliated Hospital of Yangzhou University and Yangzhou TCM Hospital from 2015 to 2017. As controls,110 healthy volunteers joined in this study. The tissues and the plasma of peripheral blood were acquired before the first treatment. The group consisted of 2 I stage, 31 II stage, 40 III stage and 37 IV stage of the patients. Ethical approval (NO.2020-YKL-017) was granted from the Ethical Committee of the hospital and fully informed consents were asked from all patients before sample collection. All participants (not just the patients) provided written informed consent, and this study was conducted in accordance with the Declaration of Helsinki.
Enzyme-Linked Immunosorbent Assay (ELISA)
The concentration of BLVRA in serum was detected with an ELISA system kit (Fengxiang Biotechnology, Shanghai, China). Each measurement was performed three times.
Immunohistochemistry (IHC)
Specimens were fixed in formalin and embedded in paraffin. Afterward, they were cut into 2–3 mm thick slides with a slicer and deparaffinized with xylene. After blocking the endogenous peroxidase activity, the sections were incubated with anti-BLVRA (Proteintech, Wuhan, China, dilution 1:100) at 4°C overnight, followed by incubation with the secondary antibodies for 30 min at room temperature. Sections were then stained with a DAB chromogenic kit (Changdao Biotechnology, Shanghai, China) and restained with hematoxylin. Cells stained brown and yellow were considered as positive. Positive results were evaluated on digital microphotographs. The percentage of positive cells was described as follows:13 0–5% for 0 score, 6–25% for 1 score, 26–50% for 2 scores, and more than 50% for 3 scores. The staining intensity was described as follows: 0 score for no staining, 1 score for weak staining, 2 scores for moderate staining, and 3 scores for strong staining. The scores from the percentage and intensity were added to an overall score. BLVRA protein with an overall score of 0–2 was defined as ‘low’, and with an overall score of 3–6 was defined as ‘high’.
Cell Lines and Cell Culture
Five human CRC cell lines and normal fetal human cells (FHC) cells were obtained from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). All cell lines were cultured in the appropriate media supplemented with 10% FBS and 1% penicillin/streptomycin, in a humidified incubator at 37°C with 5% CO2.
Cell Transfection
Lentiviral vector overexpressing BLVRA or BLVRA-specific siRNAs, as well as the appropriate negative controls (NC), were purchased from Shanghai Genechem (Shanghai, China). HT-29, and SW620 cells (1.5×105 cells per well) transfections were performed with FuGENE HD Transfection Reagent (Promega, USA).
Cell Proliferation Assay
Transfected HT-29 and SW620 cells, as well as blank controls and negative controls, were seeded in 96-well plates at 3×103 cells per well. Immediately or after culturing for various times (24 h, 48 h, or 72 h), 20 µL MTT (5 mg/mL) was added to each well and incubated for 4 h. Afterward,150 µL DMSO was added to each well and OD values were measured at 490 nm with a microreader.
Flow Cytometric Assessment of Apoptosis
Cells were collected after being cultured for 24 h in six-well plates. After the addition of 10 μL Annexin V-FITC and 5 μL PI (Fcmacs, Nanjing, China), cells were incubated at room temperature in the dark for 15 minutes. Apoptotic cells were quantified by fluorescence-activated flow cytometry (FACS).
Transwell Assays
For migration and invasion assays,1×105 cells were seeded in the upper chambers with 200 µL serum-free medium or Matrigel (BD Biosciences, San Jose, CA, USA), respectively. Corning Costar filters (Corning, NY, USA) with a pore size of 8 μm were inserted into the lower chambers with medium containing 20% FBS. After 24 h incubation, each well was dyed with a crystal violet solution (Beyotime Biotechnology, Shanghai, China) for 30 min, and cells on the top filter were wiped off with a cotton swab. The numbers of migratory or invasive cells were counted in five randomly picked fields by microscopy.
Real-Time Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted with Invitrogen Trizol (Thermo Fisher Scientific, Waltham, MA, USA) and quantified using a UV spectrophotometer. After reverse transcription (Transgen, Beijing, China), RCR amplification was performed by Real-Time PCR using the Fermentas SYBR Green PCR reagent (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer’s instructions, in an ABI 7300 Real-Time PCR system (Thermo Fisher Scientific). Results were processed using the ABI 7300 SDS software (Thermo Fisher Scientific) and normalized to GAPDH mRNA levels. Each reaction was performed in triplicate.
Immunofluorescence Staining
CRC cells were seeded into 24-well plates containing glass coverslips and fixed with 4% paraformaldehyde, permeabilized with Triton X-100, and blocked with 5% bovine serum albumin. Cells were then incubated with the primary antibody (Cell Signaling Technology, Inc, Shanghai, China; 1:200) overnight at 4°C, followed by incubation with Alexa Fluor 647-conjugated secondary antibody (Thermo Fisher Scientific) for 1 h. Cell nuclei were stained with DAPI. Images were captured under an inverted fluorescence microscope.
Western Blot Analysis
Proteins were extracted from samples with RIPA buffer (KeyGEN BioTECH, Nanjing, China) according to the manufacturer’s protocol. Total protein concentrations were measured using the BCA assay. Equal amounts of proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto PVDF membranes, which were blocked in 5% BSA for 1.5 h, followed by overnight incubation at 4°C with different antibodies (BLVRA 1:1000, cyclin D1 1:1000, Bcl2 1:1000, Bax 1:5000, survivin 1:1000, caspase-7 1:1000, E-cadherin 1:1000, vimentin 1:1000, snail 1:1000, Wnt5a 1:1000, β-catenin 1:1000, p-β-catenin 1:1000, p-GSK-3β 1:1000, p-PTEN 1:1000, C-myc 1:1000, COX-2 1:1000, and GAPDH 1:5000).After incubating with the corresponding secondary antibodies for 2 h, protein bands were visualized using a Western blotting analysis system.
Statistical Analysis
All data were analyzed with GraphPad Prism statistical software and the results were expressed as the mean ± standard deviation (SD). Analysis of statistical significance was performed with Student’s t-test for two groups and one-way ANOVA for more than two groups; p values <0.05 were considered statistically significant. NS was considered as no significance.
Results
BLVRA Is Highly Expressed in CRC Patients
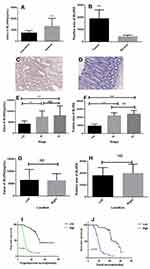
ELISA revealed that the serum level of the BLVRA protein was significantly higher in CRC patients than in healthy volunteers (Figure 1A). Moreover, tumors displayed significantly more widespread IHC staining for BLVRA than adjacent tissues (Figure 1B–D).
We also examined whether the levels of BLVRA differed among tumors of different clinical stages and positions. Results showed that the serum levels (Figure 1E) and the size of the stained area (Figure 1F) had significantly higher values in samples from stage III/IV tumors than from stage I/II tumors. With respect to position, neither the serum levels (Figure 1G) nor the size of the stained area (Figure 1H) significantly differed between right- and left-sided colon cancer. In addition, the high expression of BLVRA was associated with poor progression-free survival and overall survival of CRC patients (Figure 1I and J).
HT-29 and SW620 Cell Lines Were Selected for the Following Experiments
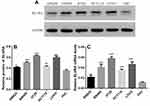
To choose the appropriate line for each experiment, their endogenous BLVRA expression levels had to be determined. Real-time polymerase chain reaction (RT-PCR) and Western blotting showed that BLVRA expression was higher in all CRC cell lines than in FHC cells. Among the CRC cell lines, SW620 exhibited the lowest and HT-29 the highest expression of BLVRA; thus, they were chosen for the overexpression and the knockdown experiments, respectively (Figure 2A–C).
Overexpression of BLVRA in SW620 Cells Promoted Proliferation, Stimulated Migration/Invasion, Accelerated EMT, and Inhibited Apoptosis
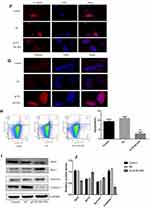
To further investigate the function of BLVRA in CRC, we infected SW620 cells with a BLVRA overexpression lentivirus or the corresponding negative control. The specific cell line was selected because its relatively low (compared to the other four tested CRC lines) endogenous BLVRA expression should make for more easily observed overexpression results. Both RT-PCR analysis and Western blotting confirmed the overexpression of BLVRA in the transfected cells (Figure 3A).
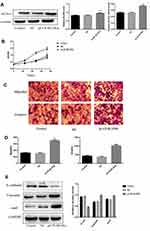 |
Figure 3 Continued |
We first examined whether BLVRA overexpression affected proliferation in CRC cells. MTT assays demonstrated that BLVRA overexpression significantly increased the proliferation of SW620 cells (Figure 3B). Next, we investigated the effect of BLVRA on migration and invasion. Transwell assays showed that overexpression of BLVRA significantly promoted both processes (Figure 3C–D).
Based on the augmenting effect of BLVRA on cell invasion and migration, we next asked whether BLVRA also affected the expression of EMT (endothelial to mesenchymal transition)-related proteins in CRC cells. Western blotting (Figure 3E) and immunofluorescence staining (Figure 3F–G) revealed that BLVRA overexpression significantly downregulated E-cadherin, whereas it upregulated vimentin and snail. These results strongly indicated that the overexpression of BLVRA could accelerate the EMT of CRC cells.
Finally, FACS analysis showed that BLVRA overexpression significantly reduced the ratio of apoptotic cells (Figure 3H). Consistently, Western blotting revealed that the transfected SW620 cells had higher expression level of the anti-apoptotic proteins, Bcl-2 and Survivin, and lower expression levels of Caspase 7 and the pro-apoptotic BAX protein (Figure 3I and J).
BLVRA Knockdown in HT-29 Cells Reduced Proliferation, Suppressed Migration/Invasion, Hindered EMT, and Promoted Apoptosis
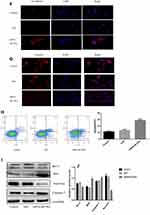
For this set of experiments, we used the HT-29 line. As mentioned above, HT-29 cells had the highest endogenous BLVRAexpression among the tested CRC lines; therefore, we expected that the results of BLVRA knockdown would be more easily observable in the specific cells. The success of the knockdown was confirmed by RT-PCR and Western blotting analysis (Figure 4A).
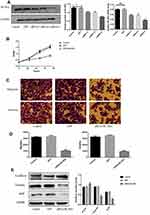 |
Figure 4 Continued |
BLVRA knockdown in HT-29 cells had the exact opposite effects compared with the BLVRA upregulation in SW620 cells. Specifically, it markedly decreased proliferation of HT-29 over time (Figure 4B), significantly reduced the migration and invasion potential of HT-29 cells (Figure 4C–D), delayed EMT (Figure 4E–G), and enhanced apoptosis (Figure 4H–J).
BLVRA Exerts Its Effects in CRC Cells via the Wnt/β-Catenin Signaling Pathway
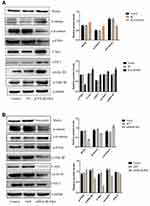
Our next step was to establish the pathway(s) that mediate the observed effects of BLVRA. Our previous experiments showed that survivin is downregulated by BLVRA. This finding, combined with the fact that survivin is a known target of the Wnt/β-catenin signaling pathway,14,15 made us focus on the said pathway.
Overexpression of BLVRA in SW620 cells increased the levels of Wnt5a and β-catenin, and reduced the levels of p-β-catenin (Figure 5A), suggesting that BLVRA indeed activated the Wnt/β-catenin signaling pathway. Additional supporting evidence was the observed increases in C-myc, COX-2, cyclin D1, and p-GSK-3β levels, all of which are positive targets of this pathway, and the reduced level of p-PTEN, which is a negative target of the pathway (Figure 5A). As expected, knocking down BLVRA in HT-29 had the opposite effects on the levels of the aforementioned proteins (Figure 5B). As a whole, these results strongly indicated that BLVRA induced the progression of CRC cells by regulating the Wnt/β-catenin signaling pathway.
Discussion
In this study, CRC tissues were found to express higher levels of BLVRA than adjacent healthy tissues. Moreover, patients had higher serum levels of the protein than healthy controls. The serum and tissue levels positively correlated with tumor stage, which means they were indicative of poor prognosis; in contrast, they did not differ between left- and right-sided CRC. Our results indicated that BLVRA had a tumor-promoting effect in CRC, prompting us to determine which biological properties of these cells were directly affected by BLVRA. In vitro overexpression and knockdown experiments using two CRC cell lines asserted that BLVRA increased proliferation and augmented the migration and invasion potential of CRC cells. These results were consistent with those of BLVRA knockdown studies in other malignancies.7,10
Apoptosis, a conserved mechanism of programmed cell death, is malfunctioning in several cancers. Emerging evidence has shown that defective apoptosis not only promoted tumorigenesis but also brings about resistance of cancer cells to treatment. In the current study, we showed for the first time that BLVRA hindered apoptosis in CRC cells by upregulating the anti-apoptotic proteins, Bcl-2 and Survivin, and downregulating the pro-apoptotic proteins, BAX (which is necessary for the activation of the mitochondria-dependent apoptosis pathway) and caspase-7 (which is an executor of apoptosis).16,17 It is worth noting that these functions may be interconnected, i.e., one mediating another. Survivin, which is also a target of the Wnt/β-catenin signaling pathway,14,15 is another anti-apoptotic gene. For example, knocking down survivin in breast cancer has been shown to induce apoptosis by upregulating caspase-7 and caspase-3. Therefore, it was possible that BVR was propitious to the emergence of tumor resistance by inhibiting apoptosis and may serve as a specific target for cancer diagnosis and treatment. This anti-apoptotic effect is consistent with a previous study reporting that BLVRA may mediate the hypoxia-induced inhibition of apoptosis.18
EMT is a pivotal step in the process toward tumor recurrence and metastasis. It involves the loss of cell-to-cell adhesion and the adoption of migratory and invasive characteristics, which is accompanied by the shift from an epithelial to a mixed epithelial/mesenchymal or mesenchymal phenotype, respectively.19 E-cadherin is a major player in cell-cell adhesion; as such, its downregulation is essential for EMT and cell migration. In contrast, vimentin, a type-III intermediate filament protein expressed in mesenchymal cells that is considered a typical EMT marker, as well as snail are upregulated during EMT.20 In our CRC lines, BLVRA downregulated E-cadherin while it upregulated vimentin and snail, suggesting that it promoted EMT in CRC by Western blotting and immunofluorescence staining. This result was consistent with those of previous studies, in which BLVRA was shown to promote EMT in breast cancer10 and NSCLC7 by repressing E-cadherin expression.
Our next step was the identification of the mechanism through which BLVRA exerted its actions in CRC cells. Beyond its namesake enzymatic activity, BLVRA participated in several signaling pathways, such as the PRC-zeta and the PI3K/AKT pathways.21,22 BVR was recently recognized as a regulator of signal transduction pathways. It has been reported that BLVRA may modulate PI3K/AKT signaling pathway in renal fibrosis. It also regulates the ERK1/2 signal pathway in breast cancer.10 On the other hand, multiple signaling pathways are known to participate in CRC progression, including Wnt/β-catenin, PI3K/AKT/mTOR, and Notch.23,24 The Wnt/β-catenin pathway is essential for development and microenvironment homeostasis in stem cells, and also plays a major role in EMT and apoptosis.25 As such, mutations in its components lead to multiple growth‐related disorders and cancer, including CRC.24,26 In the aforementioned study, we discovered BLVRA could modulate apoptosis and EMT, whether it took effects by activating Wnt/β-catenin pathway remained indeterminate. This pathway, which is tightly regulated in normal colon stem cells, can be derailed by various factors, such as the abnormal expression of Wnt. Interestingly, even non-protein regulatory factors, such as miR‐27a and miR‐590‐3p, reportedly promote the proliferation and invasiveness of colon cancer through this pathway.27,28 Canonical Wnt/β-catenin signaling is also directly linked to its extracellular secretion for tumor progression of colon CSCs.29
In this pathway, without Wnt, β-catenin cannot accumulate in the cytoplasm as it is phosphorylated by glycogen synthase kinase 3-beta (GSK3β), then ubiquitinated and degraded by the proteasome. In the presence of Wnt, the stable, non-phosphorylated β‐catenin enters the nucleus to form an active complex with lymphoid enhancer factor (LEF) and T-cell factor (TCF), thus leading to the transcriptional activation of β-catenin target genes such as the cyclin D1, c-Myc, cox-2, PTEN, and survivin. The activated Wnt signaling pathway exerts critical functions in malignant cancer progression, including the emergence of radioresistance.30 Among Wnt ligands, there are more than 19 closely related but distinct secreted cysteine‐rich glycoproteins. The basic expression level of β‐catenin is associated with a favorable or unfavorable prognosis in several solid cancers. In our study, we discovered that overexpression of BLVRA activated the Wnt/β‐catenin signaling pathway and affected its target genes to inhibit apoptosis and induce EMT. In contrast, knockdown of BLVRA inactivated the Wnt/β‐catenin signaling pathway, causing the opposite effects to its target genes compared to overexpression. Overall, the complicated matrix of this pathway’s target genes may lead to distinct roles in the progression of CRC.
To sum up, BLVRA acted as a stimulator in CRC progression, asserting this effect by regulating the Wnt/β‐catenin signaling pathway. Therefore, BLVRA could be considered a novel prognostic factor in CRC. One may go as far as to consider it as a potential target of chemotherapy, especially since it has been found to be related to chemotherapy resistance in other types of cancer, namely gliomas.31,32 However, further investigation is required in order to ascertain whether it can be utilized as a therapeutic target for colon cancer treatment.
Abbreviations
BLVR or BVR, Biliverdin reductase; BLVRA, Biliverdin reductase A; CRC, colorectal cancer; EMT, endothelial to mesenchymal transition.
Acknowledgments
This study was funded by the National Natural Science Fund (No. 81673736) as well as the Yangzhou Science and Technology Bureau (YZ2018071).
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Su W-B, Liu Z-Y. MiR-431 inhibits colorectal cancer cell invasion via repressing CUL4B. Eur Rev Med Pharmacol Sci. 2019;22:5051–5052. doi:10.26355/eurrev_201805_15062.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics. CA Cancer J Clin. 2017;67:7–30. doi:10.3322/caac.21387
3. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi:10.3322/caac.21338
4. Shang H, Wang T, Shang F, et al. Over-expression of DJ-1 attenuates effects of curcumin on colorectal cancer cell proliferation and apoptosis. Eur Rev Med Pharmacol Sci. 2019;23:3080–3087. doi:10.26355/eurrev_201904_17591.
5. Gibbs PE, Miralem T, Maines MD. Biliverdin reductase: a target for cancer therapy? Front Pharmacol. 2015;6:119. doi:10.3389/fphar.2015.00119.
6. Kubickova KN, Subhanova I, Konickova R, et al. Predictive role BLVRA mRNA expression in hepatocellular cancer. Ann Hepatol. 2016;15:881–887. doi:10.5604/16652681.1222104.
7. Liu F, Song S, Yi Z, et al. HGF induces EMT in non-small-cell lung cancer through the hBVR pathway. Eur J Pharmacol. 2017;811:180–190. doi:10.1016/j.ejphar.2017.05.040
8. Zhang B, Liang X, Shi W, et al. Role of impaired peritubular capillary and hypoxia in progressive interstitial fibrosis after 5/6 subtotal nephrectomy of rats. Nephrol. 2005;10(4):351–357. doi:10.1111/j.1440-1797.2005.00412.x
9. Subhanova I, Muchova L, Lenicek M, et al. Expression of biliverdin reductase A in peripheral blood leukocytes is associated with treatment response in HCV-infected patients. PLoS One. 2013;8(3):e57555. doi:10.1371/journal.pone.0057555
10. Zhang M, Song S, Yi Z, et al. Human biliverdin reductase promotes EMT through the ERK1/2 signal pathway in breast cancer. Eur J Phamacol. 2016;788:45–53. doi:10.1016/j.ejphar.2016.06.019
11. Arena V, Pennacchia I, Guerriero G, Mancuso C. The heme oxygenase/biliverdin reductase system in skin cancers. J Biol Regul Homeost Agents. 2015;29:259–264.
12. Zhang J, Wang K, Zhang J, et al. Using proteomic approach to identify tumor-associated proteins as biomarkers in human esophageal squamous cell carcinoma. J Proteome Res. 2011;10(6):2863–2872. doi:10.1021/pr200141c
13. TONG JD, JIAO NL, WANG YX, et al. Downregulation of fibulin-3 gene by promoter methylation in colorectal cancer predicts adverse prognosis. Neoplasma. 2011;58(5):441–448. doi:10.4149/neo_2011_05_441
14. Ma H, Nguyen C, Lee K-S, et al. Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene. 2005;24:3619–3631. doi:10.1038/sj.onc.1208433
15. Tetsu O, McCormick F. Proliferation of cancer cells despite CDK2 inhibition. Cancer Cell. 2003;3:233–245. doi:10.1016/s1535-6108(03)00053-9
16. Gavathiotis E, Reyna DE, Davis ML, et al. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi:10.1016/j.molcel.2010.10.019
17. Tian Z, Wang J, Xu M, et al. Resveratrol improves cognitive impairment by regulating apoptosis and synaptic plasticity in streptozotocin-induced diabetic rats. Cell Physiol Biochem. 2016;40:1670–1677. doi:10.1159/000453216
18. Song S, Yi Z, Zhang M, et al. Hypoxia inhibits pulmonary artery endothelial cell apoptosis via the e-selectin/biliverdin reductase pathway. Microvasc Res. 2016;106:44–56. doi:10.1016/j.mvr.2016.03.009
19. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Investig. 2009;119(6):1420–1428. doi:10.1172/JCI39104
20. Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi:10.1038/35000025
21. Lerner-Marmaros N, Miralem T, Gibbs PE, Maines MD. Regulation of TNF-alpha-activated PKC-zeta signaling by the human biliverdin reductase: identification of activating and inhibitory domains of the reductase. FASEB J. 2007;21:3949–3962. doi:10.1096/fsb2.v21.14
22. Lerner-Marmaros N, Miralem T, Gibbs PE, Maines MD. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc Natl Acad Sci U S A. 2008;105:6870–6875. doi:10.1073/pnas.0800750105
23. Mao HY, Liu SP, Kong GM, et al. FBLN3 inhibits the invasion and metastasis of colorectal cancer through the AKT/mTOR pathway. Neoplasma. 2019;66:336–342. doi:10.4149/neo_2018_180703N441
24. Qiu C-Z, Wang M-Z, Yu W-S, et al. Correlation of GOLPH3 gene with Wnt signaling pathway in human colon cancer cells. J Cancer. 2016;7:928–934. doi:10.7150/jca.13968
25. Vallée A, Lecarpentier Y, Guillevin R, Vallée J-N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim Biophys Sin. 2017;49:853–866. doi:10.1093/abbs/gmx073
26. Nusse R, Clevers H. Wnt/beta‐catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi:10.1016/j.cell.2017.05.016
27. Ba S, Xuan Y, Long ZW, et al. MicroRNA‐ 27a promotes the proliferation and invasiveness of colon cancer cells by targeting SFRP1 through the Wnt/beta‐catenin signaling pathway. Cell Physiol Biochem. 2017;42:1920–1933. doi:10.1159/000479610
28. Feng Z-Y, Xu X-H, Cen D-Z, et al. miR‐590‐3p promotes colon cancer cell proliferation via Wnt/beta‐catenin signaling pathway by inhibiting WIF1 and DKK1. Eur Rev Med Pharmacol Sci. 2017;21:4844–4852.
29. Lamichane BD, Jung SY, Yun J, et al. AGR2 is a target of canonical Wnt/b-catenin signaling and is important for stemness maintenance in colorectal cancer stem cells. Biochem Biophys Res Commun. 2019;515:600–606. doi:10.1016/j.bbrc.2019.05.154
30. Jing Q, Li G, Chen X, et al. Wnt3a promotes radioresistance via autophagy in squamous cell carcinoma of the head and neck. J Cell Mol Med. 2019;21:1–12. doi:10.1111/jcmm.14394.
31. Florczyk U, Golda S, Zieba A, et al. Overexpression of biliverdin reductase enhances resistance to chemotherapeutics. Cancer Lett. 2011;300:40–47. doi:10.1016/j.canlet.2010.09.003
32. Kim SS, Seong S, Hyeon Lim S, et al. Biliverdin reductase plays a crucial role in hypoxia-induced chemoresistance in human glioblastoma. Biochem Biophys Res Commun. 2013;440:658–663. doi:10.1016/j.bbrc.2013.09.120
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.